Functional response of Cydnodromus picanus (Acari: Phytoseiidae) on two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae)
DOI:
https://doi.org/10.15446/acag.v66n2.55054Palabras clave:
Biological control, predation, Holling's disc equation, attack rate, handling time (es)Descargas
The functional response of adult females of predatory mite Cydnodromus picanus Ragusa (Acari: Phytoseiidae) was evaluated at different egg densities (5, 10, 20, 30, 40, 50, 60, 70, 80, 100 and 150 eggs per predator) of Tetranychus urticae Koch (Acari: Tetranychidae), which had 16, 42 and 65 h since oviposition. The experimental design was in a completely randomized blocks with five replicates per treatment. The environmental conditions of the trials were 25±2°C temperature, 50±2% of relative humidity and a photoperiod of 16:8 hours (light: dark). The average consumption rates for the three kinds of prey showed no significant differences (p>0.05) being 20.56±1.02, 18.59±0.79 and 18.38±0.94 prey/predator for eggs of 16, 42 and 65 h age, respectively. Using a logistic regression, a type II functional response on C. picanus females for the three kinds of eggs was determined. The values of response parameters for C. picanus females were as follows: Attack rate (a): 0.055±0.006, 0.076±0.009 and 0.073±0.016; Handling time (Th): 0.684±0.036, 0.894±0.034 and 0.898±0.062; for eggs of 16, 42 and 65 h age, respectively. These values are within the range of variation for different species of phytoseiids. These results suggest that C. picanus could effectively regulate populations of T. urticae in the field.
Recibido: 8 de enero de 2016; Aceptado: 4 de marzo de 2016
Abstract:
The functional response of adult females of predatory mite Cydnodromus picanus Ragusa (Acari: Phytoseiidae) was evaluated at different egg densities (5, 10, 20, 30, 40, 50, 60, 70, 80, 100 and 150 eggs per predator) of Tetranychus urticae Koch (Acari: Tetranychidae), which had 16, 42 and 65 h since oviposition. The experimental design was in a completely randomized blocks with five replicates per treatment. The environmental conditions of the trials were 25±2°C temperature, 50±2% of relative humidity and a photoperiod of 16:8 hours (light: dark). The average consumption rates for the three kinds of prey showed no significant differences (p>0.05) being 20.56±1.02, 18.59±0.79 and 18.38±0.94 prey/predator for eggs of 16, 42 and 65 h age, respectively. Using a logistic regression, a type II functional response on C. picanus females for the three kinds of eggs was determined. The values of response parameters for C. picanus females were as follows: Attack rate (a): 0.055±0.006, 0.076±0.009 and 0.073±0.016; Handling time (Th ): 0.684±0.036, 0.894±0.034 and 0.898±0.062; for eggs of 16, 42 and 65 h age, respectively. These values are within the range of variation for different species of phytoseiids. These results suggest that C. picanus could effectively regulate populations of T. urticae in the field.
Key words:
Biological control, predation, Holling's disc equation, attack rate, handling time.Resumen:
La respuesta funcional de hembras adultas del ácaro depredador Cydnodromus picanus Ragusa (Acari: Phytoseiidae) se evaluó a diferentes densidades de huevos de Tetranychus urticae Koch (Acari: Tetranychidae) que tenían 16, 42 y 65 horas de edad. Las densidades evaluadas fueron: 5, 10, 20, 30, 40, 50, 60, 70, 80, 100 y 150 huevos por depredador. Las condiciones ambientales de los ensayos fueron 25±2 °C de temperatura, 50±2% de humedad relativa y un fotoperíodo de 16:8 horas (luz: oscuridad). Las tasas de consumo promedio para los tres tipos de presas no presentaron diferencias significativas (p>0,05) siendo de 20,56±1,02; 18,59±0,79 y 18,38±0,94 presas/depredador para los huevos de 16, 42 y 64 h de edad, respectivamente. Utilizando una regresión logística, se determinó una respuesta funcional de tipo II para las hembras de C. picanus para los tres tipos de presas. Los valores de los parámetros de la respuesta funcional para las hembras de C. picanus fueron: Tasa de ataque (a): 0.055±0.006; 0.076±0.009 y 0.073±0.016; Tiempo de manipulación (Th ): 0.684±0.036; 0.894±0.034 y 0.898±0.062, para los huevos de 26, 42 y 65 h d edad, respectivamente. Estos valores están dentro del rango de variación para diferentes especies de fitoseidos. Estos resultados sugieren que C. picanus podría efectivamente regular poblaciones de T. urticae a nivel de campo.
