Ex situ potential conservation of ipecacuanha (Psychotria ipecacuanha (Brot.) Stokes.), a critically endangered medicinal plant species
DOI:
https://doi.org/10.15446/acag.v66n4.60080Palabras clave:
in vitro conservation, plant limited growth, osmotic regulator, recovery phase, vigour. (en)in vitro conservation, plant limited growth, osmotic regulator, recovery phase, vigour (es)
Descargas
Psychotria ipecacuanha is a medicinal plant endangered in Colombia, due to the indiscriminate exploitation suffered several decades ago for its therapeutic importance. Its great medicinal value is due to emetine production and its derivatives, mainly in the roots. Given these concerns, the aim of this research was to evaluate different parameters for in vitro conservation of P. ipecacuanha by a limited plant growth, using as in vitro explants plant nodes. Plant growth response to 18, 23 and 25°C with and without addition to MS basal culture medium 15 and 30 gL-1 of mannitol osmotic regulator was evaluated. Registration of plant survival data (%), plant height (cm), plant development of axillary shoots, number of leaves, callus formation and vigour of developed buds and was initially performed at 3 months and then every two months until 12 months. The explants conserved for 9 months in culture medium without mannitol osmotic regulator and temperature of 18°C, showed response after transferring and reculturing in recovery culture medium for 6-8 weeks, this process had not achieved any effect on plant growth and vigour in the recovery phase. In addition, the critical parameters in the acclimatization process were determined.
P. ipecacuanha is a medicinal plant endangered in Colombia, due to the indiscriminate exploitation suffered several decades ago for its therapeutic importance. Its great medicinal value is due to emetine production and its derivatives, approached in the roots. Given these concerns, the aim of this research was to evaluate different parameters for in vitro conservation of P. ipecacuanha due to limited plant growth, using as in vitro explants plant nodes. In fact, plant growth response to 18, 23 and 25°C with and without addition to MS basal culture medium 15 and 30 gL-1 of mannitol osmotic regulator was evaluated. Registration of plant survival data (%), plant height (cm), plant development of axillary shoots, number of leaves, callus formation and vigour of developed buds and was initially performed at 3 months and then every two months until 12 months. The explants conserved for 9 months in culture medium without mannitol osmotic regulator and temperature of 18°C, showed response after transferring and reculturing in recovery culture medium for 6-8 weeks, this process had not achieved any effect on plant growth and vigour in the recovery phase. In addition, the critical parameters in the acclimatization process were determined.
Recibido: 15 de septiembre de 2016; Aceptado: 22 de mayo de 2017
Abstract
Psychotria ipecacuanha is a medicinal plant endangered in Colombia, due to the indiscriminate exploitation suffered several decades ago for its therapeutic importance. Its great medicinal value is due to emetine production and its derivatives, mainly in the roots. Given these concerns, the aim of this research was to evaluate different parameters for in vitro conservation of P. ipecacuanha by a limited plant growth, using as in vitro explants plant nodes. Plant growth response to 18, 23 and 25°C with and without addition to MS basal culture medium 15 and 30 gL-1 of mannitol osmotic regulator was evaluated. Registration of plant survival data (%), plant height (cm), plant development of axillary shoots, number of leaves, callus formation and vigour of developed buds and was initially performed at 3 months and then every two months until 12 months. The explants conserved for 9 months in culture medium without mannitol osmotic regulator and temperature of 18°C, showed response after transferring and reculturing in recovery culture medium for 6-8 weeks, this process had not achieved any effect on plant growth and vigour in the recovery phase. In addition, the critical parameters in the acclimatization process were determined.
