Population parameters and damage of Polyphagotarsonemus latus (Acari: Tarsonemidae) in Valencia orange (Citrus sinensis [L.] Osbeck) crop
DOI:
https://doi.org/10.15446/acag.v66n4.59922Palabras clave:
Broad mite, biology, infestation levels, damage, Colombia (en)Descargas
Recibido: 5 de septiembre de 2016; Aceptado: 18 de mayo de 2017
Abstract
Polyphagotarsonemus latus is one a major pest of Valencia orange in Colombia. To study its biology, it was established an experiment on young leaves of the Valencia orange at 25 ± 5°C, 70 ± 5% RH, and 12:12 L:D photoperiod. To characterize the nature of damage caused by this mite on leaves and young fruits, a trial in screenhouse conditions using young leaf and small fruits (0.1 - 0.3 cm in diameter) and fruits between 1.0-3.0 cm in diameter, which were infested with 5, 15 and 30 adult females of P. latus, was established. The total duration of life cycle was 3.2 days; the female had an oviposition period of 7.4 days and longevity 9.6 days. Life table parameters were as follows: net reproductive rate (Ro ) = 93.3, intrinsic rate of natural increase (rm ) = 1.2, finite multiplication rate (λ) = 3.7, doubling time (TD) = 0.6 days and generation time (T) = 3.4. The first signs of damage and shoot drop in newly developed shoots occurred between 7.0 and 11.8 days after infestation. Small fruit infested with 5-30 mites showed damage 2.4 days after infestation. Shoot drop started 7.0 days after infestation. In the case of fruits between 1.0 and 3.0 cm in diameter, the damage and the collapse of the structure was between 3.0 and 13.5 days, respectively. These results explain how a mite with a very short life cycle and huge biotic potential causes such severe damage in shoots and newly formed fruits of Valencia orange.
Keywords:
Broad mite, biology, infestation levels, damage, Colombia.Resumen
Polyphagotarsonemus latus es una de las principales plagas de la naranja Valencia en Colombia. Para estudiar su biología, se estableció un experimento sobre hojas jóvenes de naranja Valencia a 25 ± 5°C, 70 ± 5% de HR y fotoperiodo L: D 12:12. Para caracterizar la naturaleza del daño causado por este ácaro en hojas y frutos jóvenes, se realizó un ensayo en condiciones de invernadero utilizando hojas jóvenes y frutos pequeños (0.1 - 0.3 cm de diámetro) y frutos entre 1.0-3.0 cm de diámetro, infestados con 5, 15 y 30 hembras adultas de P. latus. La duración total del ciclo de vida fue de 3.2 días; la hembra tenía un período de oviposición de 7.4 días y una longevidad de 9.6 días. Los parámetros de la tabla de vida fueron los siguientes: tasa reproductiva neta (Ro) = 93.3, tasa intrínseca de crecimiento natural (rm) = 1.2, tasa de multiplicación finita (λ) = 3.7, tiempo de duplicación (TD) = 0.6 días y tiempo de generación = 3.4. Los primeros signos de daño y caída de brotes en brotes tiernos ocurrieron entre 7.0 y 11.8 días después de la infestación. Los frutos pequeños infestados con 5-30 ácaros mostraron daño 2.4 días después de la infestación. La caída del brote comenzó 7.0 días después de la infestación. En el caso de frutos de entre 1.0 y 3.0 cm de diámetro, el daño y el colapso de la estructura se dio entre 3.0 y 13.5 días, respectivamente. Estos resultados explican cómo un ácaro con un ciclo de vida muy corto y un enorme potencial biótico, causa tal daño severo en brotes y frutos recién formados de naranja Valencia.
Palabras clave:
Ácaros, biología, niveles de infestación, daño, Colombia.Introduction
Within the Tarsonemidae family P. latus is considered the most important pest of citrus in the tropics and in other regions of the world (Gerson, 1992). This Tarsonemidae, known as "broad mite", has a wide geographical distribution and a considerable number of host plants in tropical areas (Gerson, 1992). According to Peña & Campbell (2005), in temperate and subtropical regions, attacks plants under screenhouse conditions, and according to Rogers, Stansly, Childers, Mccoy & Nigg (2010), also attacks other citrus species such as lemon and lime. In this sense, P. latus disperses through wind, the transportation of infected plant tissues, by natural contact of plant foliage, and through foretic relationship with insects (Palevsky, Soroker, Weintrub, Mansour, Abo-Moch & Gerson, 2001). On the other hand, Ochoa, Aguilar & Vargas (1991), and Rogers, Stansly, Childers, Mccoy & Nigg (2010), consider that P. latus is the most important pest in lemons in some areas of the Caribbean.