Palabras clave:
Control biológico, depredación, Ecuación del disco de Holling, tasa de ataque, tiempo de manipulación.Introduction
Tetranychus urticae Koch (Acari: Tetranychidae) is a cosmopolitan and polyphagous pest that attacks many economic importance crops in Chile, such as vegetables, extensive cultures (cotton, corn, etc.), citrus, vine and other fruit and ornamental trees. Although different strategies for control of T. urticae are recommended worldwide, the use of natural enemies has been highlighted as a tool of first order for biological control of this pest, especially the use of predator mite Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae) (De Moraes, 2004). However, this practice has been less common that the use of chemical acaricides, which improperly used, can cause resurgence, resistance and other problems of pests due to the elimination of natural enemies (Vitelli & Sato, 2016).
Cydnodromus picanus, was described in 2000 on Citrus aurantium L. (Rutaceae) and reported only for Chile (Ragusa, 2000; De Moraes et al., 2004). Recent researches suggest C. picanus, could be considered as junior synonymy of Cydnodromus idaeus (Denmark and Muma) (Tixier et al., 2011). According to Ragusa et al. (2000), this species could be used in arid and warm agroecosystems, where is difficult to find phytoseiids that are adapted to these extreme conditions to control tetranychids. Based on classification of McMurtry & Croft (1997), C. picanus is a generalist predator belonging to type III lifestyle. In conditions of absence of prey can feed of pollen from different species (Ragusa et al. 2000), depredates on Oligonychus yothersi (McGregor) in Persea americana Mill. (Lauraceae) (Rioja & Vargas, 2009) and on Tetranychus cinnabarinus Boisduval in Dianthus caryophyllus Linn. (Caryophyllaceae) (Tello et al., 2009). The citrus are one of the more important crops in the zone of Pica, Chile (20º 29`12.4" S; 69º 19` 33.9" O), where C. picanus had been found associated to Panonychus citri (McGregor) (Ragusa et al., 2000).
The present research provides information about the predatory efficiency of C. picanus throughout functional response. The equations of the functional response are a key component of any mechanistic model, because they describe the interactions among consumers and its resources (Villemereuil & López-Sepulcre, 2011). This information is vital for the implementation of conservation programs for natural enemies and augmentation-type biological control (Ragusa et al., 2010). The effectivity of a predator is directly related to the type of its functional response. Predatory arthropods unfold one of the three typical functional responses, but these responses may vary with the crop phenology, the habitat heterogeneity, the predator age and other biotic and abiotic factors. In type I functional response, there is a lineal increase of predator attack rate respect to prey density (with a slope equal to the search efficiency), until reaching a point at which the maximum attack rate remains constant, even if the prey density increases. The response is estimated by a linear equation Na=α+βN0 ; where Na =number of prey eaten, N0 =prey density (number of prey offered), α and β are the y-intercept and the slope of prediction line, respectively. In type II functional response appears another parameter, in addition to the search efficiency, termed "handling time" (Th ) defined as the time that invests the predator in chasing, capturing, consuming and digesting a prey. In type II functional response, the number of prey consumed hyperbolically approaches to an asymptote in which the maximum attack rate is expressed (K=T/Th ). This type of response is estimated by a curved line function: Na =aN0T/(1+aN0Th), where Na =number of prey consumed, a=constant attack rate or instantaneous search rate, N0 =initial prey density, T=total available time and Th =handling time. The type III functional response is performed when the prey-consumed number approaches an asymptote as a sigmoid function (increases in the proportion of prey killed to an inflection point and then decreases similarly to type II response). The type III functional response may be derived using the model of Hassell (Hassell, 1978), where the attack rate (a) is a hyperbolic function of prey density: a=(d+hN0)/(1+cN0 ), substituting the value of the Holling equation, obtaining the type III model: Na=(dN0T+hN0 2T )/(1+cN0+dN0Th+bN0 2T h ), where Na =number of prey consumed, N0 =initial prey density, T=total time, Th =handling time and b, c, and d are constants.
