Asymbiotic seed germination and in vitro propagation of Cattleya trianae Linden & Reichb.f. (Orchidaceae)
Germinación asimbiótica de semillas y propagación in vitro de Cattleya trianae Linden y Reichb.f. (Orchidaceae)
DOI:
https://doi.org/10.15446/acag.v66n4.63597Palabras clave:
Organic additives, coconut water, Pineapple juice, in vitro propagation (en)Organic additives, coconut water, Pineapple juice, in vitro propagation (es)
Descargas
Cattleya trianae (Linden & Reichb.f., 1860), Colombian national flower, is in danger of extinction due to the destruction of its natural habitats and excessive collection for horticultural purposes. Therefore, in vitro culture is a tool for the conservation of threatened species. In this study we determined the most suitable culture medium for asimbytic seed germination and in vitro propagation of C. trianae. Initially, mature capsules were collected, the seeds were subsequently disinfected and seeded with the syringe method (Vendrame et al., 2007), to evaluate the effect of five media on the development of C. trianae after 20 weeks. The seedlings were transplanted and acclimated using different substrates. The best percentage (54.2%) of seedling formation after 20 weeks was found in MS + JP medium with significant differences (P <0.05: Tukey HSD). In this research, it is reported that the addition of organic additives to the MS medium improves the efficacy of this, and therefore, allows a greater growth and development of C. trianae under in vitro conditions.
Recibido: 27 de marzo de 2017; Aceptado: 29 de mayo de 2017
Abstract
Cattleya trianae (Linden & Reichb.f., 1860), Colombian national flower, is in danger of extinction due to the destruction of its natural habitats and excessive collection for horticultural purposes. Therefore, in vitro culture is a tool for the conservation of threatened species. In this study we determined the most suitable culture medium for asimbytic seed germination and in vitro propagation of C. trianae. Initially, mature capsules were collected, the seeds were subsequently disinfected and seeded with the syringe method (Vendrame et al., 2007), to evaluate the effect of five media on the development of C. trianae after 20 weeks. The seedlings were transplanted and acclimated using different substrates. The best percentage (54.2%) of seedling formation after 20 weeks was found in MS + JP medium with significant differences (P <0.05: Tukey HSD). In this research, it is reported that the addition of organic additives to the MS medium improves the efficacy of this, and therefore, allows a greater growth and development of C. trianae under in vitro conditions.
Key words:
Organic additives, coconut water, Pineapple juice, in vitro propagation.Resumen
Cattleya trianae (Linden y Reichb.f., 1860) flor nacional de Colombia, se encuentra en peligro de extinción, debido a la destrucción de sus hábitats naturales y a la recolección excesiva con fines comerciales y hortícolas. Por lo tanto, el cultivo in vitro es una herramienta para la conservación de especies amenazadas. En este estudio se determinó el medio de cultivo más adecuado para la germinación asimbiótica de semillas y propagación in vitro de C. trianae. Inicialmente, se colectaron cápsulas maduras, posteriormente, las semillas se desinfectaron y se sembraron con el método de la jeringuilla (Vendrame et al., 2007), para evaluar el efecto de cinco medios en el desarrollo de C. trianae después de 20 semanas. Las plántulas se trasplantaron y aclimataron utilizando diferentes sustratos (corteza de pino triturada, arcilla expandida, carbón vegetal y fibra de coco). El mejor porcentaje (54,2%) de formación de plántulas después de 20 semanas se encontró en el medio de cultivo MS+JP con diferencias significativas (P<0.05: Tukey HSD). En esta investigación se reporta que la adición de aditivos orgánicos al medio MS mejora la eficacia de éste, y por lo tanto, permite un mayor crecimiento y desarrollo de C. trianae bajo condiciones in vitro.
Palabras claves:
Aditivos orgánicos, Agua de coco, Jugo de piña, Propagación in vitro.Introduction
Orchidaceae family is one of the most diverse groups and with a high risk of extinction of the Plantae kingdom (Salazar & Gélvez, 2015; Zhang, Yan, Tian, Li & He, 2015), comprises about 25000 species integrated in 880 genera. In Colombia, there are approximately 4010 species and 260 genera (Mejía & Pino, 2010). Cattleya trianae is classified as the national flower, according to the concept issued by the Colombian Academy of History in 1936. It is endemic to Colombia, grows epiphyte or litophyte, usually on tall caracolí (Anacardium excelsum (Bertero ex Kunth) Skeels) trees, walnut (Cordia alliodora (Ruiz & Pav.) Oken), hobo (Spondias mombin L.), ceiba (Ceiba pentandra (L.) Gaertn.) and purple oak (Tabebuia rosea (Bertol.) Bertero ex A.DC.).