Keywords:
In vitro conservation, plant limited growth, osmotic regulator, recovery phase, vigour.Resumen
Psychotria ipecacuanha es una especie medicinal amenazada en Colombia, debido a la explotación indiscriminada que varias décadas atrás sufrió por su importancia terapéutica. Su gran valor medicinal se debe a la producción de emetina y sus derivados, principalmente en las raíces. Ante este panorama, el presente estudio tuvo como objetivo evaluar diferentes parámetros para la conservación in vitro de P. ipecacuanha por crecimiento limitado, usando como explantes nudos de plantas in vitro. Se evaluó la respuesta de crecimiento a 18, 23 y 25°C sin y con la adición al medio de cultivo basal MS del regulador osmótico manitol a 15 y 30 gL-1. El registro de los datos de supervivencia (%), altura (cm), desarrollo de brotes axilares, número de hojas, formación de callo y vigor de los brotes desarrollados, se realizó inicialmente a los 3 meses y luego cada dos meses hasta completar 12 meses. Una temperatura de 18°C, sin la adición del regulador osmótico manitol al medio cultivo basal MS, permitió mantener vitroplantas de esta especie hasta por nueve meses, sin verse afectado el crecimiento y vigor en la etapa de recuperación. Además se logró determinar los parámetros críticos en el proceso de aclimatización.
Palabras clave:
Conservación in vitro, crecimiento limitado, etapa de recuperación, regulador osmótico, vigorosidad.Introduction
Anthropogenic pressure, introduction of new species, as well as domesticated and invasive weeds have dramatic effects on plant diversity, which is reflected in an increasing in the number of endangered species (Cruz, González & Engelmann, 2013).
Wide varieties of plant species, including the group of medicinal plants, are in a situation of threat, mainly due to the loss of biodiversity previously mentioned. Within the medicinal species, Psychotria ipecacuanha (Brot.) Stokes (Rubiaceae), has a great importance at world level, its natural populations are distributed in countries like Nicaragua, Costa Rica, Panama, Brazil and Colombia (Ferreira, Cruz, Lima & Trindade, 2012). This species is in critical danger of extinction according to the literature reports (Oliveira, Venturini, Rossi & Hastenreiter, 2010; Souza, Martins, Santana & Oliveira, 2008). Although it has not been categorized by the International Union for Conservation of Nature (IUCN), since the beginning of the 20th century, this plant has been overexploited for its potential in traditional medicine, since it was and still is used as emetic, expectorant, amebicide, diaphoretic, among other properties (Akinboye & Bakare, 2011; Ferreira, Cruz, Lima & Trindade, 2012; Botero, Urrea & Naranjo, 2015).
Taking into consideration the above mentioned, and in order to contribute to a sustainable management of the plant genetic resource, domestication projects have been carried out in Costa Rica, Nicaragua and Panama and in Brazil conservation programs with the purpose of curbing overexploitation in natural populations (Vieira, 1999; Ocampo, 2007).
In Brazil, according to Gomes, Cavalcanti & Paulino (2009), proposed a program for conservation purposes focused on propagation and reproductive biology studies of native species as follows: agricultural incentives for growers of medicinal plants; use of different parts of the plant (such as leaves instead of bark) as a raw material for phytotherapeutic products; management and sustainable use and creation of a national certification for raw material. In this program, P. ipecacuanha is classified as one of the major medicinal plant species with potential of conservation priority. In Colombia, conservation programs involving this species have not yet been advanced. This, together with the excessive exploitation of their natural populations, makes it necessary to propose efficient propagation alternatives, reforestation, conservation and sustainable use.
It was observed that in vitro conservation, offers additional advantages to other conservation methods since the plant material can be maintained for long periods of time without problems of variability, offer a safe means for distribution and exchange, allows the maintenance of extensive collections using minimal space, cultures are not subject to environmental catastrophes and the plant material can be recovered at any time for propagation or any other purpose (Cruz, González & Engelmann, 2013).