Tello et al. (2009), proved the life parameters of C. picanus when fed with eggs, the same happened with survival, achieving 100% when fed with this stadium. For those experiments, eggs less than 20 h old were performed. In addition, the functional response of this phytoseiid was affected by the maturity of the egg offered as food.
The aim of this research was to evaluate the consumption rates of C. picanus (=C. idaeus) in relation to eggs density of T. urticae and to determine the effect of egg maturity on functional response parameters, under laboratory conditions.
Material and methods
Establishment of colonies
The bioassays were performed in the Regional Research Center INIA La Cruz (Instituto de Investigaciones Agropecuarias (INIA), La Cruz, Chile), at a temperature of 25±2°C, 50±2% of relative humidity (RH) and a photoperiod of 16:8 h (L:D). Cydnodromus picanus, was collected from trees of C. aurantium in Pica, Chile (20°29' S; 69°19' O) and bred in acrylic plates of 12x12 cm according to the methodology described by Swirski, Amitai & Dorzia (1970), in breeding rooms and fed only with pollen from Oxalis sp (Oxidales) to avoid habituation to prey. Tetranychus urticae obtained from breeding rooms located in INIA La Cruz. The colonies of this tetranychid had a time of about four months since they were collected in the field. Tetranychus urticae was reproduced on bean plants (Phaseolus vulgaris L.), that were infested with the mite when they had two true leaves. To maintain available plants, 100 pots weekly seeded with 20 bean seeds.
Consumption rate and functional response
The functional response of C. picanus on T. urticae eggs of different maturity time studied in separated bioassays. For each maturity time, one phytoseiid adult female, previously subjected to 24 h fasting, was confined in an artificial arena (black acrylic plate of 4 cm diameter) (Tello et al., 2009). These C. picanus females came from a cohort of eggs of the same age, using newly emerged females. Glue (Point Sticken Glue, Point Chile S.A.) was applied in the edges plates to prevent the mites escape. Therefore, T. urticae eggs of different maturity times (16, 42 and 65 h) were introduced as preys in the plates at densities of 5, 10, 20, 30, 40, 50, 60, 70, 80, 100 and 150 eggs per plate. T. urticae eggs were obtained by depositing on a bean leaf about 100 females to oviposit and at 16h were transferred into trial arenas, replicating this for eggs of 42 and 65 h. The number of T. urticae eggs consumed by the predator recorded at 24 h (experimental time). The observations were made using a stereomicroscope with 40X magnification (Zeiss Stemi 6V 6, Alemania).
Statistical analysis
The consumption among eggs with different maturity (16, 42 and 65 h) were compared. In addition, for each age, among different densities. Because consumption data is not normally distributed, even after applying transformations (e.g. logarithmic and square root). The non-parametric Kruskal-Wallis test with post-hoc comparison were performed to determine the type of functional response, data of each density were fitted to maximum likelihood logistic regression (CATMOD procedure, SAS Institute, Cary, NC, USA). (Equation 1).
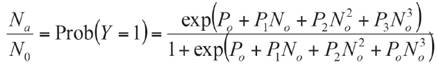
Where Y, is the dichotomous dependent variable, representing a non-consumed (Y=0) or consumed (Y=1) prey at the end of the trial, Na is the number of prey consumed, No is the number of prey available; and P0, P1, P2 and P3 are the intercept, linear, quadratic and cubic coefficient, respectively. Negative or positive significant linear coefficients (e.g., P1 ) indicate a type II or III functional response, respectively. The slope of type II response decreases (e.g., P1 is negative), while that slope of type III increases (e.g., P1 is positive). After determining the type of functional response, its parameters (attack rate and handling time) are estimated through linearization of Holling' s disc equation depending on the type of functional response (Xiao & Fadamiro, 2010). Type II equation was used: a and Th parameters were calculated using a nonlinear regression by least squares based on Gauss-Newton method (SAS Institute, Cary, NC, USA). The a/Th value indicates the effectiveness of depredation. The maximum depredation rate (K) was calculated as T/Th . To perform comparisons between a and Th parameters, standard deviation was estimated with 95% confidence interval using Jackknife statistical technique available in SAS (SAS Institute, Cary, NC, USA). Later, the parameters were compared using non-parametric Kruskal-Wallis test (α=0.05) due to heterogeneity of variances and non-normally distributed residuals.