It is known that is found in the Colombian departments of Cundinamarca, Tolima and Huila at an altitude of 1000 to 1800 m.a.s.l. (Calderón, 2007). The plant has large flowers with great ornamental and economic value (Galdiano et al., 2012). C. trianae is in the global category of the International Union for Conservation of Nature (IUCN) putting this species at risk of becoming endangered according to the red book of Colombian plants (Calderón, 2007). Due mainly to a quality habitat deterioration, therefore, many plants of this species have been illegally collected from wild populations, which can be translated into species damaging for more than 150 years and have allowed a decreasing of some subpopulations in their natural environment. In addition, orchid seeds needs special conditions to have achieved germination in their habitat, which leads to a symbiotic relationship with a mycorrhizal fungus (Chen, Goodale, Fan & Gao, 2015).
in vitro propagation is a technique that results in an important alternative for the conservation of endangered orchid species (Bhattacharyya, 2017; Chen, Goodale, Fan & Gao, 2015; Salazar, 2012). Several authors have achieved asimbiotic germination using different simple means with minerals, vitamins, hormones and sugars (Kauth et al., 2011; Arditti & Ghani, 2000; Knudson, 1946). Likewise, cultures have been improved supplementing the media with organic components (Gallo et al., 2016; Salazar et al., 2013; Salazar & Cancino, 2012). The present research aims to determine the most suitable culture medium for asymbiotic seed germination and in vitro propagation of C. trianae for the species conservation.
Materials and methods
Plant material
Mature capsules of C. trianae were collected 8 months after being manually pollinated under greenhouse conditions in the municipality of Bochamela, Norte de Santander, Colombia (Figures 1A, B, C).
To identify mature capsules, was observed the transition from green to yellow. Subsequently, the mature seeds were extracted from capsules, stored for 5 days in kraft paper sachets, and in glass vials, preserved with a desiccating agent containing silica gel (to avoid excessive moisture) at temperature of 4°C (Salazar, 2012).
Figure 1: in vitro propagation procedure of Cattleya trianae. (A) C. trianae inflorescence. (B) Green capsule of C. trianae. (C) Mature capsule of C. trianae. (D) Protocorm. (E), (F) Adapted seedlings. (G) Development of C. trianae.
Sterilization and seed sowing
The syringe method described by Vendrame et al. (2007), was used for seeds disinfection and planting. A small portion of seeds was placed in a 5 ml sterile syringe with a cloth filter. In fact, seeds were immersed for 30 seconds in 70% ethanol, followed by immersion in 0.75% (v/v) in sodium hypochlorite solution (NaOCl) at 1.0 % plus 0.1% Tween 20(r) (surfactant), for 5 minutes under constant rinsing. Subsequently, five washes were performed with sterile deionized water; the filter was withdrawn from the syringe to perform the seeding in a laminar flow chamber. Therefore, 100 seeds per species were grown in petri dishes, containing 25 mL of culture medium.
Media and culture conditions
The basal culture medium was MS using 100% macro and micronutrient concentrations with 3000 mg.L-1 Saccharose, 700 mg.L-1 agar, 100 mg.L-1 Myo-inositol and 1000 mg.L-1 activated carbon. Five basal media were tested in this study: basal MS media as control, MS supplemented with pineapple juice (MS + JP), MS with coconut water (MS + AC), MS plus 0.5 mg.L-1 indoleacetic acid (AIA) and MS with 0.5 mg.L-1 gibberellic acid (MS + GA3). Media with organic supplements were prepared by adding 200 mL.L-1 (20%) of coconut water and pineapple juice, respectively. With a pH of 6.0, it was sterilized at 15 pounds of pressure (Psi) at 121°C for 20 minutes. The culture media were incubated under controlled environmental conditions (23 ± 2°C, photoperiod 16 hours light and 8 hours darkness, with a light intensity of 25-μmol m-2s-1, given by fluorescent light and 60% relative humidity).
