Cytogenetic evaluation of chili (Capsicum spp., Solanaceae) genotypes cultivated in Valle del Cauca, Colombia
DOI:
https://doi.org/10.15446/acag.v66n4.59162Palabras clave:
Chromosome number, meiotic behavior, meiotic index, plant genetic resources conservation, ploidy level, pollen viability (en)Descargas
The genus Capsicum comprises a large group of hot and sweet peppers, with different morphological varieties and degree of fruits pungency. Due to its active principles is widely used in cooking and in traditional medicine, being sold in large worldwide. In order to provide cytogenetic information which supporting plant breeding programs, this study aimed to analyze the meiotic behavior, chromosome number and pollen viability of the three species of greater economic importance of Capsicum (C. annuum, C. frutescens and C. chinense). Therefore, fruits and flower buds were collected from the Experimental Center of the Universidad Nacional de Colombia, campus Palmira- CEUNP to the corresponding mitotic, meiosis and pollen viability analysis. Plants presented different meiotic abnormalities, where all can disrupt the process of meiosis cell division, creating unbalanced chromosome numbers and varied pollen fertility. Chromosome number variation, checking 2n=2x=24, 2n=2x=26, 2n=4x=48 to 2n=6x=72 chromosomes also occurred. A low degree of non-viable pollen grains was observed for C. frutescens, so these accession should not considered as a parental for plant breeding programs.
Recibido: 19 de septiembre de 2016; Aceptado: 4 de abril de 2017
Abstract
The genus Capsicum comprises a large group of hot and sweet peppers, with different morphological varieties and degree of fruits pungency. Due to its active principles is widely used in cooking and in traditional medicine, being sold in large worldwide. In order to provide cytogenetic information which supporting plant breeding programs, this study aimed to analyze the meiotic behavior, chromosome number and pollen viability of the three species of greater economic importance of Capsicum (C. annuum, C. frutescens and C. chinense). Therefore, fruits and flower buds were collected from the Experimental Center of the Universidad Nacional de Colombia, campus Palmira- CEUNP to the corresponding mitotic, meiosis and pollen viability analysis. Plants presented different meiotic abnormalities, where all can disrupt the process of meiosis cell division, creating unbalanced chromosome numbers and decreasing pollen fertility. Chromosome number variation, checking 2n=2x=24, 2n=2x=26, 2n=4x=48 to 2n=6x=72 chromosomes, also occurred. A low degree of non-viable pollen grains was observed for C. frutescens genotype, so these accession should not considered as a parental for breeding programs.
Key words:
Chromosome number, meiotic behavior, meiotic index, plant genetic resources conservation, ploidy level, pollen viability.Resumen
El género Capsicum comprende un gran grupo de ajís picantes y dulces con diferentes variedades morfológicas y el grado de pungencia de los frutos. Debido a sus principios activos, es utilizado ampliamente en la cocina y en la medicina tradicional, siendo comercializado ampliamente en todo el mundo. Con el fin de aportar una información citogenética apropiada que apoye los programas de mejoramiento genético vegetal, este estudio tuvo como objetivo analizar el comportamiento meiótico, el número cromosómico y la viabilidad de polen de las tres especies de Capsicum (C. annuum, C. frutescens y C. chinense) de mayor importancia económica. Frutos y botones florales fueron colectados en el Centro Experimental de la Universidad Nacional de Colombia sede Palmira - CEUNP para los correspondientes análisis mitótico, meiótico y polínico. Las plantas presentaron diferentes anormalidades meióticas, afectando la estabilidad, conllevando al desbalance cromosómico y reduciendo la fertilidad del polen. También se encontró una variación en el número de cromosomas, desde 2n=2x=24, 2n=2x=26, 2n=4x=48 hasta 2n=6x=72. Se observó un bajo grado de granos de polen viables para C. frutescens, de tal manera que tal accesión no puede ser considerada como parental para programas de mejoramiento genético.
Palabras clave:
Comportamiento meiótico, conservación de recursos fitogenéticos, índice meiótico, nivel de ploidía, número cromosómico, viabilidad de polen.Introduction
The genus Capsicum belongs to the Solanaceae family, which is represented by approximately 3000 species, distributed in 150 genera (Barth & Duarte, 2008). Capsicum comprises a large group of hot and sweet peppers with different morphological varieties and degree of fruits pungency. In fact, due to their active ingredients are widely used and marketed worldwide (Büttow, Barbieri, Neitzke, Heiden & Carvalho, 2010; Neitzke, Barbieri, Vasconcelos, Fischer, Vilella & Castro, 2014).
