Effect of soil water availability on gas exchange in young trees of Eucalyptus grandis W. Hill ex Maiden
DOI:
https://doi.org/10.15446/acag.v66n4.58194Palabras clave:
Water stress, waterlogging, Stomatal conductance, Photosynthesis, Transpiration (en)Descargas
Two outdoor trials were conducted in the department of Valle del Cauca, Colombia, in contrasting climatic conditions, to evaluate the effect of soil water availability on the gas exchange of four elite genotypes of Eucalyptus grandis as follows: 28-3, 18-3, 24A-5 and 19-1, respectively. One trial was conducted on the campus of the Universidad Nacional de Colombia located in Palmira, at 994 m.a.s.l. with an average temperature of 23.5°C (maximum of 31°C, minimum of 18°C), and 76% relative humidity (RH). The second trial was conducted at the nursery of Carton de Colombia S.A., located in Restrepo, at 1450 m.a.s.l. with an average temperature 18°C (maximum of 26°C, minimum of 12°C) and 80% RH. A split-plot design with four replicates was used. Treatments were field capacity (FC), ½ FC, ¼ FC, and soil waterlogging. At both locations, pot surfaces were covered with plastic sheeting to prevent the entrance of rainfall. Significant differences between water regimes, genotypes and locations occurred in photosynthesis rate, internal CO2 of the substomatal cavity (Ci), stomatal conductance, and transpiration rate, indicating that E. grandis has physiological defense mechanisms to drought stress such as stomata closure, evidenced by a decreasing in photosynthesis rate, stomatal conductance, and transpiration rate. A differential response was also observed between genotypes depending on the environment, indicating a genotype x environment interaction in terms of plant physiological response. Eucalyptus grandis appears to save water under water stress conditions by a decreasing in photosynthesis rate. Excess water is more limiting than water deficit.
Recibido: 15 de junio de 2016; Aceptado: 29 de noviembre de 2016
Abstract
Two outdoor trials were conducted in the department of Valle del Cauca, Colombia, in contrasting climatic conditions, to evaluate the effect of soil water availability on the gas exchange of four elite genotypes of Eucalyptus grandis as follows: 28-3, 18-3, 24A-5 and 19-1, respectively. One trial was conducted on the campus of the Universidad Nacional de Colombia located in Palmira, at 994 m.a.s.l. with an average temperature of 23.5°C (maximum of 31°C, minimum of 18°C), and 76% relative humidity (RH). The second trial was conducted at the nursery of Carton de Colombia S.A., located in Restrepo, at 1450 m.a.s.l. with an average temperature 18°C (maximum of 26°C, minimum of 12°C) and 80% RH. A split-plot design with four replicates was used. Treatments were field capacity (FC), 1/2 FC, 1/4 FC, and soil waterlogging. At both locations, pot surfaces were covered with plastic sheeting to prevent the entrance of rainfall. Significant differences between water regimes, genotypes and locations occurred in photosynthesis rate, internal CO2 of the substomatal cavity (Ci), stomatal conductance, and transpiration rate, indicating that E. grandis has physiological defense mechanisms to drought stress such as stomata closure, evidenced by a decreasing in photosynthesis rate, stomatal conductance, and transpiration rate. A differential response was also observed between genotypes depending on the environment, indicating a genotype x environment interaction in terms of plant physiological response. Eucalyptus grandis appears to save water under water stress conditions by a decreasing in photosynthesis rate. Excess water is more limiting than water deficit.
Keywords:
Water stress, waterlogging, stomatal conductance, photosynthesis, transpiration.Resumen
Se establecieron dos experimentos a la intemperie en sitios con clima contrastantes uno en el campus de la Universidad Nacional de Colombia Sede Palmira, Valle del Cauca, Colombia (994 msnm, temperatura promedio 23.50 C, temperatura máxima 310 C, temperatura mínima 180 C, H.R 76%) y el otro en el vivero de Cartón de Colombia S. A, en Restrepo, Valle del Cauca, Colombia (1450 msnm, temperatura promedio 180 C, temperatura máxima 260 C, temperatura mínima 120 C, H.R 80%) con el fin de evaluar el efecto de la disponibilidad de agua en el suelo cc (capacidad de campo) 1/2 cc, 1/4 cc y saturación sobre el intercambio de gases en 4 genotipos de Eucalyptus grandis. Se empleó un diseño de parcelas divididas con 4 repeticiones, las superficies de las macetas fueron cubiertas con tela plástica para evitar la entrada de agua lluvia. Se presentaron diferencias significativas en la tasa de fotosíntesis, Ci (CO2 interno en la cavidad subestomática), conductancia estomática y tasa de transpiración, diferencias entre genotipos y entre localidades, indicando que el E. grandis tiene mecanismos fisiológicos de defensa al estrés hídrico, como es el cierre de estomas que se evidencia con la disminución de la tasa de fotosíntesis, conductancia estomática y tasa de transpiración, también se presentó respuesta diferencial entre los genotipos según el ambiente lo que evidencia la interacción genotipo - ambiente. Se puede decir que el E. grandis economiza el agua cuando está en condiciones de estrés hídrico, disminuyendo la tasa de fotosíntesis y que el exceso de agua es una condición más limitante que el déficit.
Palabras clave:
Estrés hídrico, Anegamiento Conductancia estomática, fotosíntesis, transpiración.Introduction
Eucalyptus grandis W. Hill ex Maiden, commonly known as rose gum, occurs naturally in the coastal areas of Queensland and New South Wales in Australia (between 32 and 17 °S latitude), located at 0-900 m.a.s.l. and characterized by an average annual rainfall of 1000-1780 mm, a 3-month dry season, and temperatures ranging from a minimum of 5°C to a maximum of 35°C. The physiological efficiency, productivity, adaptability, and multiple uses of this species, also known by its synonym Eucalyptus saligna var. pallidivalvis Baker et A.C. Sm, makes it economically important. In addition, its leaves are source of important essential oils used in medicine and in manufacturing cosmetics. Its main uses, however, lies in its timber for building and construction and its short fiber for producing printer paper and cardboard (FAO, 1981).