On leaves, damage is characterized by malformation of growing tissues, including leaves, shoots and flowers. The mite injects toxic saliva causing twisted, hardened and distorted growth in the plant terminal buds (Rogers, Stansly, Childers, Mccoy & Nigg, 2010). When populations are high, infested leaves bend and turn to coppery and purple color. Damage on citrus fruit occasionally occurs on the shaded side and can go unnoticed; however, if the attack is severe, there can be discoloration and fruit drop. Fruit areas which are infested, have a burnt appearance and rough texture. Furthermore, there may be a lifting of the epidermis superficial layer, which remains to the fruit surface a silver-gray thin film (Rogers, Stansly, Childers, Mccoy & Nigg, 2010).
In fact, in a previous work, Peña & Bullock (1994), found that the attack of the mite reduces leaf area and water content in limes and sweet oranges in citrus-growing areas in Florida. Peña (1990), found in artificial infestations on lime fruits in laboratory, smaller than 2.5 cm in diameter, the mite population have allowed an increasing in few days after infestation. In screenhouse conditions, damage occurred between 10 and 14 days, and in the field conditions, between 9 and 15 days after infestation.
Colombia has a low market share of orange export. This situation is associated with, among other limiting factors, damages caused by pests such as mites, which are a serious problem in fruit crops (Ochoa, Aguilar, & Vargas, 1991). In Colombia, P. latus, was reported in 1971 on citrus (Zuluaga, 1971).
Recently, Mesa & Rodríguez (2012), reported the presence of this mite in Valencia orange in Antioquia, Caldas, Quindío, Risaralda and Valle del Cauca-Colombia, where P. latus is the responsible of losses between 37-40% on fruit quality. Given the negative impact of P. latus in the citrus production in Colombia, this study was conducted as a first step in developing an IPM program for broad mite control with the objectives of (1) determining its main biological and behavioral aspects and (2) to study the P. latus damage in different Valencia orange plant organs.
Materials and methods
Origin and colony maintenance
Specimens of P. latus were obtained 15 days before the onset of the study from a colony established with 200 - 300 mites collected from young leaves of Valencia orange (Citrus sinensis L cvar Valencia) from an orchard in commercial production in the municipality of Caicedonia (N 4º 22' 8.8" W 75° 48' 40.8"), department of Valle del Cauca-Colombia. The colony was maintained on young leaves of Valencia orange trees grafted on the same cultivar, without application of acaricides. Fifty females of P. latus were placed on each leaf with a painting brush. A population increasing was weekly checked. The colony was maintained at 25 + 5°C, 70 + 5% RH and at 12:12 (L:D) photoperiod in the Entomology and Acarology Laboratory at the Universidad Nacional de Colombia, campus Palmira, Colombia.
Life cycle and description of the experimental unit
A piece of plastic foam saturated with water was placed inside plastic petri dishes 15.0 cm in diameter and 1.5 cm height. In each piece of foam, 10 previously washed 4.0 cm2 squares of Valencia orange young leaves were deposited. A piece of paper placed on each side served as a barrier. These pieces formed the experimental unit on which three P. latus females and one male were transferred to obtain eggs. 12 h later, mites were removed, and one egg was left in each experimental unit. Petri dishes were covered with their respective lids. The size of the cohort was 100 eggs. Each replicate was checked twice per day (7:00 a.m. and 5:00 p.m.) and hatching and molting were recorded until mites reached adult stage. Additionally, immature duration, was measured.
Fecundity, longevity and life table parameters
Once mites reached adulthood, females were individually introduced in an experimental unit with a male obtained from the stock colony. These individuals were observed on a daily basis and the number of eggs laid per female was counted. With these data, survival (l x) and fecundity (m x) curves were plotted. Eggs were monitored to the adult stage. In fact, P. latus adults were mounted in a Hoyer medium and checked under a phase contrast microscope to confirm their sex and to establish the male: female ratio.
Therefore, the experimental units were maintained in similar conditions to those previously described. Data to calculate the mite life table were obtained from 30 fertilized females. The demographic parameter calculated were as follows: net reproductive rate (R0 ), intrinsic rate of increase (rm ), average generation time (T), doubling time (TD) and finite rate of increase (λ), which were calculated using the Jackknife technique implemented by , Luiz & Campanhola (2000), with the SAS statistical package (SAS, 2012) with the proposed equations by Andrewartha & Birch (1954); Rabinovich(1980). (Equations 1-5).
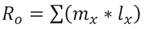 Equation1
Equation1
 Equation 2
Equation 2
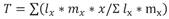 Equation 3
Equation 3
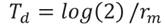 Equation 4
Equation 4
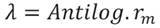 Equation 5
Equation 5
Where: l x = mite survival, and 𝑚 𝑥 = fecundity.