In vitro conservation techniques are grouped into two categories as follows: 1) slow growth, where the accessions are maintained as tissues or plants on nutrient media and conditions that manage to minimize growth and development in vitro. The most widely used way of retarding plant growth is through the reduction of the culture temperature combined with a decreasing in light intensity or incubation of the culture medium under dark conditions. Other modifications include dilution of the mineral elements from the culture medium; reduction of sugar concentration; changes in the nature and/or concentration of plant growth regulators and the addition of osmotic regulators in the culture medium; 2) Cryopreservation, where the plant material is stored for long periods in liquid nitrogen, maintaining the viability and genetic stability of the plant material, which have allowed to conserve the full potential of plant function and development (Normah, Chin & Reed, 2013).
Given these concerns, in order to establish a protocol for in vitro conservation by a limited plant growth, this research aimed to evaluate the effect of different temperatures and concentrations of the osmotic regulator mannitol on the plant development from nodal segments of P. ipecacuanha.
Materials and methods
Plant material
The plant material consisted of in vitro plants obtained from apices and nodes from individuals of different populations of P. ipecacuanha sampled from Urabá- Antioquia, Colombia according to the "Agreement No.50 of access to plant genetic resources without commercial interest".
in vitro propagation
From the in vitro developed plants subcultures were performed every four weeks from apices and nodes, which were grown in the culture medium composed of the MS salts (Murashige & Skoog, 1962) to half its concentration, supplemented with sucrose (20 gL-1) and gelrite (Gellan Gum Powder, Phyto Technology Laboratories(r)) 2.8 gL-1, adjusting the pH to 5.8 before autoclaving at 121°C and 15 lb pressure for 15 min. The plant material was maintained in growth room at 23 ± 1°C and constant white light of 20 µmolm-2s-1.
Effect of temperature and mannitol on in vitro plant growth
In vitro plants in the multiplication stage were devoid of their leaves and apex, ensuring that 2 cm nodes were the explants to evaluate. The plant growth response was evaluated at 18, 23 and 25°C ± 1, respectively, with the addition to the culture medium of the osmotic regulator mannitol (Phyto Technology Laboratories, LLCTM) at 15 and 30gL-1, (Ma15 and Ma30, respectively), for nine treatments including control (Ma0). The light intensity was maintained between 20-30 μmolm-2s-1 and a photoperiod of 16/8 light/dark conditions. The sample size was 14 glass bottles (6.5 cm x 8.0 cm) per treatment and per temperature, each with 5 nodes. The experiment was performed in triplicate.
Plant survival (%), plant height (cm), number of leaves and nodes, and traits such as development of axillary shoots, rooting, vigour of newly developed shoots and presence of callus, were recorded. Data were recorded initially at 3 months and then every 2 months for a total of 12 months. For data collection, at the proposed time a total of 10 plants per treatment were randomly selected, which after recording the information were transferred into a MS basal culture medium and to standard conditions, 23 ± 1°C and submitted to a constant white light, conditions in which they remained 4 months, registering every two months the variables listed above to determine the plant recovery capacity.
Acclimatization assay
In order to evaluate the response to the hardening process of vitroplants submitted to conservation treatments, in this preliminary experiment the plant survival and ex vitro growth of vitroplants of P. ipecacuanha, were evaluated with a size greater than or equal to 3 cm in plant height, with 4 leaves on average and the presence of roots, which were planted in pots containing previously sterilized nursery soil. The greenhouse conditions were as follows: 26±2°C, HR 60-70% approximately and a level of polyshade of 50%.
Under these conditions, growth in height, number of new leaves and surviving plants out of a total of 40 selected for the trial were recorded every three days for three months.
Data analysis
A factorial design was applied where the response variables were as follows: plant height (cm), number of leaves and nodes, and factors were temperature and treatment with mannitol.
Due to the normality assumptions were not met with the Shapiro Wilk test and homogeneity of variance with the Barlett test, nonparametric statistical analyzes were performed. Statistical analysis was performed both for the conservation stage (up to 9 months) and for the recovery stage (up to 4 months). The Kruskal Wallis test was applied to compare the different treatments in both stages. In addition, Pearson correlation tests were performed between the plant height, number of leaves and nodes variables, for both stages. The tests were considered significant for an error α = 0.05. The data were processed in the statistical package R version 3.0.3 (r).