The taxonomy of this genus is still confused, there is controversy as to the number of species, ranging from 25 (Büttow et al., 2010), 30 (Moscone, Scaldaferro, Grabiele, Cecchini, Garcia, Jarret, Daviña, Ducasse, Barboza & Ehrendorfer, 2007) and 31 (Martins, Pereira, Souza & Costa, 2010) described species, being classified as domesticated (five species), semi-domesticated and wild (Souza, Martins & Pereira, 2011).
The origin center of this genus is the Tropical America (Pickersgill, 1997). Spanish and Portuguese people were the first to discover this kind in the Americas, one of the products most commonly used in cooking and traditional medicine for these people (Ribeiro, Lopes, Carvalho, Henz & Reifschneíder, 2008). In cooking, as constituents of salads and condiments; in traditional medicine, in the fight and prevention of diseases (Signorini, Renesto, Machado, Bespalhok & Monteiro, 2013), some countries like Peru and Mexico use peppers in large-scale, because of the color characteristics and assets of the fruit principles (Teodoro, Garcia & Corona, 2007).
According to Rufino & Penteado (2006), Asia is the continent that more grows peppers, with approximately 89% of the total cultivated. India, Korea, Thailand, China, Vietnam, Sri Lanka and Indonesia are the most prominent production countries. Production of the United States and Mexico is around 7%, followed by countries in Europe, Africa and Middle East, with 4% of the total cultivated peppers. In Brazil, the production of peppers is growing, being an important diversity center, and harboring domesticated, semi-domesticated and wild species, with emphasis on the cultivation of domesticated species C. annuum, with greater economic importance in this country (Rufino & Penteado, 2006; Neitzke et al., 2014).
Among the widely consumed species and commercially cultivated are C. annuum (pepper, sweet pepper); C. baccatum (finger-to-girl in-hat-friar); C. chinense (pepper-of-smell, pepper-goat, murici); C. frutescens (chilli pepper) and C. pubescens (hairy pepper, rocoto), which is a no cultivated species into Brazil (Carvalho, Maciel, Beckman & Poltronieri, 2014).
The basic chromosome number usually found in domesticated species, C. annuum, C. frutescens, C. chinense and C. baccatum is x=12 (2n=24) (Moscone et al., 2007), but in some wild species as C. buforum, C. campylopodium and C. cornutum, a number of x=13 (2n=26) was found (Moscone et al., 2007; Pozzobon & Wittmann, 2006; Teodoro, Garcia & Corona, 2007).
Given these concerns, this study aimed to analyze cytogenetically C. annuum, C. frutescens and C. chinense genotypes from Valle del Cauca, Colombia. In addition, chromosome number, meiotic behavior, meiotic index and pollen viability were studied, in order to detect chromosome abnormalities related to the ploidy level and pollen fertility. Since, to amplify cytogenetic information for this group of plants, which have allowed a basis for plant breeding programs.
Materials and methods
We collected flower buds, flowers and fruits of plants of three species of Capsicum: C. annuum (Cayenne), C. frutescens (Tabasco) and C. chinense (Habanero) grown at the Experimental Center at the Universidad Nacional de Colombia, campus Palmira - CEUNP, in the municipality of Candelaria, Valle del Cauca, Colombia.
The plant materials collected were stored in plastic bags and transferred to the cytogenetics laboratory of the Universidad Nacional de Colombia, campus Palmira, for the meiosis analysis in flower buds, and mitosis in root meristems. Then, floral buds were fixed in Carnoy I solution (3:1 ethanol:glacial acetic acid), for a maximum time of 3 to 4 hours, transferred to fresh solution and then preserved under refrigeration until the moment of preparation of the slides. To prepare the slides, technique of squash were employed; the anthers were macerated into a drop of Carnoy I solution and stained by acetic carmine 2%.
Chiasmata frequency were analyzed at 10 cells in diakinesis of each species. Spindle patterns were evaluated at meiosis II (metaphase, anaphase and telophase, respectively). The meiotic index (MI), analyzing a minimum of 100 tetrads of microspores. Pollen viability was counted in 400 grains for each genotype.