Climate change and global warming trends, largely attributed to the increased concentration of atmospheric CO2 (from 50 to 60 µmol mol-1 between 1983 and 2013), make it increasingly important to understand plant physiology and the overall dynamics of the local environment and ecosystems (IPCC, 2013).
In this understanding of plant physiology, one can observe climate change and global warming are mainly attributed to the higher concentration of atmospheric CO2, since plants require it for photosynthesis, therefore, it is very important to understand the plant physiology and the dynamics of the local environment and the ecosystems. This knowledge serves as the basis for initiating and implementing plans for adaptation and mitigation of the effects of climate change on ecological, economic and social policies, since trees permanently fix C and store it on stems. Due to E. grandis is a rapidly growing tree with high production potential, high photosynthesis rates and good adaptability to adverse weather conditions and should therefore be considered when designing CO2 capture strategies (Mejía de Tafur, Burbano, García & Baena, 2014; Stephenson et al., 2014).
Studies conducted by Rolando & Little (2008), in tree nurseries at the Institute for Commercial Forestry Research (ICFR) in Pietermaritzburg, South Africa, indicated that E. grandis responds to water stress by lowering stomatal conductance, an indication of stomata closure and, lower photosynthesis rate with the subsequent economy of water, but at the expense of tree growth (Mejia de Tafur, Zapata, Urrego, Ibarra, Leal, 2017). Therefore, a research conducted by El-Sharkawy, Cock & Hernández (1985), at the International Center for Tropical Agriculture (CIAT) in Palmira, Colombia, found that E. deglupta Blume and E. citriodora Hook reduced stomatal conductance and photosynthesis rate by more than 50% in conditions of atmospheric vapor pressure deficit (VPD) of 3-4 kPa as compared with the values obtained with a VPD of 1.0-1.5 kPa without leaf water potential (Ψ) showing direct sensitivity to atmospheric moisture.
Whitehead & Beadle (2004), and Almeida, Soares, Landsberg & Rezende, (2007), indicate that E. grandis has several mechanisms to tolerate water stress such as lower leaf area index (LAI) in periods of drought, near-vertical arrangement of leaves, high stomatal sensitivity to VPD, deep rooting ability, and osmotic adjustment to maintain turgor in leaves. The performance of eucalyptus depends mainly on the number of leaves and their health and consequently on LAI (Hubbard, Stape, Ryan, Almeida & Rojas, 2010; Merchant et al., 2007). In the case of E. grandis, the tree retains its leaves during the 1-year growing season. However, a significant number of leaves survive for more than two years (Whitehead & Beadle, 2004), which is important because the tree does not have to use photoassimilates to form new leaves for photosynthesis. The loss of leaves in response to water stress also contributes to reduced growth, even though water loss simultaneously decreases (Mejia de Tafur et al., 2017).
There are Eucalyptus species that are tolerant to stress caused by excess water, which forms adventitious roots and can be found in swamps and in marginal saline areas, although the growth of E. grandis decreased under soil saturation conditions, some genotypes do present tolerance. Research conducted by Close & Davidson (2003), in E. nitens, Sena & Kozlowski (1980), in E. camaldulensis and E. globulus, and Lima, Jarvis, & Rhizopoulou (2003), in E. grandis, E. urophilla, E. camaldulensis, E. torelliana and E. phaeotrica, show the formation of adventitious roots and aerenchyma in response to periods of prolonged waterlogging, in addition to reducing the photosynthesis rate and significantly decreasing plant height and root and leaf production in trees of E. robusta (Clemens, Kirk, & Mills, 1978).
E. grandis is usually grown in intertropical areas, with prolonged and severe seasonal droughts that are foreseen to suffer modifications in their rainfall distribution patterns. In view of the economic and ecological importance of this species and its capacity to adapt to adverse conditions, this study evaluates the photosynthetic response of four genotypes of E. grandis under conditions of shortage and waterlogging.
Materials and methods
Trials were established at two sites located in the department of Valle del Cauca, Colombia, with bimodal rainfall distribution, with the main dry season between June and August and the secondary dry season between December and March. The first trial was established at Universidad Nacional de Colombia, campus Palmira, Colombia (3° 30′ 45.13″ N, 76° 18′ 30.25″ W), located at 994 m.a.s.l, with an annual rainfall of 895 mm, an average temperature of 23.5°C (minimum 18-19°C, maximum 29-31°C), 70-76% relative humidity (RH), and 4 h day-1 of sunlight during the rainy season and 6 h day-1 during the dry season. The second trial was established at the nursery of Carton de Colombia S.A. in Restrepo (3° 49′ N, 76° 07′ W), located at 1450 m.a.s.l. with an annual rainfall of 1100 mm, an average temperature of 18°C (minimum 12°C, maximum 26°C), 80% RH, and 5 h day-1 of solar radiation.
The response of four elite genotypes of E. grandis (28-3, 18-3, 24A-5, and 19-1) to soil water availability at field capacity (FC), 1/2 FC, 1/4 FC, and soil waterlogging was evaluated at both sites. Genotypes were selected by Carton de Colombia SA for their adaptability to different environmental conditions. In Palmira, seedlings were planted in pots 60 cm tall and 30 cm in diameter using soil from the site. In Restrepo, seedlings were planted in pots 90 cm tall and 57 cm in diameter, using the soil substrate used by Carton de Colombia S.A. in the nursery (33% sawdust, 33% charcoal, and 33% soil).
Trials were established out in the open, using a split-plot design with four replicates, the main plot evaluating soil water availability (FC, 1/2 FC, 1/4 FC, waterlogging) and the subplots, the genotypes (28-3, 18-3, 24A-5, 19-1), for a total of 64 experimental units per trial. Pots were covered with plastic sheeting to prevent the entrance of rain water.
The water retention curve was determined in both soil and substrate to calculate the volume of water to be applied to each treatment. The soil waterlogging treatment kept a water surface 1 cm deep. Treatments were induced 15 days after planting when plants were 30 cm tall.
A portable ADC LCpro+ open photosynthesis system was used to evaluate the photosynthesis rate. Assessments were performed on mature, fully expanded leaves of branches of the upper third of the tree. Readings were conducted in Palmira at 26, 34, 46, and 62 days after transplanting (DAT) and in Restrepo, at 36, 45, 80, 100, 131, and 156 DAT, respectively. Trees were harvested at 130 DAT in Palmira and at 213 DAT in Restrepo location to assess biomass accumulation in the stems. Data were analyzed by ANOVA using SAS (r) 9.3 software. WUE was calculated by dividing the transpiration rate per photosynthesis rate.