Results
In general, the response variables evaluated at 18, 23 and 25°C without and with the addition to the culture medium of the osmotic regulator mannitol at 15 and 30gL-1, presented statistical differences. Plants kept for 12 months, that survived and had achieved a very low or zero survival rate in the recovery stage, therefore, they were not taken into account for statistical analysis.
Conservation stage
At this stage, the temperature and mannitol showed interaction in the evaluated response variables. The plant survival rate was maintained between 45.4-100%, with the lowest value being recorded at 23°C for plants that remained for 9 months in storage in the medium with 30 gL-1 mannitol. At 9 months, the lowest average plant height (0.57cm) was found at 18°C in Ma30 and the highest was reached at 25°C in Ma0 (4.97cm). In fact, comparisons made for this variable showed significant differences among treatments with mannitol and the evaluated temperatures (Figure 1, A1 and A3). As for time, there were no significant differences between 5, 7 and 9 months (Figure 1, A2), which formed a group that presented differences with respect to 3 months.
For the number of leaves, the lowest mean value recorded at 9 months was 0.70 at 25°C in Ma30, the highest average number of leaves (8.20) was recorded at 23°C in the treatment without mannitol. No significant differences were found for the number of leaves between the evaluated temperatures (Figure 1, B1). The culture media Ma15 and Ma30 formed a group, which presented statistical differences with Ma0 (Figure 1, B3). However, in terms of the conservation times, there was a difference between data obtained at 5 months with respect to the other times (Figure 1, B2).
Figure 1: Average plant heigh (A), number of leaves (B) number of nodes (C) obtained in the different evaluated treatments: Temperature°C (A1, B1, C1). Time/months (A2, B2, C2) and manitol gL-1 (A3, B3, C3).
In the means recorded for the number of nodes, at 9 months the lowest average value (1.10) was found at 18°C in Ma30 and the highest (4.30) at 23°C in the Ma0 treatment. For this variable, statistical differences were presented for both factors, temperatures 23 and 25°C formed a group that presented statistical differences with the temperature of 18°C (Figure 1, C1). Among the mannitol treatments, there were significant differences (Figure 1, C3). With regard to time, it was found that 5, 7 and 9 months formed a statistically different group with respect to 3 months (Figure 1, C2).
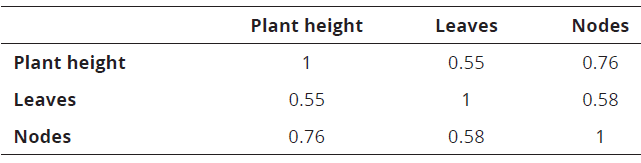
In the correlation test between the three response variables, a linear association was found between the plant height of the newly developed shoots with the number of leaves and nodes (Table 1).
Table 1: Correlation between the different variables evaluated in vitro conservation stage
Plant recovery stage
At this stage, temperature and mannitol showed interaction for the response variables, number of nodes and leaves but not for plant height with p values of 0.0435, 0.0042 and 0.1392, respectively. The percentage of plant survival including all treatments ranged from 0 to 100%, with the lowest value being recorded at 18°C, at 2 months in the recovery medium and only for plants that remained for 7 months in Ma15 conservation.
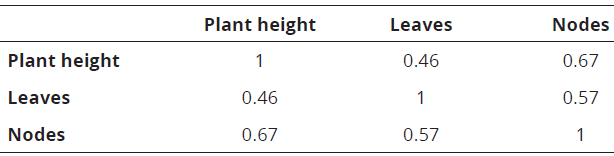
Regarding the different evaluated treatments, the highest average value for plant height in the recovery stage was reached at 4 months (3.74 cm), in plants, which were only 3 months in Ma0 conservation at 18 ° C. For 23 and 25°C, the highest values of this variable were obtained after 2 months of recovery, after having been in storage for 7 months. The treatments with mannitol showed significant differences for plant height, number of leaves and number of nodes, reaching the highest value in Ma0. In the correlation test among the evaluated variables, a linear association was found between the plant height and the newly developed shoots with the number of leaves and nodes (Table 2).