For the root meristem analysis, the seeds were germinated in Petri dishes, with moistened filter paper, at 25°C. The onset of germination varied from 5 to 10 days to C. annuum and C. frutescens, and 10 to 20 days to C. chinense. Roots were subjected to a pretreatment by using 8-hydroxyquinoline (8-HQ) solution followed by cooling for 2 hours at 14°C. Thereafter, the roots were washed with distilled water three times for 5 minutes to eliminate 8-HQ. Then, this material was fixed in 3:1 Carnoy I solution and stored under refrigeration for slide mounting. For slides preparation, the rootlets were washed in distilled water and hydrolyzed 1N HCl for 20 minutes at room temperature to the degradation of the cell wall. After hydrolysis, were washed again in distilled water. The rootlets were cut vertically, macerated, stained with orceine 2% and viewed under an optical microscope with objective at 10, 40 and 100x.
To determine the morphological floral structure patterns of the Capsicum 10 flowers were preliminarily evaluated, registering the variables as floral radius, length of peduncle, number and color of the petals, and number of stamens, respectively.
The quantitative and morphological traits were studied according to the standard procedure of recording data in chili crop and were analyzed by using the GLM procedure from Statistical Analysis Systems-SAS (r). To identify significant difference among treatments and statistical significance for all comparisons was made at p<0.05. Tukey's multiple range test was used to compare the mean values of treatments.
Results
Both mitosis and meiosis analysis for C. annuum, C. frutescens and C. chinense, confirmed chromosome number 2n=2x=24 (Figures 1 a, b, c), In the genotypes of C. annuum and C. frutescens, there was a numerical variation of 2n=26 chromosomes (Figure 1d), suggesting that resulting from a process of aneuploidy or an upward disploidy, causing an increasing in chromosome number.
Figure 1: Mitotic cells in prometaphase (a) and metaphase (b) in C. annuum, with 2n=24; meiosis (diakinesis), C. frutescens with 2n=2x=24 (c) and 2n=2x=26 (d).
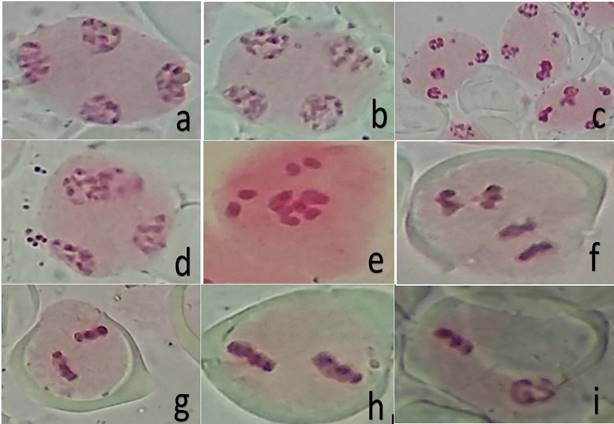
Some chromosomal abnormalities as additional nucleoli (Figure 2a-b), micronuclei (Figure 2c) and nuclear reconstitution at telophase II (Figure 2d) possibly leading to triad; irregular segregation in both metaphase and anaphase II (Figures 2e-f), and asyncronic chromosome division (Figure 2i) during meiosis II were observed in C. annuum and C. frutescens.
Figure 2: Spindles patterns and chromosomal abnormalities during meiosis II in C. annuum (a-e) and C. frutescens (f-i). a) parallel spindles in telophase II (TII), with additional nucleoli; b) perpendicular spindles in TII, with additional nucleoli; c) TII with micronuclei; d) nuclear restitution in TII, which possibly giving rise to an unreduced gamete; e) early ascension of chromosomes at metaphase II (MII); f) perpendicular spindle at anaphase II, with lagging chromosome; g) convergent spindle at MII; h) parallel spindle at MII; i) asynchronous division (prophase-metaphase) in parallel spindle.
C. frutescens presented polyploid cells with 2n=4x=48 to 2n=6x=72, which could be the result of defective cell wall formation during pre-meiotic mitosis or processes of endomitosis (Figures 3a, 3b).