Results
During the trials, average atmospheric CO2 concentration in Palmira was 383 ppm (± 13.6), average chamber temperature of 33.27°C (± 3.82) and average leaf temperature of 35.82 °C (± 4.02). In Restrepo, average atmospheric CO2 concentration was 380 ppm (± 12.5), average chamber temperature, 27.42°C (± 3.89), and average leaf temperature, 29.59°C (± 3.82). Highly significant differences (<0.001) in all response variables were found between levels of soil water availability in Palmira location (Table 1).
* Ci: Internal CO2 in the sub-stomatal cavity. **Values with the same letter are statistically equal.Table 1: Photosynthetic response of E. grandis to soil water availability in Palmira, Valle del Cauca, Colombia (average temperature of 23.5°C). Highly significant statistical differences were observed. Data correspond to the average of all genotypes per treatment.
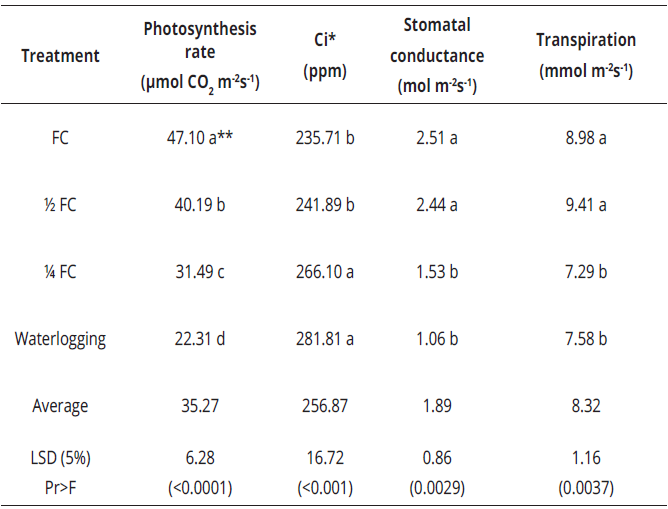
The highest photosynthesis rate was with the FC treatment, followed by the 1/2 FC, 1/4 FC, and waterlogging treatments, indicating that E. grandis has physiological mechanisms, such as stomata closure, that allows it to respond to water stress. Differences were found among genotypes regarding the different evaluated test variables. Genotype 28-3 presented the highest photosynthesis rate (38.71 µmol CO2 m-2s-1) and 24A-5, the lowest (31.91 µmol CO2 m-2 s-1). Using as reference the average stomatal conductance among treatments (1.89 mol m-2s-1) (Figure 1).
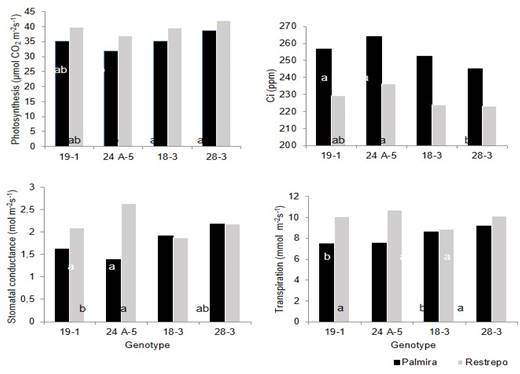
Figure 1: Gas exchange in E. grandis genotypes in Palmira (average temperature of 23.5°C) and Restrepo (average temperature of 18°C), department of Valle del Cauca, Colombia. Data correspond to the average of all treatments per genotype.
In fact, the average stomatal conductance among genotypes (1.78 mol m-2s-1) and the average transpiration rate among treatments (8.32 mmol m-2s-1) (Table 1) and among genotypes (8.21 mmol m-2s-1), the stomatal conductance in genotypes 28-3 (2.18 mol m-2s-1) and 18-3 (1.92 mol m-2s-1) and the transpiration rate of 28-3 (9.18 mmol m-2s-1) and 18-3 (8.63 mmol m-2s-1) were higher than the overall average, whereas those of genotypes 19-1(Conductance 1.63 mol m-2s-1, transpiration rate 7.49 mmol m-2s-1) and 24A-5 (Conductance 1.39 mol m-2s-1, transpiration rate 7.52 mmol m-2s-1) were lower. In Restrepo, the rate of photosynthesis and stomatal conductance were significantly lower when the ground was waterlogged and thus, the Ci was higher in this treatment. The transpiration rate, however, was significantly higher at 1/2 FC, followed by 1/4 FC and FC, and lower in the waterlogging treatment (Tables 2,3).
**Values with the same letter are statistically equal.Table 2: Effect of water stress on gas exchange in E. grandis genotype 19-1 in Palmira, Restrepo, and Palmira-Restrepo (P+R), Valle del Cauca, Colombia.