Table 2: Correlation between the different evaluated variables in the recovery stage
Morphological traits
A morphological trait of the developed plants from the submitted nodes to different temperature treatments and the mannitol osmotic regulator, were differentiated in terms of plant height, leaf size and coloration (Figure 2). Plants maintained at 18°C with and without mannitol during the conservation stage presented a pale green coloration with chlorotic zones, which became more acute with time. The recovery of these plants under standard conditions took at least 4 months to reach the vigour of the control plants (23°C without mannitol), while those that remained at 23 and 25°C, which had achieved this response variable within 2 months of being transferred into the recovery culture medium. On the other hand, was found an excessive leaf growth and root formation in plants maintained at 25°C without mannitol.
Figure 2: Morphological traits of plants under different conservation treatments for 7 months
In the plant materials conserved at 23 and 25°C, callus formation was found around the nodal segments and abundant roots, mainly at 25°C, at this temperature, from 5 months, the vegetative apices and the ends of the leaves starts to become necrotic.
The percentage of plant survival began to decrease more drastically at 9 months of conservation stage at all evaluated temperatures, mainly in the treatment without mannitol (Ma0) and at 25° C, due to nutrients depletion in the culture medium, resulting in a rapid increasing in the foliar mass and consequently, its subsequent senescence. Before that time, 91.8% of the plants transferred to recovery culture medium managed to survive and differences were noted in plant height, number of nodes and leaves (Figure 3).
Figure 3: Morphological traits of the plants in the recovery stage after 2 months
Acclimatization assay
During the first 4 weeks of acclimatization, plants still protected with plastic cups drilled to facilitate gas exchange and have allowed a gradual decreasing in the relative humidity, plant growth and newly developed leaves. After this time, they were deprived of the coverage, finding that they were able to stay turgid, which reflects the water absorption capacity of the substrate and regulation of the transpiration process. However, the fungi presence, insects and the necrosis of the apices and edges of the leaves, which finally reduced the population to 50%, which was evidenced. Under this situation, plants with a size smaller than 5 cm were unable to recover or maintain, only those with larger sizes and presenting internodes could be maintained during the test period.
The average plant survival rate at the end of the assay was 30% with an average plant growth of 1.1 cm in plant height without the newly developed shoots and in some plants, the development of 1-2 new leaves (Figure 4a).
Figure 4: Plant traits in the hardening process. A. plants in development with newly developed leaves. B. Apical necrosis.
It is important to highlight the high susceptibility of these plants to the attack of fungi and insects (mainly mites) and therefore the need to carry out phytosanitary controls, which were not contemplated in this test. In the case of apical necrosis (Figure 4b), it was found to be favored with a high relative humidity (greater than 70%), which increases the susceptibility to the biotic agents indicated above, which coincides with the behavior observed in the mother plants maintained in semicontrolled conditions in greenhouse.
This is a preliminary stage of the acclimatization process for P. ipecacuanha with a view to improving the hardening percentages in subsequent trials in which different types of substrate are considered, among others, the supplementation of these with mycorrhizas and/or PGPB-plant growth promoting bacteria, as well as other environmental conditions.
Discussion
Among the criteria to be considered when deciding the validity of a methodology for in vitro conservation is the plant survival and an adequate plant growth rate, as well as the recovery ability and their vigour after conservation time. i.e, that under standard plant growth conditions, the plant material recovery is appropriate.