Transplanting and acclimatization
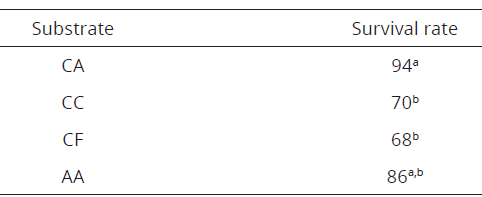
Six months later, seedlings of in vitro culture were taken, with roots and more than two leaves (seedlings more than 3 cm in length), then the seedlings were transplanted in polystyrene containers with a pine bark substrate (1: 1), bark substrate with carbon (CC; 1: 1), pine bark and coconut fiber (CF; 1: 1) and single expanded clay (AA). Therefore, seedlings were placed in transparent plastic boxes to perform the acclimatization process, gradually decreasing relative humidity for 30 days after transplanting, under greenhouse conditions at a temperature of 25 ± 2ºC. The survival percentage in acclimatization for three months after transplanting was recorded.
Experimental design and statistical analysis
A completely randomized 6 x 5 factorial analysis (six developmental stages and five culture media) was performed with 5 replicates (each with 100 seeds). All data were statistically analyzed by analysis of variance (ANOVA). Therefore, Means were compared by Tukey's HSD (Honest Significant Difference) test to determine significant differences at a level of P<0.05.
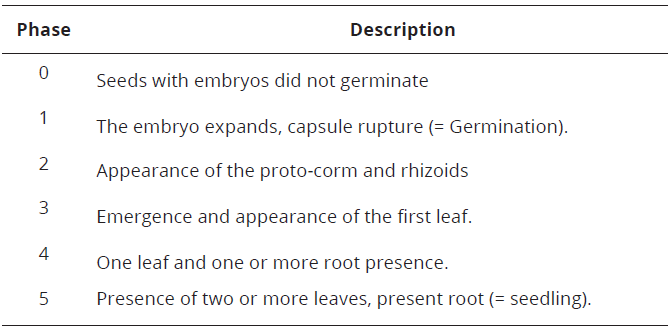
Seed germination process to seedling formation was evaluated at 20 weeks of planting, through the development stages of orchids adapted by Johnson & Kane, 2007 (Table 1). Statgraphic Centurion(r) software version 16 was used for the statistical analysis.
Source: Adapted from: Johnson & Kane (2007).Table 1: Development phases of orchid seeds
The survival percentage in acclimatization for three months after transplantation was recorded with a completely randomized experimental design, with five replicates and 10 seedlings per substrate.
Results
The germination process started when the expansion and capsule (15 days) rupture were visualized, with an average of 95% (phase 1; Tables 1, 2). The highest percentage was found in MS + GA3 culture medium, with no significant differences (P<0.05: Tukey HSD; Table 2).
Table 2: Effect of the culture medium on the germination percentage and protocorms formation of C. trianae
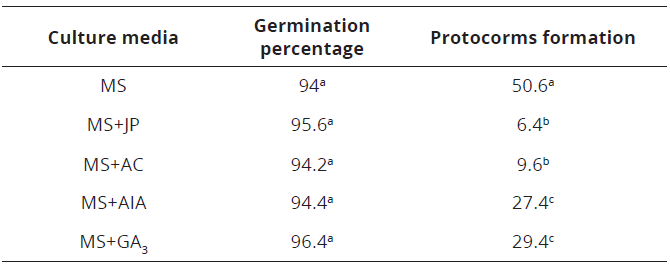
In phase two, the highest protocorms percentage was found in the MS culture medium (Tables 2,3; Figure 1D).
Table 3: Effect of the culture medium on germination and seedling formation of C. trianae culture at 20 weeks.
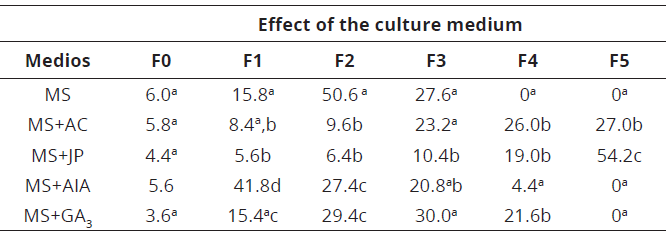
The means values with different letter of each column indicate statistically significant differences, according to Tukey HSD test (P≤0.05).