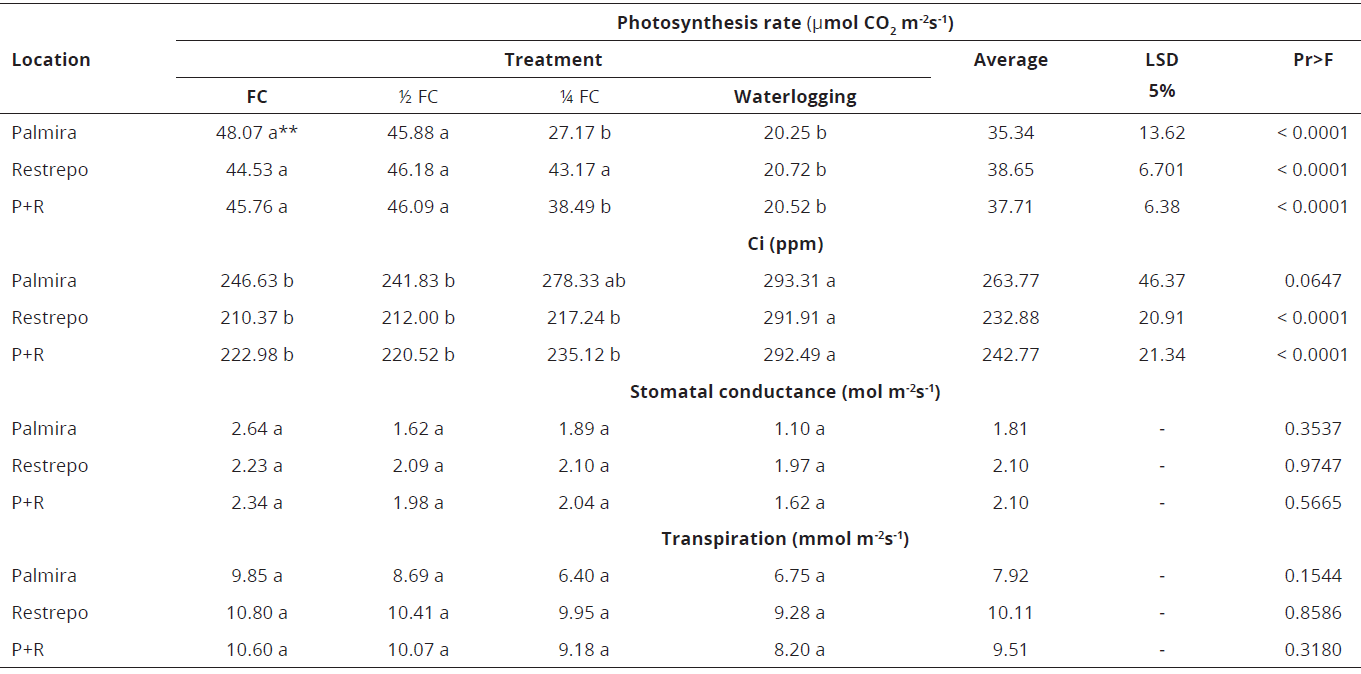
*Ci: Internal CO2 in the sub-stomatal cavity. **Values with the same letter are statistically equalTable 3: Photosynthetic response of E. grandis to soil water availability in Restrepo, Valle del Cauca, Colombia (average temperature of 18 °C). Highly significant statistical differences were observed. Data correspond to the average of all genotypes per treatment.
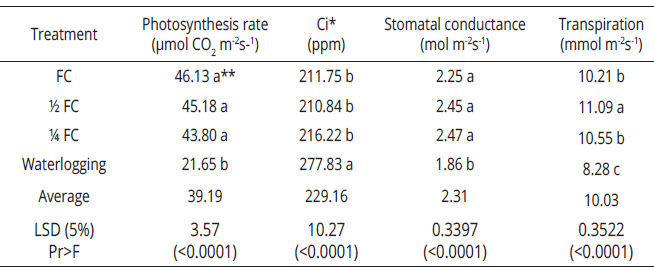
Averaging the data of Restrepo and Palmira indicated that the highest photosynthesis rate occurs in the FC treatment, followed by the 1/2 FC, 1/4 FC, and waterlogging treatments. The highest photosynthesis occurred in genotype 28-3 and the lowest in genotype 24A-5; the Ci was higher with lower photosynthesis, and transpiration rate was higher in genotypes 24A-5 and 28-3. Stomatal conductance, on the other hand, presented no significant differences. There were significant differences in all test variables between the Palmira and Restrepo (Tables 1, 2). The lower transpiration rate could possibly be attributed to the lower VPD in Restrepo due to the lower temperature. In other words, E. grandis requires less water in Restrepo for its physiological functions and is therefore more efficient in its use. In Palmira, the genotype 19-1 the rates of photosynthesis were significantly lower in the 1/4 FC and waterlogging treatments. In Restrepo, all treatments except the waterlogging had high rates of photosynthesis, like one another (Table 3). This indicates that plant response differs depending on environmental conditions, evidencing the interrelationship between soil water availability x environment on plant physiology. In Palmira, genotype 24A-5 showed the highest photosynthesis rate at FC, followed by 1/2 FC (Table 4).