Figure 3: Poliploidy in C. frutescens; a. 2n=6x=72; b. 2n=4x=48
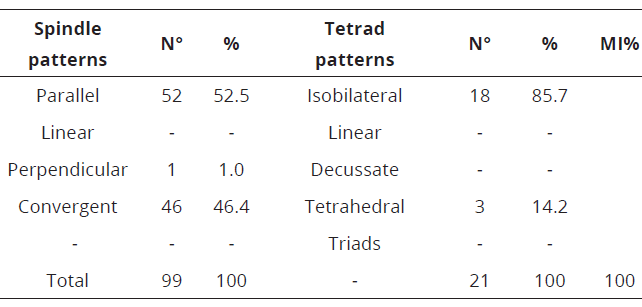
The meiotic index (MI) of the three evaluated species of Capsicum can be seen in Table 1 and 2. C. annuum, showed 98.7% and C. frutescens 100%, respectively.
Table 1: Spindle patterns at meiosis II, tetrads of microspores and meiotic index in C. annuum.
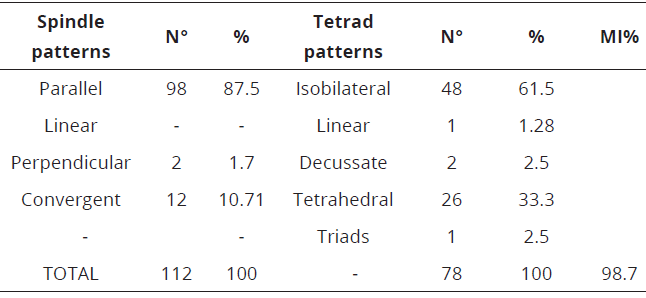
Table 2: Spindle patterns at meiosis II, tetrads of microspores and meiotic index in C. frutescens.
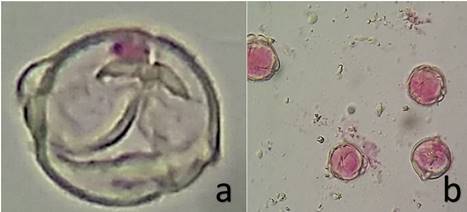
It was found a pollen fertility equal to 94.6% in C. annuum, while C. frutescens showed 19.8% and C. chinense, 97.0% (Table 3, Figure 4). The genotypes of C. annum and C. chinense, because they have a high pollen fertility rate, are recommended as parental in breeding programs. C. frutescens genotype can be considered as a male sterile.
Table 3: Pollen fertility of C. annuum, C. frutescens and C. chinense.
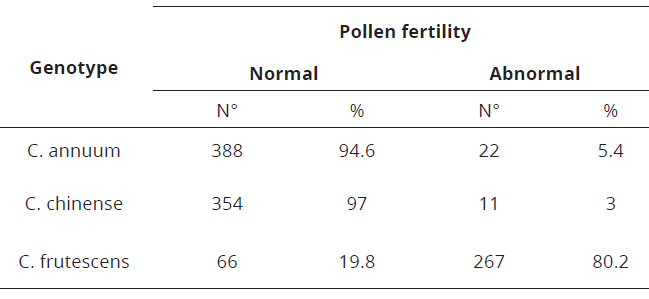
Figure 4: Pollen grains in C. annuum; a. no viable; b. viable
The genotype of C. frutescens showed a high degree of no viable pollen, as to be regarded a male-sterile, since pollen is the final product of male meiosis not recommended as a parental. This large number of no viable grains shows a meiotic instability of this species, or a post-meiotic event which leads to partial or complete sterility.
The number of floral structures of the species of C. annuum and C. frutescens varied between 5 and 7, while in C. chinense this variation was higher, 4 to 7 (Tables 4-6; Figure 5).
Table 4: Floral structure in C. annuum.
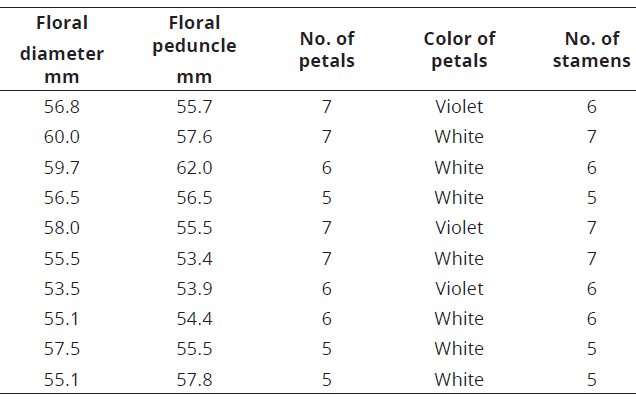
Table 5: Floral structure in C. frutescens.