Valencia orange phenology and damage caused by P. latus
The experiment was carried out under semi-controlled conditions in an antiaphid screenhouse in Palmira, Valle del Cauca, 980 m a. s. l. at 25 oC and 70% R.H. One-year-old Valencia orange trees grafted on Citrandarin "Sunky x English" rootstock, were selected from each of which there were selected young leaves, small green fruits with diameters between 0.1 - 0.3 cm (age: 9.5 days after flowering) and fruits with diameters between 1.0 - 3.0 cm (60 days after flowering).
However, with a painting brush, 5, 15 and 30 P. latus females, were properly placed. Uninfested structures were used as control groups. After infestation, it was daily measured percentage of affected fruit surface, day of appearance of first damage symptom and day of structure abscission due to the mite damage. During the experiment, photographs of the damaged organs were taken. The experiment on young leaves was developed under a completely randomized design with one leaf phenological stage, four infestation levels (0, 5, 15 and 30 females/structure, respectively) and 30 replicates per treatment. In the case of fruits, was developed a completely randomized design with two phenological stages of the fruit, four infestation levels (0, 5, 15 and 30 females/ structure, respectively) and ten replicates per treatment. Uninfested fruits were used as control groups. Data were subjected to analysis of variance (ANOVA) using the GLM procedure from Statistical Analysis Systems (SAS, 2012). To identify significant difference among treatments and statistical significance for all comparisons was made at p<0.05. Tukey's multiple range test was used to compare the mean values of treatments.
Results
Life cycle and developmental stages
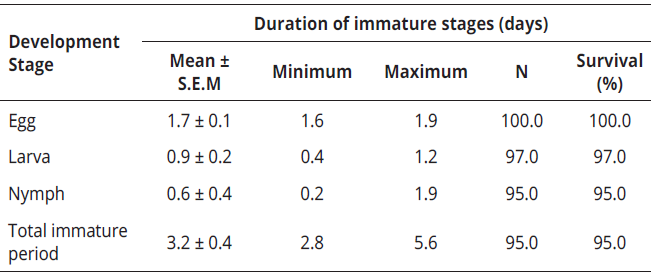
It was already expected that P. latus would sucessfully develop from eggs to adults when fed on valencia orange, which takes 3.2 days. The average duration of incubation period was 1.7 days and the larval stage lasted 0.9 days. The quiescent nymph "pupa" lasted 0.6 days; in fact, the immature survival was 95% (Table 1). Sex ratio (female: male) was 2.4:1.
Table 1: Mean duration (days ± S.E.M.) of the immature stages of P. latus on Valencia orange young leaves at 25 ± 5°C, 70 ± 5% RH and 12:12 (L : D) photoperiod
Reproductive, longevity, fecundity periods and life table parameters
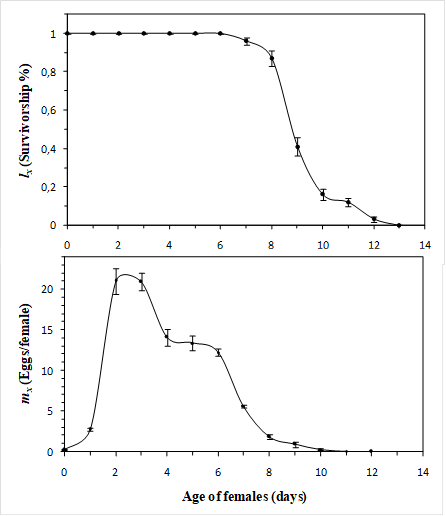
P. latus males starts their reproductive activity in search of a pupa stage of development, which they raise with the help of their last pair of legs, which is fixed with the male genital papilla to their body, in turn, is a posterior extreme and it is loaded as they walk until the adult female emergence. In general, P. latus female, requires a pre-oviposition period of 1.3 days. However, preoviposition of P. latus is almost immediately after emerging. Average duration of oviposition period is relatively short (7 days). The survival period (lx) follows a normal curve in which young P. latus female survival is very high, in the case of the fecundity, most egg production peak occurs in the first and second day of the P. latus female life (Figure 1).
Figure 1: Survivorship (l
x
) and fecundity (m
x
) curves of P. latus on Valencia orange young leaves at 25 + 5°C, 70 + 5% RH and 12H:12H (L:D) photoperiod. Error bars represent mean standard error.
On average, the longevity of P. latus female was 9.6 days (Table 2).