In the appearance and development of the first leaf (phase 3), the MS + JP culture medium (10.4%; Table 3) was observed with a lower percentage. Therefore, means which had the presence of the initial root (phase 4), were higher to lower percentage MS + AC, MS + GA3, MS + JP, MS + AIA, respectively. In contrast, the MS culture medium, which had no roots present (Table 3). In the seedlings formation (phase 5), pineapple medium was found to be more efficient with significant differences (P <0.05: Tukey HSD; 54.2%) in seedling development and growth of C. trianae (Table 3; Figure 1G), followed by the MS + AC culture medium, which generated a percentage of 27% (Table 3). It is important to note that after the final evaluation of C. trianae seeds, the culture media MS, MS + AIA and MS + GA3, did not form seedlings at 20 weeks (Table 3).
In transplanting and acclimatization of C. trianae, which was in vitro seedlings propagated, the most suitable substrate for plant adaptation (Figures 1E, F) was determined, which had achieved 94% of survival percentage in the crushed pine bark substrate and expanded clay (CA, Table 4). Additionally, the AA substrate with 86% efficacy and with less effectiveness in the CF substrate with 68%.
Tabla 4: Supervivencia de C. trianae en diferentes sustratos después de 12 semanas de haber sido trasplantadas.
Discussion
Orchid species have different needs for mineral nutrients for germination and seedling development. For this reason, the most suitable in vitro culture medium for each of them is studied. Arditti & Ghani (2000), consider phase 1 as the first stage in the germination process in orchid seeds. Similar research with Cattleya mendelli reported a high degree of germination of 95.7% in the MS + AC culture medium (Salazar, 2012). However, germination varies considerably, and in many species, have a decreasing in storage longevity (Vendrame et al., 2007). In addition, can be inferred that germination is influenced not only by the culture medium, but by seeds maturity and quality. Studies carried out by Salazar & Cancino (2012), found similarities in protocorms formation, where the highest percentage was found in the MS culture medium in this orchid species: Prosthechea vespa (Vell.) W.E.Higgins and Sobralia klotzscheana Rchb.f. In this research, a beneficial effect of using organic additives to the culture medium is demonstrated for seedlings germination, formation and development, respectively. This is in concordance with other authors, where the effectiveness of the organic components (pineapple juice and coconut water) have allowed a verification in the in vitro culture of a great variety of orchid species. (Gallo et al., 2016; Salazar et al., 2013). Nonetheless, Pedroza (2009), found that the addition of AIA (0.5 mg.L-1) to the MS culture medium, promoted the development phases of Epidendrum elongatum. In the same way, Coello et al. (2010), highlights the importance of the GA3 hormone in orchids germination and growth. However, it should be noted that despite the difficulty in analyzing its composition and the lack of information in the literature about the effects of organic components, such as coconut water and pineapple juice, these are rich in energy, vitamins, amino acids and phytohormones. Recent studies have shown that coconut water in the culture medium significantly stimulates the formation of roots and shoots in Dendrobium lasianthera J.J.Sm. orchid species (Wida et al., 2017). However, in this research the most efficient culture medium was that supplemented with pineapple juice.
In transplanting and acclimatization, the seedlings grew vigorously with a high survival degree, which demonstrates the importance of using suitable substrates, which had achieved a good water retention and humidity. Santos et al. (2016), obtained a high survival percentage using vermiculite as substrate. Conversely, is convenient to use substrates of easy availability and of low costs to optimize the production.
Conclusions
This study demonstrated that the addition of organic additives such as coconut water and pineapple juice to in vitro culture medium optimizes the growth and development of C. trianae, orchid species, which could be applied to mass-scale propagation as well as ex situ species conservation of endangered orchids in their natural habitats. Given these concerns, the use of organic supplements could be an effective alternative for reducing the high costs generated by the use of plant hormones in the culture medium.
Acknowledgments
To Universidad Francisco de Paula Santander for their valuable collaboration
References
Referencias
Arditti, J. & Ghani, A. (2000). Numerical and physical properties of orchid seeds and their biological implications. New Physiologist, 145(3), 367-421. http://www.jstor.org/stable/2588806.
Bhattacharyya, P., Kumar, V. & Staden, J. (2017). Assessment of genetic stability amongst micropropagated Ansellia africana, a vulnerable medicinal orchid species of Africa using SCoT markers. S Afr J Bot, 108, 294–302. https://doi.org/10.1016/j.sajb.2016.11.007
Calderón, E. (2007). Libro Rojo de plantas de Colombia. Vol. 6. Orquídeas primera parte, Instituto Alexander von Humboldt – Ministerio de Ambiente, Vivienda y Desarrollo Territorial. Calderòn, S.E. (Eds.). Bogotá, Colombia. 832p. http://www.humboldt.org.co/es/test/item/280-libro-rojo-de-plantas-de-colombia-vol-6-orquideas-primera-parte-serie-libros-rojos-de-especies-amenazadas.