Results and discussion
Consumption rate
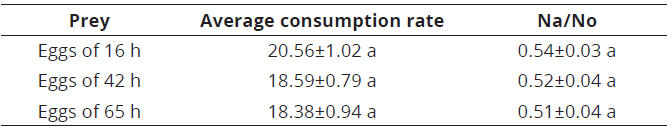
The average consumption rate of C. picanus did not show significant differences among the three types of eggs offered [KW=4.04; gl=2; P=0.131]. This indicates that the maturity time of eggs offered as prey have no influence in the preference by C. picanus. The predator consumed 54, 52 and 51% of initial egg density at 16, 42 and 65 h, respectively, not showing significant differences [KW =1.166; gl=2; P=0.556] (Table 1).
Equal letters in the same column indicate no significant differences according to Kruskal-Wallis test (P<0.05); Na=number of prey consumed; No=initial prey density; SE=standard error.Table 1: Average consumption rate (±SE) and proportion of prey consumed (±SE) for Cydnodromus picanus fed with Tetranychus urticae eggs of different maturity times
From a density of 50 eggs.predator-1, the age of eggs did not affect the predation by C. picanus; 5 (KW=0.32, gl=2, P=0,3679), 10 (KW=2.33, gl=2, P<0.1503), 20 KW=0.43, gl=2, P=0.7884), 30 (KW=4.76, gl=2, P<0.0913), 40 (KW=0.04, gl=2, P=0.9807) and 50 (KW=1.35, gl=2, P=0.5053). The Kruskal-Wallis test did not show significantly differences (P>0.05) for those prey densities (Table 2). Differences showed densities of 60 (KW=12.49, gl=2, P<0.05), 70 (KW=8.75, gl=2, P<0.05) and 100 (KW=8.54, gl=2; P<0.05) eggs.predator-1. The number of attacked eggs per capita was significantly affected by prey density for each egg maturity (16h: KW=54.87, gl=10, P< 0.0001), 42h: KW=43.23, gl=10, P<0.0001 and 65h: KW=46.31, gl=10, P<0.0001).
Means in the same row followed by different uppercase letters are significantly different; means in the same column followed by different lowercase letters are significantly different (P<0.05; Kruskal-Wallis Test). SE=standard error.Table 2: Prey consumption (mean ± SE) for Cydnodromus picanus when fed with Tetranychus urticae eggs of three maturity times at different prey densities

Functional response
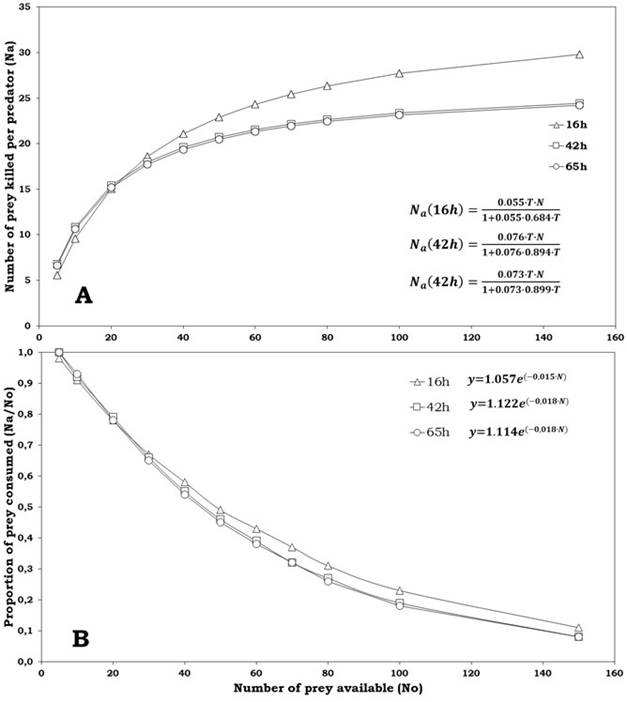
The logistic regression delivered a significantly negative linear parameter (P1 <0) for the three classes of prey offered (eggs of 16, 42 and 65 h), suggesting that C. picanus performs a type II functional response for the three types of prey (Table 3). The functional response curves (Figure 1A), showed a high proportion of prey consumed at lower densities in the three kinds of prey. The proportion of eggs consumed by predator (Na/N0 ) declined with the increase of prey density (Figure 1B); this implies that there is an inverse density-dependence, being confirmed by logistic regression (Table 3) that, for all ages of eggs, presented a negative linear coefficient (P1 <0) and a positive quadratic coefficient (P2 >0), both significant (P<0.001; Table 3).