The growth rate and plant development is dependent on the surrounding temperature and each species has a temperature range represented by a minimum, a maximum and an optimum (Hatfield & Prueger, 2015). In this research, the effect of temperature on the limitation or non-growth of P. ipecacuanha vitroplants was evident. The evaluated plant growth parameters showed that at 18°C, the plant growth is limited, which have allowed the plant material to be preserved for up to 9 months, according to the evaluation time performed in this research. Similar to this result, in Coffea arabica L., belonging to the same family of P. ipecacuanha, Rubiaceae, obtained the best results in the treatment at 20°C in a culture medium supplemented with only sucrose (20 gL-1) and reported a plant survival rate greater than 90% after a period of 8 months in conservation stage (Bertrand, Noirot & Charrier, 1992). These authors point out, as a general rule, that the minimum storage temperature of a plant depends on the geographical conditions under which the species grows. Furthermore, Silva, Gomes & Scherwinski (2012), suggest that the storage temperature should be between 4 and 10°C for those cultures that normally grow at 20-25 ° C and between 15 and 20°C for those growing at 30°C. In the conservation of papaya (Carica papaya L.) apices, which grows in natural conditions at temperatures of approximately 30°C, Suksa, Kataoka, Fujime & Subhadrabandhu (1997), achieved high survival rates after 12 months of culture at 16°C.
For P. ipecacuanha specifically, Chaudhuri & Jha (2008), describe the maintenance of vitroplants under reduced growth conditions at a temperature of 22 ± 2°C for more than 12 years, also using kinetin (0.25 mgL-1) in the culture medium during the duration of the study. According to these authors, these plants did not present genetic variation in the number of chromosomes. Taking into account the use of regulators, the number of subcultures and the time they remained in vitro conditions, this study is not a reference to take into account since it is not possible to assure genetic stability in plants, which is an indispensable requirement in a conservation program.
Regarding the use of osmotic regulators, Rayas, Cabrera, Santos, Basail, López, Medero & Beovides (2013), stated that minimal growth of in vitro cultures can be achieved by changes in the osmotic potential of the cells in the culture medium. This potential of the culture medium has a direct effect on the explants, as it is more negative, the less the water absorption and the pressure of cellular turgor necessary for the cell division and as a consequence there will be a low availability of nutrients, thus affecting the increase. In this work, although mannitol in the conservation stage benefited the minimum growth of plants at all temperatures, in the recovery stage, the percentage of mortality increased specifically in those that remained for more than 5 months in treatments with mannitol.
Mannitol has been reported as an osmotic agent that promotes stress in in vitro explants, which leads to limiting plant growth. This sugar alcohol is not metabolized by plant tissues and generally cannot be used as a carbon source (Hassan, Stino, Gomaa & Al-Mousa, 2014).
On the contrary, in Uncaria tomentosa (Willd. ex Schult.) DC., also belonging to the Rubiaceae family, it was found that mannitol at 4 and 6% in the in vitro preservation medium, causes oxidation and non-survival of most explants before a period of 4 months, (Cruz, González & Engelmann, 2013). Similarly, Lemos, Ferreira, Calheiros, Ramalho & Alburquerque (2002), found in sugarcane that mannitol also had harmful effects during the storage period.
In some plant species, the use of growth regulators in conservation media has been evaluated. However, established protocols have often resulted in changes in gene expression, in vitro physiological disorders, and abnormal plant growth. Therefore, the relevance of using them should be evaluated. Bertrand, Noirot & Charrier (1991), described the use of BAP in different concentrations (0, 1.3 and 4.4 μM) in coffee, finding for the 4 evaluated species this regulator to 1.3 μM, ensured minimum plant growth for 6 months and preserved according to the authors, their traits stability.
It is important to highlight in the present study that at 7 months of conservation, although the two evaluated parameters, temperature and osmotic regulator, contributed to retard the plant growth, the plant recovery was affected to a greater extent with the use of mannitol at 18°C of temperature. However, the plants that remained for 9 months in conservation, only in those maintained at 18°C and in mannitol free medium, the plant recovery percentage was 100%. The longer time required for the plant recovery conserved at 18°C was to be expected, considering that the natural plant growth conditions of P. ipecacuanha ranged from 23 and 28°C, which is typical of the tropical humid forest.