Chen, Y., Goodale, U., Fan, X. & Gao, J. (2015). Asymbiotic seed germination and in vitro seedling development of Paphiopedilum spicerianum: An orchid with an extremely small population in China. Global Ecol Conserv, 3, 367–378. https://doi.org/10.1016/j.gecco.2015.01.002
Coello, C., Miceli, C., Orantes, C., Dendooven, L. & Gutiérrez, A. (2010). Plant growth regulators optimization for in vitro cultivation of the orchid Guarianthe kinneri (Bateman) Dressier & W.E. Higgins. Gayana Bot, 67(1), 19-26. http://dx.doi.org/10.4067/S0717-66432010000100003
Galdiano, R., Mantovani, C. & Gertrudes E. (2012). Propagação in vitro de Cattleya trianaei (Linden & Reichenbach fil.) (Orchidaceae) em meios de culturas e com doses de fertilizante comercial. Comunicata Scientiae, 3(3), 210-214. https://repositorio.unesp.br/handle/11449/73601.
Gallo, F., Souza, L., Milaneze, M. & Almeida, O. (2016). Seed structure and in vitro seedling development of certain Laeliinae species (Orchidaceae). Revista Mexicana de Biodiversidad, 87(1), 68–73. https://doi.org/10.1016/j.rmb.2016.01.005
Johnson, T. & Kane, M. (2007). Asymbiotic germination of ornamental Vanda: in vitro germination and development of three hybrids. Plant Cell Tiss Org, 91(3), 251–261. https://doi.org/10.1007/s11240-007-9291-7
Kauth, P., Kane, M. & Vendrame, W. (2011). Comparative in vitro germination ecology of Calopogon tuberosus var. tuberosus (Orchidaceae) across its geographic range. In Vitro Cell Dev Biol – Plant, 47(1), 148–
https://doi.org/10.1007/s11627-010-9316-5
Knudson, L. (1946). A nutrient for germination of orchid seeds. American Orchid Society Bulletin, 15, 214–217.
Mejía, H. & Pino, T. (2010). Diversity of orchids epiphytes in a tropical rain forest (Bh-T) of Departament Chocó, Colombia. Acta biol Colomb, 15(2), 37-46. http://www.scielo.org.co/pdf/abc/v15n2/v15n2a3.pdf.
Pedroza, J. (2009). The effect of activated charcoal, indol acetic acid (IAA) and benzyl amino purine (BAP) on Epidendrum elongatum Jacq protocorm-like body (PLB) development in vitro conditions. Rev colomb biotecnol, 6(1), 17-32. http://www.scielo.org.co/pdf/biote/v11n1/v11n1a03.pdf.
Salazar, S. (2012). Germinación asimbiótica de semillas y desarrollo in vitro de plántulas de Cattleya mendelii Dombrain (Orchidaceae). Acta Agron, 61(1), 69-78. http://www.scielo.org.co/pdf/acag/v61n1/v61n1a09.pdf.
Salazar, S. & Cancino, G. (2012). Evaluación del efecto de dos suplementos orgánicos en la germinación in vitro de orquídeas nativas de la provincia de Pamplona, Colombia. Rev colomb biotecnol, 14(1), 53-59. http://revistas.unal.edu.co/index.php/biotecnologia/article/view/31703/32917.
Salazar, S., Zulay, A. & Barrientos, F. (2013). Evaluation of different in vitro culture media in the development of Phalaenopsis hybrid (Orchidaceae). Rev colomb biotecnol, 15(2), 97-105. http://dx.doi.org/10.15446/rev.colomb.biote.v15n2.41268
Salazar, S. & Gélvez, J. (2015). Determining the viability of orchid seeds using the Tetrazolio and Carmín Índigo tests. Rev Cienc, 19, 59-69. http://www.scielo.org.co/pdf/rcien/v19n2/v19n2a04.pdf.