* Ci: Internal CO2 in the sub-stomatal cavity. **Values with the same letter are statistically equal.Table 4: Effect of water stress on gas exchange in E. grandis genotype 24A-5 in Palmira, Restrepo, and Palmira-Restrepo (P+R), Valle del Cauca, Colombia.
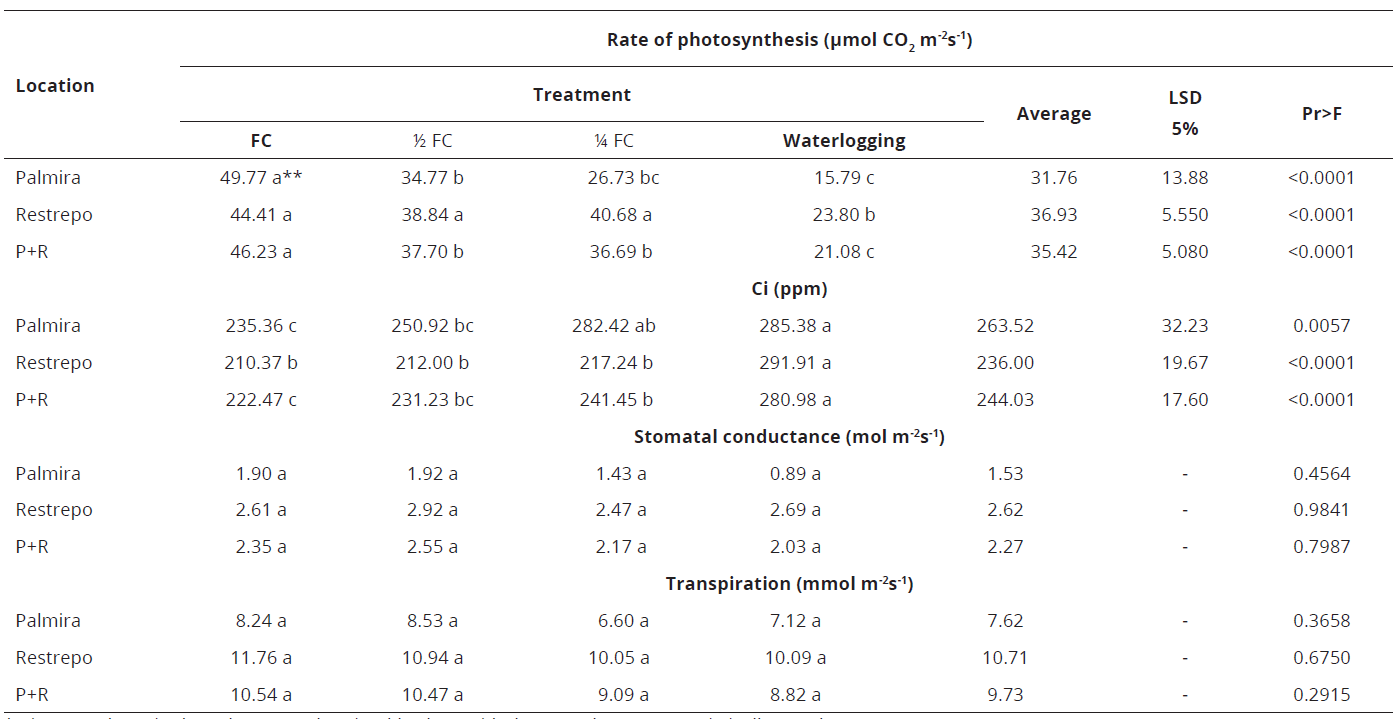
The lowest rates were observed in the 1/4 FC and waterlogging treatments. However, significant differences in Ci only occurred between the FC and waterlogging treatments. There were no significant differences among treatments in stomatal conductance and transpiration rate. In Restrepo, however, at normal temperatures, the photosynthesis rate was only significantly lower in waterlogging conditions and therefore the Ci was higher. Three groups were formed when data of Palmira and Restrepo were combined as follows: (1) the FC treatment, presenting the highest photosynthesis; (2) the 1/2 FC and 1/4 FC treatments; and (3) the waterlogging treatment. Therefore, the Ci was lower in the FC treatment. As in previous cases, the other response variables presented no significant differences among themselves. This indicates that excess water is a limiting condition for gas exchange and suggests the influence of biochemical and/or anatomical factors inside the mesophyll in response to water stress (Table 4).
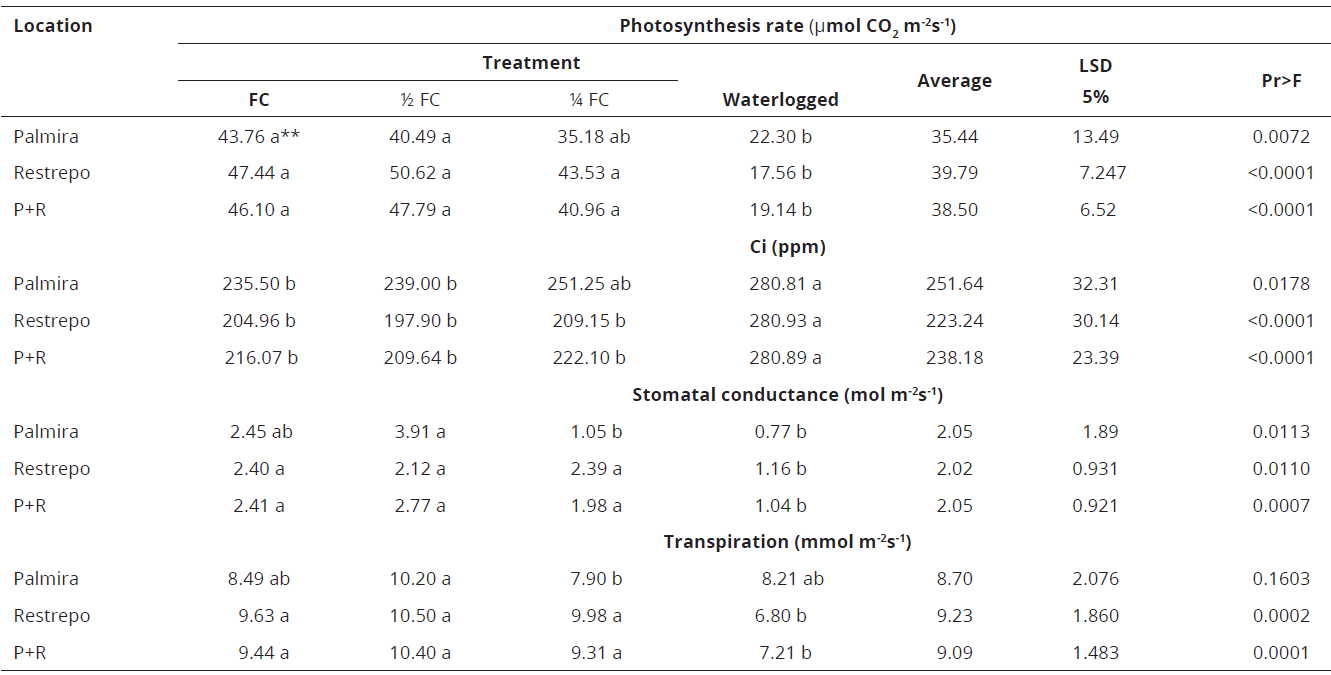
In Palmira, genotype 18-3 presented a lower rate of photosynthesis at 1/4 FC and in the waterlogging treatment, higher stomatal conductance at 1/2 FC followed by FC, and transpiration is higher at 1/2 FC. In Restrepo, the photosynthesis rate and other response variables were only affected by waterlogging. The same result was obtained when the data from Palmira + Restrepo locations, were combined (Table 5).
* Ci: Internal CO2 in the substomatal cavity. **values with the same letter are statistically equal.Table 5: Effect of water stress on gas exchange in E. grandis genotype 18-3 in Palmira, Restrepo, and Palmira-Restrepo (P+R), Valle del Cauca, Colombia.