According to the results obtained in this research, another important alternative for this species would be to preserve for 7 months only, at a temperature of 23°C and to use in the culture medium mannitol 15 or 30 gL-1. However, the formation of nodular callus and abundant roots must be considered since it can later lead to deformations or regeneration of adventitious buds, which are not desirable in this case.
It should be emphasized that this is the first conservation work carried out in Colombia for P. ipecacuanha and is the starting point for future studies on long term conservation, using cryopreservation.
Conclusion
From the results obtained in the present research, the proposed in vitro conservation protocol for P. ipecacuanha is storage at 18°C in the culture medium without the addition of mannitol for 9 months. Given these concerns, the average plant growth rate is 1.3cm, there is no root formation and in the plant recovery stage, the plants had achieved a survival rate of 100% and a multiplication rate of 1.9. With a view to achieving times greater than one year, it is proposed to evaluate the reduction of the amount of salts in the medium, growth inhibiting substances, among other factors involved in limited plant growth as a conservation measurement.
Acknowledgments
To Colciencias and CODI for financial support for the development of the project, to Universidad de Antioquia-UdeA, Antioquia, Colombia and to the Biotechnology Research Group of the same university.
References
Referencias
Akinboye, S. & Bakare, O. (2011). Biological activities of emetine. Open Nat Prod J, 4, 8-15. https://doi.org/10.2174/1874848101104010008
Bertrand, D. A., Noirot, M. & Charrier, A. (1991). Minimal growth in vitro conservation of coffee (Coffea spp.). 1. Influence of low concentrations of 6-benzyladenine. Plant Cell Tiss Org, 27, 333-339. https://doi.org/10.1007/BF00157599
Bertrand, D.A., Noirot, M. & Charrier, A. (1992). Slow growth in vitro conservation of coffee (Coffea spp.). 2: Influences of reduced concentrations of sucrose and low temperature. Plant Cell Tiss Org, 31, 105-110. https://doi.org/10.1007/BF00037693
Botero, C., Urrea, A., Naranjo, E. (2015). Potencial de regeneración de Psychotria ipecacuanha (Rubiaceae) a partir de capas delgadas de células. Acta Biol Col, 20 (3), 181-192. http://dx.doi.org/10.15446/abc.v20n3.47354
Chaudhuri, K. & Jha, B. (2008). Conservation and production of Ipecac (Cephaelis ipecacuanha Rich.) plants from long term shoot cultures. Plant Tiss Biotech, 18 (2), 157-164. http://dx.doi.org/10.3329/ptcb.v18i2.3646
Cruz, C.A., González, A.T. & Engelmann, F. (2013). Biotechnology and conservation of plant biodiversity. Resources, 2, 73-95. http://dx.doi.org/10.3390/resources2020073
Ferreira, J.W.S., Cruz, M.P., Lima, d.S.L. & Trindade, M.M.F. (2012). Use and importance of quina (Cinchona spp.) and ipeca (Carapichea ipecacuanha (Brot. L. Anderson): Plants for medicinal use from the 16th century to the present. J Herbal Med, 2 (4), 103-112. http://dx.doi.org/10.1016/j.hermed.2012.07.003
Gomes, d.M.J., Cavalcanti, d.A.E. & Paulino, d.A.U. (2009). Native medicinal plants commercialized in Brazil, priorities for conservation. Environ Monit Assess, 156, 567-580. http://dx.doi.org/10.1007/s10661-008-0506-0
Hassan, N., Stino, G., Gomaa, H. & Al-Mousa, R. (2014). In vitro medium-term germplasm conservation and genetic stability of grape (Vitis vinifera L.). J Hortic Sci Ornamental Pl, 6 (1), 09-17. http://dx.doi.org/10.5829/idosi.jhsop.2014.6.1.1133
Hatfield, J. & Prueger, J. (2015). Temperature extremes: Effect on plant growth and development. Weather and Climate Extremes, 10, 4-10. http://dx.doi.org/10.1016/j.wace.2015.08.001
Lemos, E., Ferreira, M., Calheiros, L., Ramalho, C. & Alburquerque, M. (2002). Conservación in vitro de germoplasma de caña de azúcar. Pesq Agropec Bras, 37 (10), 1359-1364. http://dx.doi.org/10.1590/S0100-204X2002001000002
Murashige, T. & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol, 15, 473-497. http://dx.doi.org/10.1111/j.1399-3054
Normah, M., Chin, H. & Reed, B. (2013). Conservation of Tropical Plant Species. Heidelberg Springer (Eds.). New York, USA. p. 538.