Table 2: Life table parameters for P. latus reared on Valencia orange young leaves at 25 + 5oC, 70 + 5% RH and 12:12 (L:D) photoperiod
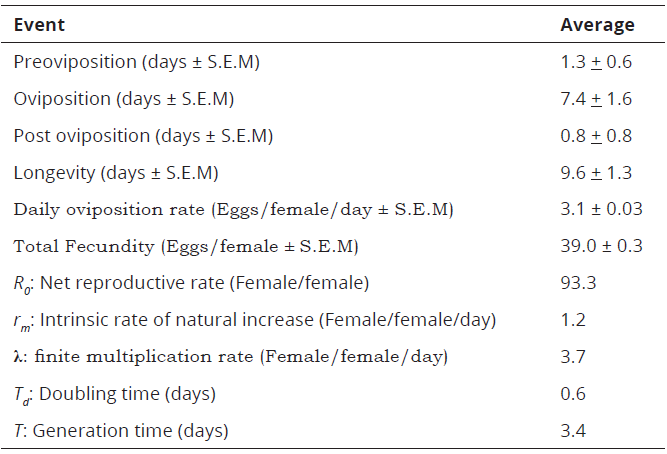
In this study, P. latus produced 39.0 eggs.female-1, which is equivalent to 3.1 eggs.female-1 per day. Conversely, P. latus showed a net reproductive rate (Ro) of 93.3, an intrinsic rate of natural increase (rm) of 1.2, a finite multiplication rate (λ) of 3.7, a generation time (T) of 0.6 and a doubling time (TD) of 3.4 days, respectively (Table 2).
Damage of P. latus in young leaves
First damage signs caused by P. latus on newly developed Valencia orange shoots are characterized by a change in the epidermis color tone, which loses brightness and becomes opaque, giving the impression of being dehydrated. Therefore, leaves twist or curl along their edges, starting from the base to the apex, at this point the highest feeding and oviposition activities of females occurs. As time passes, damage is more noticeable and the leaf color turns into a brown spot that starts mainly on the mid-rib. If damage is severe, the shoot drops off (Figure 2).
Figure 2: Damage caused in Valencia orange shoots by P. latus: A, B, D and E. Tissue distortion, twisting or curling towards its back, sheaf of leaves tanning. C. Intervenal yellowing. F. Leaf drop. G. Healthy shoot.
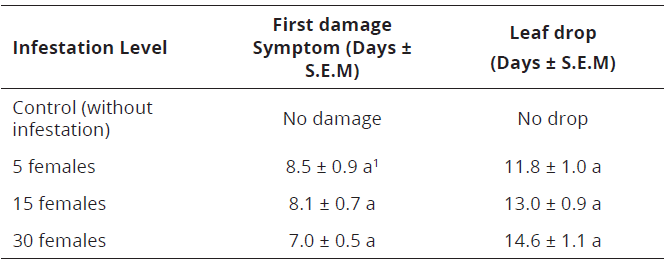
No differences were found between days of appearance of first symptoms (F=13.5; df=3; P<0.30) and leaf drop (F= 31.8; df=3; P<0.25) according to the level of infestation of P. latus in Valencia orange shoots (Table 3). These symptoms occurred between 7.0 and 11.8 days after infestation.
Table 3: Days of appearance of first damage symptoms and newly developed leaf drop in Valencia orange young leaves affected by three infestation levels of P. latus under screen house conditions at 25°C and 70% R.H in Palmira Valle del Cauca, Colombia
Damage of P. latus in young fruit
First signs of damage caused by P. latus on Valencia orange fruits with a diameter between 0.1-0.3 cm, are characterized by a change in the epidermis color tone, which loses brightness and becomes opaque. Later, there are parallel scissions that start at the base of the fruit, giving the impression of tissue dehydration. The highest feeding and oviposition activity of the females takes place in this area. Over time, damage is more noticeable and it becomes a brown spot at the base of the fruit (Figure 3).
In the case of fruits with larger diameter (1.0-3.0 cm), damage is characterized by the change in the epidermis coloration, which becomes whitish and opaque, with mummified appearance; this damage may, initially, occupy small areas, but as time passes, can cover all the fruit (Figure 3).
There were differences between days of appearance of first symptoms (F= 8.5; df= 2; P<0.0001) and fruits drop (F= 8.6; df= 2; P<0.0006) according to the level of infestation of P. latus females and the infested structure type (Table 4).
Figure 3: Damage symptoms at 25oC and 70% R.H in Palmira-Valle del Cauca, Colombia caused by P. latus in Valencia orange fruits with a diameter between 0.1-0.3 cm (age: 9.5 days after flowering) and 1.0-3.0 cm (60 days after flowering): A: Healthy small fruit; B: damage caused by 15 females after 7 days of infestation on small fruit; C: Damage caused by 30 females after 24 hours of infestation on small fruit; D: Healthy large fruit E: Damage caused by 15 females after 7 days of infestation on large fruit; F: Damage caused by 30 female after 24 hours of infestation on large fruit.