Figure 1: Consumption of Tetranychus urticae eggs (A) and proportion of prey consumed (B) per Cydnodromus picanus females according to provided density (fitted equations inside plots)
a Significant at P<0.001, SE=standard error.Table 3: Selection of functional response model based on maximum likelihood analysis of parameter estimations (±SE) using the proportion of eggs consumed as a polynomial function of offered eggs density

The comparison of functional response curves revealed significant differences among the three kinds of eggs offered (a: KW=11.94, gl=2, P<0.05; Th : KW=11.51, gl=2, P<0.05). The maximum number of prey consumed was significantly higher for eggs of maturity of 16 h (Table 4). The estimated parameters of functional response for C. picanus, for the three types of prey, showed that the attack rate was higher for eggs of 42 h and 65 h than for eggs of 16 h. On the other hand, C. picanus showed the lowest handling time (Th ) for eggs of 16 h (Table 4). The slightly higher a/Th values recorded for C. picanus on eggs of 42 and 65 h suggest that this predator was more effective on consuming that sort of T. urticae eggs.
Values with equal letters in the same column are significantly not different according to Kruskal-Wallis test (P<0.05); a=search or attack rate; Th
=handling time (day);
R2
=determination coefficient; a/Th
=depredation efficiency; K=maximum depredation rate.Table 4: Values of R2 and parameters (mean ± SE) of functional response of Cydnodromus picanus adult females on Tetranychus urticae eggs of different maturity times estimated by disc equation.

The average eggs consumption rate is similar to those obtained for adult females of this phytoseiid species. Ragusa et al. (2000) indicates a daily consumption of about 16 eggs of T. urticae at 26±1°C and 70±5% HR. Tello et al. (2009) registered the same consumption value (16 eggs.day-1) with T. cinnabarinus eggs at 29.44±1.47°C and 42.35±5.01% RH.
Cydnodromus picanus, showed a type II functional response for each maturity time of eggs. This type of functional response is registered for different phytoseiid especies, including: Amblyseius californicus (Mc-Gregor) (Gotoh, Nozawa & Yamaguchi, 2004); Neoseiulus longispinosus (Evans) (Rahman, Babu, Roobakkumar & Perumalsamy, 2012); Phytoseiulus persimilis Athias-Henriot, Galendromus occidentalis (Nesbitt) (Xiao & Fadamiro, 2010); Neoseiulus cucumeris (Oudemans) (Madadi et al., 2007). In general, the results obtained in this study for a and Th parameters of functional response for C. picanus are within the range of variation for different phytoseiid especies (Table 5).
Table 5: Functional response parameters (a and Th
) for different phytoseiid species depredating on Tetranychus urticae eggs in a range between 15°C and 35°C

The search (or attack) rate and the handling time are parameters used to determine the magnitude of the functional responses. This parameter could be affected by different factors, such as the predator velocity, the prey movements and the spent time in subduing each prey (Hassell, 1978). In this research, the handling time for eggs of 16 h was significantly lower than for eggs of 42 and 65 h, which could be explained for the presence of a higher amount of vitellus and the easiness to extract it. When eggs mature, the embryonic development makes diminish the amount of vitellus, making difficult its extraction and making to take longer time to predator in feeding. Cabrera, Donohuea, Khalil, Sonenshinec & Roea (2009), pointed that there is no available studies describing the characteristics of proteins in the yolk of mite eggs. According to these authors, in newly oviposited tetranychids eggs (<3 h), the embryo development would be minimal and the proteins would be more available for predators. Therefore, we can deduce that as time progress and egg maturity with it; the eggs of 42 and 65 h present higher embryo development, so the amount of vitellus must be minimal and thus of lower nutritional value.