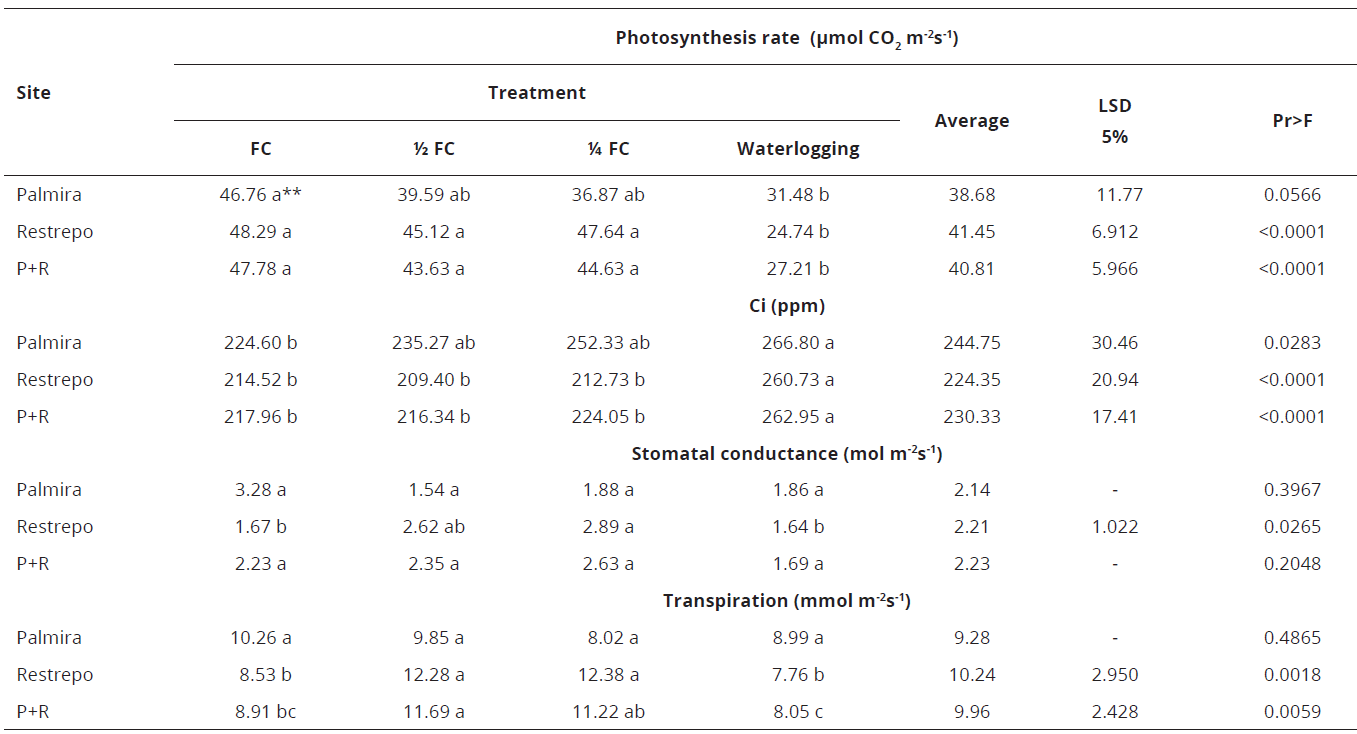
Genotype 28-3 has a lower photosynthesis rate in the waterlogging treatment at both sites and, thus, higher Ci. There are some differences in stomatal conductance and transpiration rate among treatments, depending on environmental conditions. When data from Palmira and Restrepo locations were combined, transpiration rate was higher at 1/2 and 1/4 FC without affecting stomatal conductance, which suggests tolerance to water deficit (Table 6).
* Ci: Internal CO2 in the sub-stomatal cavity. **Values with the same letter are statistically equal.Table 6: Effect of water stress on gas exchange in Eucalyptus grandis genotype 28-3 in Palmira, Restrepo, and Palmira-Restrepo (P+R), Valle del Cauca, Colombia.
The photosynthesis rate of four genotypes was significantly reduced in the 1/4 FC and waterlogging treatments when grown in Palmira (high temperature), except for genotype 24A-5, which also reduced its rate of photosynthesis at 1/2 FC, and genotype 28-3, which only presented differences between the FC and the waterlogging treatment. When grown in Restrepo, genotypes presented statistically similar rates of photosynthesis in the FC, 1/2 FC, and 1/4 FC treatments, which were higher than those of the waterlogging treatment. The effect of temperature and genotype on the physiological response of the plant to water stress is therefore confirmed (Table 7).
**Values with the same letter are statistically equal.Table 7.Water: use efficiency (WUE) in Palmira and Restrepo, Valle del Cauca Colombia, and for both sites combined.
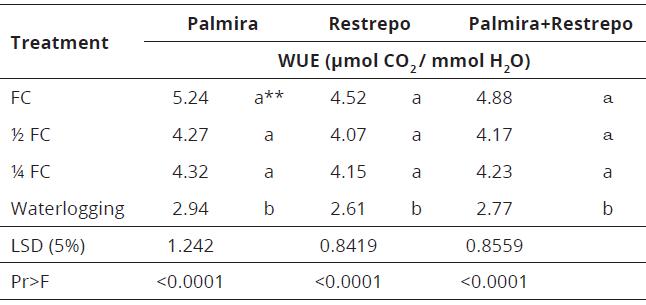
The WUE model showed significant differences among treatments (0.0001). Data indicate the plants under waterlogging were less efficient in water use as they fix less CO2 per mmol of water consumed than other treatments, which could be attributed to the fact that the rate of photosynthesis decreased more than that obtained with the FC treatment (≈50%) than the transpiration rate (≈20%), suggesting that the plant must adjust its physiology to spend proportionately more water as a defense to excess water.
Discussion
The average photosynthesis rate was 35.17 µmol CO2 m-2s-1 in Palmira and 39.55 µmol CO2 m-2s-1 in Restrepo, both high values if compared with the average values found in C3 species (10-24 µmol CO2 m-2s-1) and with those found by Osorio & Pereira (1994), in E. globulus and E. nitens, and Whitehead & Beadle (2004), Mendes, de Paula, Scarpinatti & de Paula (2013), and El-Sharkawy et al. (1985), in E. deglupta and E. citriodora.
Lima et al. (2003), found in several Eucalyptus species (E. grandis, E. urophilla, E. camaldulensis, E. torelliana and E. phaeotrica) photosynthesis rates between 25-30 µmol CO2 m-2s-1 with atmospheric CO2 concentrations of 700 ppm (± 30) in the dry season, whereas rates of photosynthesis were 10-15 µmol CO2 m-2s-1 at normal concentrations of 350 ppm (± 30).