Santos, S., Smidt, E., Padial, A. & Fortes L. (2016). Asymbiotic seed germination and in vitro propagation of Brasiliorchis picta. Afr J Biotechnol, 15(6), 134-144. http://dx.doi.org/10.5897/AJB2015.15043
Vendrame, W., Carvalho, V. & Dias, J. (2007). In vitro germination and seedling development of cryopreserved Dendrobium hybrid mature seeds. Scientia Horticulturae, 114(3), 188–193. https://doi.org/10.1016/j.scienta.2007.06.006
Wida, E., Hariyanto, S. & Wulan, Y. (2017). In vitro propagation of the endangered medicinal orchid, Dendrobium lasianthera J.J.Sm through mature seed culture. Asian Pac J Trop Biomed, 7(5), 406-410. http://dx.doi.org/10.1016/j.apjtb.2017.01.011
Zhang, Z., Yan, Y., Tian, Y., Li, J. & He, J. (2015). Distribution and conservation of orchid species richness in China. Biol Conserv, 181, 64–72. http://dx.doi.org/10.1016/j.biocon.2014.10.026
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Quezia Rocha Navarro, Diego de Oliveira Corrêa, Alexandre Behling, Miguel Daniel Noseda, Luciana Lopes Fortes Ribas. (2023). Effect of microalga Desmodesmus subspicatus and plant growth regulators on the in vitro propagation of Cattleya warneri. Plant Cell, Tissue and Organ Culture (PCTOC), 153(1), p.77. https://doi.org/10.1007/s11240-022-02442-x.
2. Seir Antonio Salazar Mercado, Edinson Alexander Botello Delgado, Jesús David Quintero Caleño. (2020). Optimización de la prueba de tetrazolio para evaluar la viabilidad en semillas de Solanum lycopersicum L.. Ciencia & Tecnología Agropecuaria, 21(3), p.1. https://doi.org/10.21930/rcta.vol21_num3_art:1344.
3. Quezia Rocha Navarro, Diego de Oliveira Corrêa, Alexandre Behling, Miguel Daniel Noseda, Érika Amano, Rogério Mamoru Suzuki, Luciana Lopes Fortes Ribas. (2021). Efficient use of biomass and extract of the microalga Desmodesmus subspicatus (Scenedesmaceae) in asymbiotic seed germination and seedling development of the orchid Cattleya warneri. Journal of Applied Phycology, 33(4), p.2189. https://doi.org/10.1007/s10811-021-02442-y.
4. Vespasiano Borges de Paiva Neto, Mateus de Aguiar Torrezan, Manoela Aparecida Vieira da Silva, Daly Roxana Castro Padilha, Jerônimo Constantino Borel, Monica Cristina Rezende Zuffo-Borges. (2022). Self-pollination of the orchid Cycnoches haagii from Brazilian Cerrado results in albino phenotype seedlings. Ornamental Horticulture, 28(1), p.85. https://doi.org/10.1590/2447-536x.v28i1.2411.
5. Jovana Ostojić, Mirjana Ljubojević, Tijana Narandžić, Magdalena Pušić. (2022). In vitro culture conditions for asymbiotic germination and seedling development of Anacamptis pyramidalis (L.) Rich. and Gymnadenia conopsea (L.) R. Br.. South African Journal of Botany, 150, p.829. https://doi.org/10.1016/j.sajb.2022.08.036.
6. Daniela Alba-Patiño, Fabian Martínez-Hernández, Juan Francisco Mota Poveda. (2021). Determination of Sites of Special Importance for the Conservation of Threatened Orchid Species in Colombia. Mediterranean Botany, 42, p.e67589. https://doi.org/10.5209/mbot.67589.
7. Jurghen Hernando Cárdenas Guarín, Alina Katil Sigarroa Rieche, Seir Antonio Salazar Mercado. (2022). Aplicación de semillas artificiales como método de conservación in vitro de orquídeas. Revista Mutis, 12(1) https://doi.org/10.21789/22561498.1818.
8. S N Salsabila, K Fatimah, S Noorhazira, T S T A B Halimatun, M Aurifullah, Z Suhana. (2022). Effect of Coconut Water and Peptone in Micropropagation of Phalaenopsis amabilis (L.) Blume Orchid. IOP Conference Series: Earth and Environmental Science, 1102(1), p.012002. https://doi.org/10.1088/1755-1315/1102/1/012002.
9. Seir Antonio Salazar Mercado, Jesús David Quintero Caleño, Laura Yolima Moreno Rozo. (2020). Improvement of the methodology of the tetrazolium test using different pretreatments in seeds of the genus Epidendrum (Orchidaceae). Journal of Seed Science, 42 https://doi.org/10.1590/2317-1545v42231028.