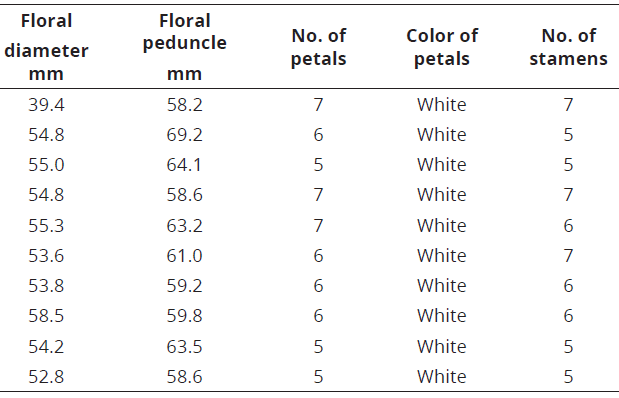
Table 6: Floral structure in C. chinense.
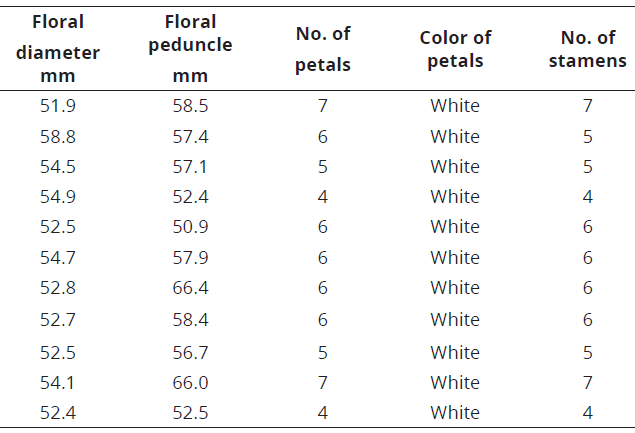
Figure 5: Floral structures in Capsicum. a. C. annuum , numbers ranging from 5 to 7; b. C. frutescens, numbers between 5 and 7; c. C. chinense with a greater variation, between 4 and 7.
Discussion
Both mitosis and meiosis analysis for C. annuum, C. frutescens and C. chinense, confirmed chromosome number 2n=2x=24 (Figures 1 a, b, c), similarly to reported by Shopova (1966); Souza et al. (2011); Pozzobon et al. (2015).
In addition, Moscone et al. (2007), reported the same chromosome number for the three species. Pozzobon & Wittmann (2006), and Teodoro, Garcia & Corona (2007), reported the same chromosome number for some wild species, among them C. buforum, C. campylopodium, C. cornutum, C. villosum var. villosum, C. schottianum and C. pereirae.
Following Moscone et al. (2007), the chromosome basic number of Capsicum would be x=12, where x=13 would have arisen with the evolution of the genus. According to Pozzobon & Wittmann (2006), the genus has two lines of separate evolution, one of the wild species with x=13 chromosomes, and the ancestral number of domesticated, x=12, emerged after the loss of a pair of chromosomes.
These abnormalities were also reported by Caetano (2003), in maize and Caetano, Sandoval, Posada, Caetano-Nunes & Lagos (2008), in Vasconcellea. By other hand, Martins, Pereira, Souza & Costa (2010), reported an early chromosome ascension and laggards, which may be lost during cell division, resulting in aneuploid cells.
Pickersgill (1977), reported a chromosome number of 2n=4x=48 for C. annuum, indicating the occurrence of polyploidy, or a natural tetraploid.
This ploidy level phenomenon, could facilitate the emergence of self-fertilization of these plants the self-incompatibility as a result of the same process, affecting pollen fertility and reducing the likelihood of genetic variability (Bateman, 1952).
According to Love (1951), meiotic index larger than 90% indicate elite materials to be used as parental in plant breeding programs. Martins et al. (2010), and Pozzobon, Bianchetti, Santos, Carvalho, Reifschneider & Ribeiro (2015), observed pollen viability higher than 90% for the species C. chinense and C. annuum, C. frutescens. Conversely, Pozzobon, Souza, Carvalho & Reifschneider (2011), found a large number of unviable pollen grains, suggesting that due to the high temperature and physical factors, can influence the low viability of this species.