E. grandis has physiological mechanisms, such as stomata closure, that allows it to respond to water stress. This is confirmed by stomatal conductance and transpiration data as these were lower with increasing water stress, whereas internal CO2 (Ci) was higher with decreasing photosynthesis rates (Figure 1) (Mendes et al., 2013; Mejia de Tafur et al., 2014).
Stomatal conductance was similar in all genotypes, but the transpiration rate was lower in genotypes 19-1 and 24A-5, which means less water loss due to stomata closure, but at the expense of CO2 fixation, which explains the lower rate of photosynthesis in the genotypes (Figure 1) (Mendes et al., 2013; Osorio & Pereira, 1994), which confirms the effect of genotype and indicating that E. grandis has in place physiological mechanisms, which have allowed to respond to water stress (Almeida et al., 2007).
Considering that the physiological stress caused by waterlogging is due to water absorption inhibition by hypoxia and/or anoxia, which in turn causes water deficit, it is evident that E. grandis has stress defense mechanisms in place, closing stomata to reduce water loss by transpiration. (Tables 1, 2). (Close, 2003; Clemens et al., 1978).
The highest Ci occured under waterlogging and in high soil water deficit, which could be attributed to either reduction in carboxylation rate of Rubisco and/or with an increasing in oxygenation reaction (ie. increased photorespiration). This is supported by the significant reduction in photosynthetic rates. Given these concerns as well as stomatal conductance, which was lower in the waterlogging treatment, and transpiration, which decreased with both water deficit and excess water, evidenced in a stomata closure as a response mechanism to water stress. (Tables 1, 2). (El-Sharkawy, Cock & Held, 1984).
The highest photosynthesis rate occurred in Restrepo location, in fact, the lowest Ci as a faster CO2 fixation leads to quicker depletion and therefore less accumulation in the substomatal cavity. The lower photosynthesis rate and the higher Ci in eucalyptus grown in Palmira, as compared with Restrepo location, indicates that significant anatomical and/or biochemical changes could possibly have occurred inside the mesophyll of Palmira-grown trees. Stomatal conductance is also higher, which indicates an increasing in stomatal opening (El-Saharkawy et al., 1984).
Data on Ci show significant differences in Restrepo location, being higher in the waterlogging treatment, which agrees with the lower photosynthesis rate. No significant differences were detected among treatments for stomatal conductance and transpiration, which can be attributed to the influence of biochemical and/or anatomical factors within the mesophyll on CO2 assimilation (Rolando & Little, 2008; El Sahrkawy et al., 1985).
Stomatal conductance and transpiration rate were the same in all treatments (Table 3), indicating that this genotype require more water in Palmira conditions due to the high temperatures. (Lima et al., 2003).
It can be concluded that the evaluated genotypes respond to water supply depending on climatic factors and that this response depends on the genotype. Data suggest that biochemical and/or anatomical factors affect the photosynthetic response of plants to water stress. In all cases, the greatest limitation occurs in the waterlogging treatment (El-Sharkawy et.al, 1984,1985).
Overall, Ci values relate to the rate of photosynthesis, being higher when photosynthesis is lower. Stomatal conductance and transpiration rate also present higher values when the plant has a good water supply. In principle, one could say that in Palmira genotypes 19-1, 18-3, and 28-3 support water reduction better than 24A-5, but in Restrepo location, all genotypes showed a similar trend (Tables 2, 3, 4, and 5). (Hubbard et.al, 2010).
Likewise, in the waterlogging treatment, the photosynthetic capacity is more easily affected due to an internal factors , which are related to water absorption, transportation, and loss (from the stoma outward) in the root-leaf continuum, respectively (Table 6) (Hubbard et.al, 2010; El- Sharkawy, 2007).
Conclusions
The results indicate that E. grandis had achieved physiological mechanisms, which have allowed to defend itself from water stress (both deficit and excess), decreasing stomatal conductance and transpiration rate, which suggests stomata closure, but with the consequent reduction in photosynthesis rate. In fact, WUE species is only reduced when subjected to excess water, making this condition more limiting than water deficit.
Given these concerns, E. grandis responds to an increasing concentration of atmospheric CO2, soil water availability, and possibly temperature with high rates of photosynthesis, making this species a good alternative when addressing atmospheric carbon fixation.
Acknowledgements
The authors thank the Universidad Nacional de Colombia-Palmira Campus, to the Soils Program, and Carton de Colombia S.A, for their support and for financing this research. Our thanks also go to Marzory Andrade Bernal for her collaboration in the statistical analyses of trial data and to Dr. Mabrowk el Sharkawy for his teachings
References
Referencias
Almeida, A.C., Soares, J.V., Landsberg, J.J., & Rezende, G.D. (2007). Growth and water balance of Eucalyptus grandis hybrid plantations in Brazil during a rotation for pulp production. For Ecol Manage, 251 (1-2), 10–21. https://doi.org/10.1016/j.foreco.2007.06.009
Clemens, J., Kirk, A.M., & Mills, P.D. (1978). The resistance to waterlogging of three Eucalyptus species: Effect of waterlogging and an ethylene-releasing growth substance on E. robusta, E. grandis and E. saligna, Oecología, 34 (2), 125–131. https://doi.org/10.1007/BF00345161
Close, D.C., Davidson, N.J., 2003. Long-term waterlogging: Nutrient, gas exchange, photochemical and pigment characteristics of Eucalyptus nitens Saplings. Russ. J. Plant Physiol. 50(6), 843–847. https://doi.org/10.1023/B:RUPP.0000003284.25827.95
Lima, D.P.W., Jarvis, P., & Rhizopoulou, S. (2003). Resposta estomática ao aumento da concentração do CO2 atmosférico e ao estresse hídrico de espécies de Eucalyptus. Sci. Agric. 60(2), 231–238. http://dx.doi.org/10.1590/S0103-90162003000200005
El-Sharkawy, M.A. (2007). Physiological characteristics of cassava tolerance to prolonged drought in the tropics: implications for breeding cultivars adapted to seasonally dry and semiarid environments. Braz. J. Plant Physiol. 19(4), 257–286. http://dx.doi.org/10.1590/S1677-04202007000400003
El-Sharkawy, M.A., Cock, J.H., Held, A. (1984). Water efficiency of cassava. II. Differing sensitivity of stomata to air humidity in cassava and other warm-climate species. Crop Sci 24 (3), 503–507. https://doi.org/10.2135/cropsci1984.0011183X002400030018x
El-Sharkawy, M.A., Cock, J.H., Hernández, A. de P. (1985). Stomatal response to air humidity and its relation to stomatal density in a wide range of warm climate species. Photosynth Res 7 (2), 137–149. https://doi.org/10.1007/BF00037004
Hubbard, R.M., Stape, J., Ryan, M.G., Almeida, A.C., Rojas, J. (2010). Effects of irrigation on water use and water use efficiency in two fast growing Eucalyptus plantations. For Ecol Manage 259 (9), 1714–1721. https://doi.org/10.1016/j.foreco.2009.10.028
Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V. & Midgley, P.M. (2013). Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC (Eds.). Bern, Switzerland. https://www.ipcc.ch/publications_and_data/publications_and_data_reports.shtml.