Table 4: Days of appearance of first damage symptoms and drop of Valencia orange fruits affected by two infestation levels of P. latus females under screenhouse conditions (25oC and 70% R.H) in Palmira-Valle del Cauca, Colombia.
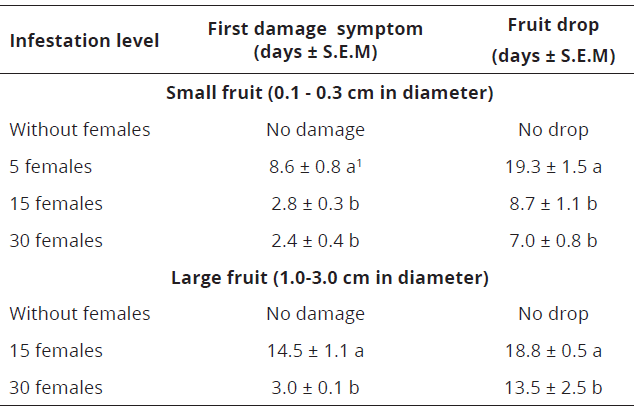
In fact, in that small fruits (0.1 - 0.3 cm in diameter) infested with 15 and 30 females of P. latus show earlier symptoms of damage and abscission of structure than those fruits infested with five females, in which damage and fruit drop occur 8.6-19.3 days after infestation, respectively.
In the case of fruits, 1.0-3.0 cm diameter with infestation levels of 15 females, the first symptoms of damage and subsequent collapse of the structure occur 14.5-18.1 days after initial infestation, if the level of infestation increases to 30 females, the first symptoms of damage and the collapse of the structure occurs significantly faster, 3.0 and 13.5 days, respectively.
Discussion
In this study, morphological characteristics of P. latus adults were similar to those observed by Ochoa, Aguilar & Vargas (1991), P. latus eggs are oval, whitish with the chorion covered by longitudinal rows of white tubercles, occupying about half the female body (Norton, Kethley, Johnston & O'Connor, 1993). The pupa stage corresponds to a quiescent phase, which develops in the larval cuticle. This developmental stage is also known as "pharate female" or quiescent nymph (Norton, Kethley, Johnston & O'Connor, 1993). Duration of the egg stage was, on average, 1.7 days, equivalent to more than half the time of the mite full development cycle, being similar to findings of Vieira & Chiavegato (1999), and Ferreira, Oliveira, Haji & Gondim (2006), in plants of C. limon (2.2 days) and grapevine (3.9 days), respectively.
Total life cycle of P. latus was 3.2 days (Table 1), which coincides with the observations of Vieira & Chiavegato (1999), who worked with young fruits of C. limon under weather conditions of 27.2 ± 0.5 °C and 68.2 ± 1.2% RH.
Similar to those in this study (25 ± 5 °C, 70 + 5% RH and 12:12 L:D h photoperiod) and Vieira & Chiavegato (1999), at 27°C and 67.6% RH and an identical photoperiod. High survival (>90%), high percentage of adult females compared to males (2.4:1) and short development time (3.2 days) are characteristics of this species which ensure its survival in different stages of development.
Figure 1, shows the cohort survival, which follows a normal curve in which immature and young P. latus female survival is very high, a fact that guarantees a stable population in terms of the age distribution and permanent reproduction and multiplication expectations. Under the conditions of this study (25 + 5 °C, 70 + 5% RH and 12:12 L:D photoperiod) a preoviposition period of 1.3 days was registered (Table 2), a similar value to those recorded by Vieira & Chiavegato (1999), in cotton leaves (1.1 days ) and lemon young fruits (0.9 days). However, in hosts plants such as grapevine, where had achieved preoviposition periods of 0.3 days (Ferreira, Oliveira, Haji & Gondim, 2006). Oviposition period lasted, on average, 7 days and was similar to that found by Vieira & Chiavegato (1999), (6.8 days on young lemon fruits). After oviposition, females can live up to 0.8 additional days, on average. However, on grapevine crops this average could extend up to 1.1 days (Ferreira, Oliveira, Haji & Gondim, 2006).
In this study, P. latus longevity on leaves of the Valencia orange was 9.6 days, these results are comparable in variability to the report by Vieira & Chiavegato (1999), in young lemon fruits. However, longevities of 13.6 days in cotton (Vieira & Chiavegato, 1999) and of 15.8 days in grapevine (Ferreira, Oliveira, Haji & Gondim, 2006), have been recorded.