Moscone et al. (2007), suggest that changes may occur in chromosome number and morphology of the genus Capsicum, making necessary cytogenetic studies for chromosomal determination of this group, which has distinct genetic variation.
Conclusion
The occurrence of differences in ploidy level, including polyploidy of Capsicum annuum, C. chinense and C. frutescens, is believed to be an outcome of morphological traits changes seen in the flowers, with the number of floral structures.
Acknowledgments
We would like to thank to GIRFIN - Grupo de Investigación en Recursos Fitogenéticos Neotropicales, Universidad Nacional de Colombia Sede Palmira, Colombia.
References
Referencias
Barth, O. M. & Duarte, S. G. (2008). Morfologia polínica de espécies arbóreas de Solanaceae do Estado de Santa Catarina, Brasil. Hoehnea, 35(3), 379-386. http://dx.doi.org/10.1590/S2236-89062008000300005
Bateman, A. J. (1952). Self-incompatibility systems in Angiosperms. Heredity, 6, 285–310. http://dx.doi.org/10.1038/hdy.1952.40
Büttow, M. V.; Barbieri, R. L.; Neitzke, R. S.; Heiden, G. & Carvalho, F. I. F.(2010). Diversidade genética entre acessos de pimentas e pimentões da Embrapa Clima Temperado. Ciênc Rural, 40 (6),1264-1269. http://dx.doi.org/10.1590/S0103-84782010000600004
Caetano, C. M. (2003). La aplicabilidad de la citogenética en Zea mays L.: genes mutantes meióticos. Revista de Ciencias Agrícolas, 20 (1-2), 27-49.
http://revistas.udenar.edu.co/index.php/rfacia/article/view/668/1184
Caetano, C. M.; Sandoval, C. L.; Posada, C. A.; Caetano, D. G. & Lagos, T. C. (2008). Citogenética de especies de Vasconcellea (Caricaceae). Acta Agron, 57 (4), 241-245.
http://revistas.unal.edu.co/index.php/acta_agronomica/article/view/9260/9908.
Carvalho, A. V.; Maciel, R. A.; Beckman, J. C. & Poltronieri, M. C. (2014). Caracterização de genótipos de pimentas Capsicum spp. durante a maturação. Boletim de Pesquisa e Desenvolvimento 90. 1ª edição. Embrapa Amazônia Oriental (Eds.). 21p. https://ainfo.cnptia.embrapa.br/digital/bitstream/item/99243/1/BDP90.pdf.
Love, R. M. (1951). Varietal differences in meiotic chromosome behavior of Brazilian wheats. Agronomy, 43, 72-76. https://dl.sciencesocieties.org/publications/aj/abstracts/43/2/AJ0430020072?access=0&view=pdf.
Martins, K. C.; Pereira, T. N. S.; Souza, S. A. M. & Costa, F. R. (2010). Meiose e viabilidade polínica em acessos de Capsicum annuum e Capsicum baccatum. Ciênc Rural, 40 (8), 1746-1751. http://dx.doi.org/10.1590/S0103-84782010000800012
Moscone, E. A.; Scaldaferro, M. A.; Grabiele, M.; Cecchini, N. M.; Garcia, Y. S.; Jarret, R.; Daviña, J. R.; Ducasse, D. A.; Barboza, G. E. & Ehrendorfer, F. (2007). The evolution of chili peppers (Capsicum - Solanaceae): a cytogenetic perspective. Acta Hortic, 745, 137-169. http://dx.doi.org/10.17660/ActaHortic.2007.745.5
Neitzke, R. S.; Barbieri, R. L.; Vasconcelos, C. S.; Fischer, S. Z.; Vilella, J. C. B. & Castro, C. M. (2014). Caracterização morfológica e estimativa da distância genética de acessos de pimenta do banco ativo de germoplasma de Capsicum da Embrapa Clima Temperado. Boletim de Pesquisa e Desenvolvimento 178. Embrapa Clima Temperado(Eds.). 43p.