Ocampo, R. (2007). Ipecacuanha, un producto no maderable cultivado bajo el bosque en Costa Rica. Agronomía Costarricense, 31 (1), 113-119. https://revistas.ucr.ac.cr/index.php/agrocost/article/view/6826/6513.
Oliveira, L., Venturini, B., Rossi, A., Hastenreiter, S. (2010). Clonal diversity and conservation genetics of the medicinal plant Carapichea ipecacuanha (Rubiaceae). Genet Mol Biol, 33, 86–93. http://dx.doi.org/10.1590/S1415-47572009005000096
Rayas, A., Cabrera, M., Santos, A., Basail, M., López, J., Medero, V. & Beovides Y. (2013). Efecto del manitol y el nitrato de plata en la conservación in vitro de la malanga (Xanthosoma spp.). Rev Colomb Biotecnol, 15(1), 167-171. http://revistas.unal.edu.co/index.php/biotecnologia/article/view/28620/41781.
Silva, R.C., Gomes, L.Z. & Scherwinski, P.J.E. (2012). Short-term storage in vitro and large-scale propagation of grapevine genotypes. Pesq Agropec Bras, Brasília, 47 (3), 344-350. http://dx.doi.org/10.1590/S0100-204X2012000300005
Souza, M., Martins, E., Santana, T. & Oliveira, L. (2008). Reproductive studies in Ipecac (Psychotria ipecacuanha (Brot.) Stockes; Rubiaceae): Pollen development and morphology. Braz Arch Biol Techn, 51(5), 981-989. http://dx.doi.org/10.1590/S1516-89132008000500015
Suksa, P., Kataoka, I., Fujime, Y. & Subhadrabandhu, S. (1997). Effect of temperature, growth retardants and osmotic potential on growth of Papaya shoots conserved in vitro. Jpn Trop Agr, 41(1), 7-13. http://dx.doi.org/10.11248/jsta1957.41.7
Vieira, R. (1999). Conservation of medicinal and aromatic plants in Brazil. In: J. Janick (Eds.), Perspectives on new crops and new uses. ASHS Press, Alexandria, VA. pp. 152–159. http://dx.doi.org/10.1.1.408.4779
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Arne Sinnesael, Olivier Leroux, Steven B. Janssens, Erik Smets, Bart Panis, Brecht Verstraete, Peter Mergaert. (2019). Is the bacterial leaf nodule symbiosis obligate for Psychotria umbellata? The development of a Burkholderia-free host plant. PLOS ONE, 14(7), p.e0219863. https://doi.org/10.1371/journal.pone.0219863.
2. Rosa Armijos-González, Pablo Ramón, Augusta Cueva-Agila. (2024). Cinchona officinalis L. ex situ conservation by in vitro slow growth and cryopreservation techniques. Plant Cell, Tissue and Organ Culture (PCTOC), 158(1) https://doi.org/10.1007/s11240-024-02784-8.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Acta Agronómica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Política sobre Derechos de autor:Los autores que publican en la revista se acogen al código de licencia creative commons 4.0 de atribución, no comercial, sin derivados.
Es decir, que aún siendo la Revista Acta Agronómica de acceso libre, los usuarios pueden descargar la información contenida en ella, pero deben darle atribución o reconocimiento de propiedad intelectual, deben usarlo tal como está, sin derivación alguna y no debe ser usado con fines comerciales.