Conclusion
This research compared the average consumption rates and the functional response of predator mite C. picanus fed with T. urticae eggs of different maturity times. The results show that adult females of C. picanus exhibita type II functional response and a proportion of prey consumed that ranged between 51% and 54% for the three types of prey offered. Studies of predation in the field, with different crops and preys (tetranychid species), are necessary to consider this predator as a potential biological control agent in arid zones of Chile.
Acknowledgements
The authors thank to Arturo Prat University and to the Doctorate Program in Agriculture in Arid-Desert Environments at Renewable Natural Resources Faculty in Prat University
References
Referencias
Ahn, J.J., Kim, K.W. & Lee, J.H. (2010). Functional response of Neoseiulus californicus (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae) on strawberry leaves. J Appl Entomol, 134(2), 98–104. http://dx.doi.org/10.1111/j.1439-0418.2009.01440.x
Cabrera, A.R., Donohuea, K.V., Khalil, S.M.S., Sonenshinec, D.E., & Roea, R.M. (2009). Characterization of vitellin protein in the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). J Insect Physiol, 55(7), 655–661. http://dx.doi.org/10.1016/j.jinsphys.2009.04.006
De Moraes, G.J., McMurtry, J.A., Denmark, H.A. & Campos, C.B. (2004). A revised catalog of the mite family Phytoseiidae. Zootaxa, 1(434), 1-494. http://dx.doi.org/10.11646/zootaxa.434.1.1
Fantinou, A.A., Baxevani, A., Drizou, F., Labropoulos, P., Perdikis, D.,... (2012). Consumption rate, functional response and preference of the predaceous mite Iphiseius degenerans to Tetranychus urticae and Eutetranychus orientalis. Exp Appl Acarol, 58(2), 133–144. http://dx.doi.org/10.1007/s10493-012-9557-6
Farazmand, A., Fathipour, Y. & Kamali, K. (2012). Functional response and mutual interference of Neoseiulus californicus and Typhlodromus bagdasarjani (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). Int J Acarol, 38(5), 369–376. http://dx.doi.org/10.1080/01647954.2012.655310
Gotoh, T., Nozawa, M. & Yamaguchi, K. (2004). Prey consumption and functional response of three acarophagous species to eggs of the two-spotted spider mite in the laboratory. Appl Entomol Zool, 39(1), 97–104. http://dx.doi.org/10.1303/aez.2004.97
Hassell, M.P. (1978). The dynamics of arthropod predator-prey systems. Monogr Popul Biol, 13(3-7). 1-237.
Kuştutan, O. & Çakmak, I. (2009). Development, fecundity, and prey consumption of Neoseiulus californicus (McGregor) fed Tetranychus cinnabarinus Boisduval. Turk J Agric For, 33, 19–28. http://dx.doi.org/10.3906/tar-0806-39
Landeros, J., Cerda, P., Badii, M.H., Aguirre, L.A., Cerna, E.,... (2013). Functional response of Neoseiulus californicus on Tetranychus urticae on apple leaves. Southwest Entomol, 38(1), 79–84. http://dx.doi.org/10.3958/059.038.0108
Madadi, H., Enkegaard, A., Brodsgaard, H. F., Kharrazi-Pakdel, A., Mohaghegh, J.,…(2007). Host plant effects on the functional response of Neoseiulus cucumeris to onion thrips larvae. J Appl Entomol, 131(9–10), 728–733. http://dx.doi.org/10.1111/j.1439-0418.2007.01206.x
Ragusa, S., Vargas, R., Tsolakis, H. & Ashbach, R. (2000). Laboratory studies on the influence of various food subtances on some biological and life-table parameters of Cydnodromus picanus Ragusa (Parasitiformes, Phytoseiidae) associated with citrus trees in the Chilean desert. Phytophaga, 10, 1-23.