Observed fecundity was 39 eggs.female-1, 3.1 eggs per P. latus female per day. In a previous research work carried out by Vieira & Chiavegato (1999), with other hosts plants of the genus Citrus, maintained at 27°C, specifically with Sicilian lemon (C. limon), found fecundities of 24.9 and 58.9 eggs per P. latus female, respectively, which shows that this host is very suitable for P. latus population development and have allowed a possible explanation why there are so high populations of the pest in this crop.
Under the conditions of this experiment, the species generation growth rate was 120% (Table 2), which is difficult to find in another species. Results show that indicators of its life table (R o =93.3, rm=1.2, λ=3.7, T= 0.6 and TD= 3.4 days) were higher compared with those achieved by Ferreira, Oliveira, Haji & Gondim (2006), in grapevine (R o= 30.12, rm=0.314, T=10.81 and λ=1.37).
Symptoms described in this study for the attack of P. latus on young leaves of Valencia orange (Figure 2), confirms findings of Jeppson, Keifer, & Baker (1975), who described a variety of symptoms and specific reactions due to the possible toxin that P. latus injects. According to these researchers, some of the symptoms are confused with viral diseases, herbicides or stress problems due to Magnesium deficiency. The most significant damages occur on the plants growing points where the tissues are turgid (Jeppson, Keifer, & Baker, 1975).
According to the results of the experiments on newly developed shoots (Table 3), independent of the number of mites, the first symptoms of damage occurred between 7 and 8.5 days after infestation.
Nevertheless, the fall of the structures occurred between 11.8 and 14.6 days, which indicates damage severity on young tissues, up to the point that infestations of five P. latus females can cause shoots drop in a short time, which is concordance to Gerson (1992).
In the present study, we found that damage symptoms from the attack of P. latus in some host plants may occur very quickly, suggesting that few mites are sufficient to cause damage. In fact, symptoms of damage caused by P. latus on Valencia orange fruits with diameters between 0.1 - 0.3 cm, which were characterized by the change in the epidermis tone and the blackening appearance at the fruit base (Figure 3), which is consistent with the findings of Peña (1990), in lime fruits. Initial symptoms of damage and fruit drop occur first in fruits with diameter between 0.1 - 0.3 cm (Table 4); especially with 15 and 30 females.
With these population levels, in less than 3 days, the impact of P. latus on fruit drop is observed, which occurs 7-8 days after the attack. These results provides more accurate and reliable estimates of the first stages of fruit formation (0.1- 0.3 cm) are very sensitive to the mite attack and confirm findings of Brown & Jones (1983), and Peña (1990), who worked with lime fruits, found that P. latus prefers those with small diameters. De acuerdo con Jeppson, Keifer & Baker (1975), who indicates mite preference for young tissues and indicate that P. latus populations in field conditions, should be evaluated at this phenological stage, in order to establish action thresholds.
In the case of fruits with larger diameter (between 1.0 - 3.0 cm), was only found a change in the epidermis coloration, which became whitish and opaque (Figure 3).
In this phenological fruit stage with the evaluated levels of infestation as an extension in the time of appearance of damage symptoms and fall of the fruit, are listed in Table 4, which confirms the reports of Jeppson, Keifer & Baker (1975), these researchers found that mites cannot feed on old and fibrous tissues.
Results obtained in this study, provides more important information to explain how a mite with such a very short life cycle, high oviposition rate and huge biotic potential causes such severe damage so quickly in both young tissues and shoots and in newly formed fruits. If we keep in mind is considered that young shoots and fruits with diameters between 0.1-0.3 cm, and infestations of 5 and 15 mites can present damage in 8.6 and 2.8 days, respectively, it is clear that P. latus is a pest in Valencia oranges as recorded by Mesa & Rodríguez (2012), for this citrus-producing areas in Colombia.
In the past, Bassett (1981), considers that the presence of a small number of individuals of P. latus is sufficient to cause economic damage in the host plant; results of this study provide basic information on the establishment of action thresholds against P. latus.
Given these concerns, the biological parameters of the mite and plant structures most affected by control measures at the appropriate timing in field conditions, which had achieved the first steps in establishing IPM strategies for mites in this crop.
For the first time in Colombia, establishing artificial infestation trials with P. latus on Valencia orange organs, with results that approximate the density of this mite found under true field conditions. However, is still necessary to determine the population densities levels of P. latus that justify control, taking into account observations by Peña (1990), on density variations of P. latus in the crop associated with the presence of natural enemies, inter specific competition with other species of mites such as Phyllocoptruta oleivora Ashmead (Acari: Eriophyidae) and environmental conditions.