Pickersgill, B. (1997). Genetic resources and breeding of Capsicum. Euphytica, 96(1), 129-133. https://doi.org/10.1023/A:1002913228101
Pozzobon, M.T. & Wittmann, M.T. (2006). A meiotic study of the wild and semi domesticated Brazilian species of genus Capsicum L. (Solanaceae). Cytologia, 71 (3), 275-287. http://doi.org/10.1508/cytologia.71.275
Pozzobon, M. T.; Souza, K. R. R.; Carvalho, S. I. C. & Reifschneider, F. J. B. (2011). Meiose e viabilidade polínica em linhagens avançadas de pimenta. Hortic Bras, 29 (2), 212-216. http://www.scielo.br/pdf/hb/v29n2/a13v29n2.pdf.
Pozzobon, M. T.; Bianchetti, L. B.; Santos, S.; Carvalho, S. I. C.; Reifschneider, F. J. B. & Ribeiro, C. S.C. (2015). Comportamento meiótico em acessos de Capsicum chinense Jacq. do banco de germoplasma da Embrapa, Brasil. Rev Bras Biociênc, 13 (2), 96-100. http://www.ufrgs.br/seerbio/ojs/index.php/rbb/article/view/3239/1278.
Ribeiro, C. S.; Lopes, C. A.; Carvalho, S. I. C.; Henz, G. P. & Reifschneíder, F. J. B. (2008). Pimentas Capsicum. EMBRAPA/MAPA (Eds.). 4(18).12 p. https://www.embrapa.br/documents/1355126/2250572/EDI%C3%87%C3%83O+18i.pdf/82e27ace-f1a5-4f95-9215-9207a6c1b7de.
Rufino, J. L. S. & Penteado, D. C. S. (2006). Importância econômica, perspectivas e potencialidades do mercado para pimenta. Informe Agropecuário, Belo Horizonte, 27(235), 7-15.
Signorini, T.; Renesto, E.; Machado, M. F. P. S.; Bespalhok, D. N. & Monteiro, E. R. (2013). Diversidade genética de espécies de Capsicum com base em dados de isozimas. Hortic Bras, 31(4), 534-539. http://dx.doi.org/10.1590/S0102-05362013000400005
Shopova, M. (1966). Studies in the genus Capsicum. Chromosoma, 19(3), 340-348. https://doi.org/10.1007/BF00326922
Souza, S. A. M.; Martins, K. C. & Pereira, T. N. S. (2011). Polimorfismo cromossômico em Capsicum chinense Jacq. Ciênc Rural, 41 (10),1777-1783. http://dx.doi.org/10.1590/S0103-84782011001000017
Teodoro-Pardo, C. V.; García-Velázquez, A.; Corona-Torres, T. (2007). Polimorfismo cromosómico en Capsicum annuum l. (Solanaceae) en recolectas de Puebla, Morelos y Querétaro, México. Agrociencia, 41(8), 873-881. http://www.redalyc.org/articulo.oa?id=30220203007
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Timir Baran Jha, Biplab Kumar Bhowmick. (2021). Conservation of floral, fruit and chromosomal diversity: a review on diploid and polyploid Capsicum annuum complex in India. Molecular Biology Reports, 48(7), p.5587. https://doi.org/10.1007/s11033-021-06355-4.
2. Cristina Rueda‐Uribe, Alexander Chautá, Tamsin L. Woodman, Eloisa Lasso, Roxibell C. Pelayo, Laura Milena Manrique‐Garzón, Marcia C. Muñoz, Rebekka Allgayer, Tia‐Lynn Ashman, Greta Bocedi, David F.R.P. Burslem, Pedro A. Camargo‐Martínez, María Ángela Echeverry‐Galvis, Catalina González‐Arango, Cecile Gubry‐Rangin, Lesley T. Lancaster, Kara K.S. Layton, Fabio Manfredini, Carlos Martel, Lia Montti, Alexander S.T. Papadopulos, Robert A. Raguso, Jonathan Ready, Alejandro Rico‐Guevara, Camila Rocabado, Justin M. J. Travis. (2025). Pollination ecology in the tropical Andes: moving towards a cross‐scale approach. Biological Reviews, 100(6), p.2312. https://doi.org/10.1111/brv.70049.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Acta Agronómica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Política sobre Derechos de autor:Los autores que publican en la revista se acogen al código de licencia creative commons 4.0 de atribución, no comercial, sin derivados.
Es decir, que aún siendo la Revista Acta Agronómica de acceso libre, los usuarios pueden descargar la información contenida en ella, pero deben darle atribución o reconocimiento de propiedad intelectual, deben usarlo tal como está, sin derivación alguna y no debe ser usado con fines comerciales.