Mejía de Tafur, M. S., Zapata, C. M., Urrego, J. B., Ibarra, D. M. & Leal, J.J. (2017). Efecto del estrés hídrico sobre la acumulación y distribución de biomasa en Eucalyptus grandis W. Hill ex Maiden. Acta Agron. 66 (1), 56-62. https://doi.org/10.15446/acag.v66n1.52628
Mejia de Tafur, M.S., Burbano, R.A., García, M.A. & Baena, D. (2014). Respuesta fotosintética de Eucalyptus grandis W. Hill a la disponibilidad de agua en el suelo y a la intensidad de luz. Acta Agron. 63(4), 311–317. https://doi.org/10.15446/acag.v63n4.43598
Mendes, H.J., de Paula, N.F., Scarpinatti, E.A., de Paula, R.C. (2013). Respostas fisiológicas de genotipos de Eucalyptus grandis x E. urophylla a disponibilidade hidrica e abudacao potassica. Cerne 19(4), 603–611. http://dx.doi.org/10.1590/S0104-77602013000400010
Merchant, A., Callister, A., Arndt, S., Tausz, M., Adams, M. (2007). Contrasting physiological responses of six Eucalyptus species to water deficit. Ann Bot. 100 (7), 1507–1515. https://doi.org/10.1093/aob/mcm234
NOAA. National Oceanic and Atmospheric Administration. United States Department of Commerce (2015). Greenhouse gas Benchmarck reached. http://research.noaa.gov/News/NewsArchive/LatestNews/TabId/684/ArtMID/1768/ArticleID/11153/Greenhouse-gas-benchmark-reached-.aspx.
Osorio, J., Pereira, J.S. (1994). Genotypic differences in water use efficiency and (13) C discrimination in Eucalyptus globulus. Tree Physiol. 14 (7-8-9), 871–882. https://doi.org/10.1093/treephys/14.7-8-9.871
Rolando, C.A., Little, K.M. (2008). Measuring water stress in Eucalyptus grandis Hill ex Maiden seedlings planted into pots. S. Afr. J. Bot. 74(1), 133–138. https://doi.org/10.1016/j.sajb.2007.08.004
Sena, A.R. & Kozlowski, T.T. (1980). Effects of flooding on Eucalyptus camaldulensis and Eucalyptus globulus seedlings. Oecologia 46 (2), https://doi.org/139–142. 10.1007/BF00540117
Stephenson, N.L., Das, A.J., Condit, R., Russo, S.E., Baker, P.J., Beckman, N.G., Coomes, D.A., Lines, E.R., Morris, W.K., Rüger, N., Álvarez, E., Blundo, C., Bunyavejchewin, S., Chuyong, G., Davies, S.J., Duque, A., Ewango, C.N., Flores, O., Franklin, J.F., Grau, H.R., Hao, Z., Harmon, M.E., Hubbell, S.P., Kenfack, D., Lin, Y., Makana, J.R., Malizia, A., Malizia, L.R., Pabst, R.J., Pongpattananurak, N., Su, S-H., Sun, I-F., Tan, S., Thomas, D., van Mantgem, P.J., Wang, X., Wiser, S.K. & Zavala, M.A. (2014). Rate of tree carbon accumulation increases with tree size. Nature 507 (7490), 90–93. https://doi.org/10.1038/nature12914
Whitehead, D., Beadle, C.L. (2004). Physiological regulation of productivity and water use in Eucalyptus: A review. For. Ecol. Manage 193 (1-2), 113–140. https://doi.org/10.1016/j.foreco.2004.01.026
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Muhammad Asif, Muhammad Farrakh Nawaz, Irfan Ahmad, Muhammad Haroon U. Rashid, Taimoor Hassan Farooq, Muhammad Kashif, Sadaf Gul, Qian Li. (2023). Detrimental Effects of Induced Soil Compaction on Morphological Adaptation and Physiological Plasticity of Selected Multipurpose Tree Species. Plants, 12(13), p.2468. https://doi.org/10.3390/plants12132468.
2. João Everthon da Silva Ribeiro, Ester dos Santos Coêlho, Francisco Romário Andrade Figueiredo, Francisco Thiago Coelho Bezerra, Thiago Jardelino Dias, Mário Luiz Farias Cavalcanti, Manoel Bandeira de Albuquerque. (2023). Características morfofisiológicas em plantas de Erythroxylum pauferrense Plowman sob estresse hídrico. Ciência Florestal, 33(2) https://doi.org/10.5902/1980509865886.
3. Richard Lasprilla V. , Sara Mejía de Tafur, Enrique A. Torres Prieto. (2023). ¿Influyen las casas de malla en la evapotranspiración de referencia (ETo)? . Acta Agronómica, 71(1), p.47. https://doi.org/10.15446/acag.v71n1.96491.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Acta Agronómica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Política sobre Derechos de autor:Los autores que publican en la revista se acogen al código de licencia creative commons 4.0 de atribución, no comercial, sin derivados.
Es decir, que aún siendo la Revista Acta Agronómica de acceso libre, los usuarios pueden descargar la información contenida en ella, pero deben darle atribución o reconocimiento de propiedad intelectual, deben usarlo tal como está, sin derivación alguna y no debe ser usado con fines comerciales.