Acknowledgements
We would like to thank to Grupo de Investigación en Acaro logia. Facultad de Ciencias Agropecuarias. Universidad Nacional de Colombia campus Palmira- Valle del Cauca, Colombia. To Ministerio de Agricultura y Desarrollo Rural. To generation strategies project for the integrated management of mites that affect fruit quality in Valencia orange for a competitive production in Colombia. To Norbey Marin.
References
Referencias
Andrewartha, H.G. & Birch, L.C. (1954). The distribution and abundance of animals. The University of Chicago Press (Eds.). Chicago, Illinois, USA. 793p.
Bassett, P. (1981). Observations on broad mite (Polyphagotarsonemus latus) (Acari: Tarsonemidae) attacking cucumber. Proc Brit Crop Protect Conf – Pests Dis, 1, 99 – 103.
Brown, R.D. & Jones, V.P. (1983). The broad mite on lemon in southern California. Cal Agricul, 27(7), 21 – 22. https://ucanr.edu/repositoryfiles/ca3707p21-72310.pdf.
Ferreira, R., Oliveira, J., Haji, F. & Gondim, Jr. M. (2006). Biologia, exigências térmicas e tabela de fertilidade do Ácaro branco Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae) en videira (Vitis vinífera L) cv Itália. Neotrop Entomol, 35(1), 126-132. http://dx.doi.org/10.1590/S1519-566X2006000100017
Gerson, U. (1992). Biology and control of the broad mite, Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae). Exp Appl Acarol, 13(3), 163 – 178. https://doi.org/10.1007/BF01194934
Jeppson, L.R., Keifer, H.H. & Baker, E.W. (1975) Mites injurious to economic plants. University of California Press (Eds.). Berkeley-California, USA.
Maia, A., Luiz, A.J. & Campanhola, C. (2000). Statistical inference on associated fertility life table parameters using jackknife technique: computational aspects. J Econ Entomol, 93(2), 511-518. https://doi.org/10.1603/0022-0493-93.2.511
Mesa, N.C. & Rodríguez, I. (2012). Ácaros que afectan la calidad del fruto de los cítricos en Colombia. pp. 163-171. En: Cítricos: cultivo, poscosecha e industrialización. Caldas: Corporación Universitaria Lasallista (Eds.). 367 p.
Norton, R., Kethley, J., Johnston, D. & O’Connor, B. (1993). Phylogenetic perspectives on genetic systems and reproductive modes of mites. In: Wrensch D, Ebbert M (Eds.). Evolution and diversity of sex ratio in insects and mites. Chapman Hall, Nueva Yok, USA. pp 8 – 99.
Ochoa, R., Aguilar, H. & Vargas, C. (1991). Ácaros fitófagos de América Central: Guía ilustrada. Centro Agronómica Tropical de Investigación y Enseñanza Manual Técnico No. 6. CATIE, Turrialba, Costa Rica. 251 p. http://orton.catie.ac.cr/repdoc/A6971e/A6971e.pdf.
Palevsky, E., Soroker, V., Weintrub, P., Mansour, F., Abo-Moch, F. & Gerson, U. (2001). How species-specific is the phoretic relationship between the broad mite, Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae) and its insect host. Exp Appl Acarol, 25(3), 217 – 224. https://doi.org/10.1023/A:1010645315630
Peña, J.E. (1990). Relationships of broad mite (Acari: Tarsonemidae) density to lime damage. J Econ Entomol, 83(5), 2008–2015. https://doi.org/10.1093/jee/83.5.2008
Peña, J.E. & Bullock, R.C. (1994). Effects of broad mite, Polyphagotarsonemus latus feeding on vegetative plant growth. Fla Entomol, 77, 180 – 184.
Rabinovich, J.E. (1980). Introducción a la ecología de poblaciones animales. Consejo Nacional para la Enseñanza de la Biología. Compañía Editorial Continental, México. 313p.
Rogers, M.E., Stansly, P.A., Childers, C.C., Mccoy, C.W. & Nigg, H.N. (2010). Florida citrus pest management guide: Rust mites, spider mites, and other phytophagous mites. University of Florida. IFAS (Eds.). New York, USA. 603p. http://edis.ifas.ufl.edu/cg002.