Rahman, V., Babu, A., Roobakkumar, A. & Perumalsamy, K. (2012). Functional and numerical responses of the predatory mite, Neoseiulus longispinosus, to the red spider mite, Oligonychus coffeae, infesting tea. J Insect Sci, 12, 125. http://dx.doi.org/10.1673/031.012.12501
Rioja, T.S. & Vargas, R.M. (2009). Life table parameters and consumption rate of Cydnodromus picanus Ragusa, Amblyseius graminis Chant, and Galendromus occidentalis (Nesbitt) on avocado red mite Oligonychus yothersi (McGregor) (Acari: Phytoseiidae, Tetranychidae). Chilean J Agric Res, 69(2), 160–170. http://dx.doi.org/10.4067/S0718-58392009000200005
Saker, I., Dahiah, H., Mofleh, M. & Basheer, A. (2015). Functional response of the predatory mite, Typhlodromus athiasae Porath and Swirski (Acari: Phytoseiidae) to the two spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) infesting bean. Egypt J Biol Pest Co, 25(1), 1-5.
Seiedy, M., Saboori, A., Allahyari, H., Talaei-Hassanloui, R. & Tork M. (2012) Functional response of Phytoseiulus persimilis (Acari: Phytoseiidae) on untreated and Beauveria bassiana—treated adults of Tetranychus urticae (Acari: Tetranychidae). J Insect Behav, 25(6), 543–553. http://dx.doi.org/10.1007/s10905-012-9322-z
Tello, V., Vargas, R., Araya, J., & Cardemil, A. (2009). Biological parameters of Cydnodromus picanus and Phytoseiulus persimilis raised on the carmine spider mite, Tetranychus cinnabarinus (Acari: Phytoseiidae, Tetranychidae). Cienc Inv Agr, 36(2), 277-290. http://dx.doi.org/10.4067/S0718-16202009000200012
Tixier, M-S., Tsolakis, H., Ragusa, S., Poinso, A., Ferrero, M., Okassa, M.,… (2011). Integrative taxonomy demonstrates the unexpected synonymy between two predatory mite species: Cydnodromus idaeus and C. picanus (Acari: Phytoseiidae). Invertebr Syst, 25, 273–281. http://dx.doi.org/10.1071/IS11025
Villemereuil, P.B. & López-Sepulcre, A. (2011). Consumer functional responses under intra- and inter-specific interference competition. Ecol Model, 222(3), 419-426. http://dx.doi.org/10.1016/j.ecolmodel.2010.10.011
Vitelli, M. & Sato, M. (2016). Pyrethroid resistance in Phytoseiulus macropilis (Acari: Phytoseiidae): cross-resistance, stability and effect of synergists. Exp Appl Acarol, 68, 71–82. http://dx.doi.org/10.1007/s10493-015-9984-2
Xiao, Y. & Fadamiro, H. (2010). Functional responses and prey-stage preferences of three species of predacious mites (Acari: Phytoseiidae) on citrus red mite, Panonychus citri (Acari: Tetranychidae). Biol Control, 53(3), 345–352. http://dx.doi.org/10.1016/j.biocontrol.2010.03.001
Xiao, Y., Osborne, L.S., Chen, J., & McKenzie, C.L. (2013). Functional responses and prey-stage preferences of a predatory gall midge and two predacious mites with two-spotted spider mites, Tetranychus Urticae, as host. J Insect Sci, 13(8), 1-12. http://dx.doi.org/10.1673/031.013.0801
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Jacinto Benhadi-Marín, José Alberto Pereira, David Barreales, José Paulo Sousa, Sónia A.P. Santos. (2018). A simulation-based method to compare the pest suppression potential of predators: A case study with spiders. Biological Control, 123, p.87. https://doi.org/10.1016/j.biocontrol.2018.05.007.
2. Maryam Mumtaz, Vattakandy Jasin Rahman, Tahseen Saba, Tingting Huang, Yuxin Zhang, Chunxian Jiang, Qing Li. (2023). Functional response of Neoseiulus californicus (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae) at different temperatures. PeerJ, 11, p.e16461. https://doi.org/10.7717/peerj.16461.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Acta Agronómica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Política sobre Derechos de autor:Los autores que publican en la revista se acogen al código de licencia creative commons 4.0 de atribución, no comercial, sin derivados.
Es decir, que aún siendo la Revista Acta Agronómica de acceso libre, los usuarios pueden descargar la información contenida en ella, pero deben darle atribución o reconocimiento de propiedad intelectual, deben usarlo tal como está, sin derivación alguna y no debe ser usado con fines comerciales.