SAS. Institute Inc. (2012). SAS User’s guide version 12.1. SAS Institute Cary North (Eds.). Carolina, USA. 22p. https://support.sas.com/documentation/onlinedoc/stat/121/intro.pdf
Vieira, M. & Chiavegato, L. (1999). Biologia de Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae) em limão siciliano (Citrus limón Burm). An Soc Entomol Bra, 28(1), 27-33. http://dx.doi.org/10.1590/S0301-80591999000100002
Zuluaga, J.I. (1971). Lista preliminar de ácaros de importancia en Colombia. Acta Agron, 21(3), 119-132. http://revistas.unal.edu.co/index.php/acta_agronomica/article/view/48546/49742.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Marc Cabedo‐López, Joaquín Cruz‐Miralles, David Peris, M. Victoria Ibáñez‐Gual, Víctor Flors, Josep A. Jaques. (2021). The response of citrus plants to the broad mite Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae). Agricultural and Forest Entomology, 23(4), p.411. https://doi.org/10.1111/afe.12442.
2. Vivian Ovando-Garay, Rebeca González-Gómez, Eugenia Zarza, Alfredo Castillo-Vera, Martha Elena de Coss-Flores, Feng ZHANG. (2022). Morphological and genetic characterization of the broad mite Polyphagotarsonemus latus Banks (Acari: Tarsonemidae) from two Mexican populations. PLOS ONE, 17(4), p.e0266335. https://doi.org/10.1371/journal.pone.0266335.
3. Rana AKYAZI, Mete SOYSAL, Yunus Emre ALTUNÇ, Duygu AKYOL. (2022). Polyphagotarsonemus latus (Banks, 1904) (Acari: Tarsonemidae)’un kontrolünde Nicotiana tabacum L. (Solanaceae), Allium sativum L. (Amaryllidaceae) ve arap sabununun etkinliği. Turkish Journal of Entomology, 46(2), p.211. https://doi.org/10.16970/entoted.1079195.
4. Neenu Augustine, Upasna Selvapandian, Chethan Badakegudlu Ramaiah, Suresh Ramakrishna Jambagi, Thiruvengadam Venkatesan, Muthugounder Mohan. (2024). Resistance to fenazaquin in broad mite, Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae): Realized heritability, risk assessment and cross‐resistance. Journal of Applied Entomology, 148(3), p.279. https://doi.org/10.1111/jen.13187.
5. Muhamad Firdaus Syahmi Sam-on, Shuhaimi Mustafa, Mohd Termizi Yusof, Amalia Mohd Hashim, Ku Nur Azwa Ku Aizuddin. (2024). Exploring the Global Trends of Bacillus, Trichoderma and Entomopathogenic Fungi for Pathogen and Pest Control in Chili Cultivation. Saudi Journal of Biological Sciences, 31(8), p.104046. https://doi.org/10.1016/j.sjbs.2024.104046.
6. Nubia Murcia Riaño, Mauricio Fernando Martínez, Javier Orlando Orduz-Rodríguez, Liliana Ríos-Rojas, Yeison López Galé, Marlon José Yacomelo Hernández, Arturo Carabalí Muñoz, Takumasa Kondo, María Cristina García Muñoz, Jairo López González, Lumey Pérez Artiles, Diana Milena Rodríguez Mora, José Mauricio Montes Rodríguez, Mónica Betancourt Vásquez, Isaura Viviana Rodríguez Torres, Juliene Andrea Barreto Rojas, Rubilma Tarazona Velásquez, Diana Mayerly Mateus Cagua, Heberth Augusto Velásquez Ramírez, Hover Beltrán López, Yeinny Carolina Pisco Ortiz, Leonardo Álvarez Ríos, Clever Gustavo Becerra Romero, Blanca Lucía Botina Azaín, Liliana Carolina Castillo Villamor, Edwin Oswaldo Rojas Barbosa, Jhon Mauricio Estupiñán Casallas, Andrea Onelia Rodríguez Roa, Nora Cristina Mesa. (2020). Modelo productivo de lima ácida Tahití (Citrus × latifolia Tanaka ex Q. Jiménez) para Colombia. https://doi.org/10.21930/agrosavia.model.7403435.
7. Neenu Augustine, Upasna Selvapandian, Thiruvengadam Venkatesan, Nagappa Srinivasa, Annabathula Mohan Rao, Benherlal Palayyan Saraswathy, Muthugounder Mohan. (2024). Evaluation of reference genes for expression studies in the broad mite, Polyphagotarsonemus latus (Acari: Tarsonemidae). Applied Entomology and Zoology, 59(1), p.31. https://doi.org/10.1007/s13355-023-00848-3.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Acta Agronómica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Política sobre Derechos de autor:Los autores que publican en la revista se acogen al código de licencia creative commons 4.0 de atribución, no comercial, sin derivados.
Es decir, que aún siendo la Revista Acta Agronómica de acceso libre, los usuarios pueden descargar la información contenida en ella, pero deben darle atribución o reconocimiento de propiedad intelectual, deben usarlo tal como está, sin derivación alguna y no debe ser usado con fines comerciales.