10. Seir Antonio Salazar Mercado, Hanner Alejandra Maldonado Bayona. (2020). Evaluation of the cytotoxic potential of sodium hypochlorite using meristematic root cells of Lens culinaris Med. Science of The Total Environment, 701, p.134992. https://doi.org/10.1016/j.scitotenv.2019.134992.
11. Seir Antonio Salazar-Mercado, Carlos Andrés Torres-León, Jhan Piero Rojas-Suárez. (2019). Cytotoxic evaluation of sodium hypochlorite, using Pisum sativum L as effective bioindicator. Ecotoxicology and Environmental Safety, 173, p.71. https://doi.org/10.1016/j.ecoenv.2019.02.027.
12. Seir Antonio Salazar Mercado, Jesús David Quintero Caleño, Víctor Jhoel Bustos Urbano. (2020). Implementación de la prueba de tetrazolio en las semillas de Raphanus sativus L. Revista Facultad de Ciencias Básicas, 15(2), p.7. https://doi.org/10.18359/rfcb.3831.
13. Seir Antonio Salazar Mercado, Edison Alexander Botello Delgado, Jesús David Quintero Caleño. (2020). Efecto de pretatamientos en la prueba de tetrazolio en semillas de Epidendrum barbaricum Hágsater & Dodson. Acta Agronómica, 68(4), p.306. https://doi.org/10.15446/acag.v68n4.79619.
14. José Tonatiuh Gutiérrez-Zavala, Irene Ávila-Díaz, Rosa Elia Magaña-Lemus. (2021). Desarrollo in vitro de la orquídea epífita Erycina hyalinobulbon (Orchidaceae), endémica de México, para promover su conservación. Acta Botanica Mexicana, (128) https://doi.org/10.21829/abm128.2021.1808.
15. Michele C. Nadal, Nayara M. Mota, Evandro A. Fortini, Ricardo T. de Faria, Joyce Dória, Michele V. dos Reis. (2025). Breeding of Ornamental Crops: Potted Plants and Shrubs. Advances in Plant Breeding Strategies. 7, p.3. https://doi.org/10.1007/978-3-031-80060-3_1.
16. Seir Antonio Salazar Mercado, Edison Alexander Botello Delgado. (2020). Effect of the medium composition on the asymbiotic germination and in vitro development of the Laeliocattleya hybrid. South African Journal of Botany, 135, p.80. https://doi.org/10.1016/j.sajb.2020.08.011.
17. Seir Antonio Salazar Mercado, Yordan Arley Palencia Delgado. (2025). Manejo de diferentes pretratamientos para optimizar la viabilidad de semillas de orquídeas. Acta Agronómica, 72(4), p.346. https://doi.org/10.15446/acag.v72n4.112433.
18. Edy Setiti Wida Utami, Sucipto Hariyanto. (2020). Organic Compounds: Contents and Their Role in Improving Seed Germination and Protocorm Development in Orchids. International Journal of Agronomy, 2020, p.1. https://doi.org/10.1155/2020/2795108.
19. Seir Antonio Salazar Mercado, Yuri Manuelita Osorio Jaimes. (2022). Inclusion of organic components in culture medium to improve the in vitro propagation of Cattleya warscewiczii and Cattleya gaskelliana. South African Journal of Botany, 148, p.352. https://doi.org/10.1016/j.sajb.2022.05.002.
20. Sibel Sütlüoğlu, Özlem Aksoy. (2024). Cytotoxic Effects of Disinfectants in Tap Water on Hordeum vulgare L. and Eisenia fetida Savigny. Kocaeli Journal of Science and Engineering, 7(2), p.158. https://doi.org/10.34088/kojose.1329925.
21. A. Vera-Aguilar, M.A. Ramírez-Mosqueda, H.E. Lee-Espinosa, R.C. Llarena-Hernández, M.V. Rodríguez-Deméneghi, Joaquín Murguía-González. (2022). Efficient protocol for in vitro propagation of Laelia anceps ssp. anceps white variant from asymbiotic seed germination. South African Journal of Botany, 149, p.376. https://doi.org/10.1016/j.sajb.2022.06.013.
22. Muthab Hussien, Viktoriya Kryuchkova, Ekaterina Raeva-Bogoslovskaya, Olga Molkanova. (2022). Clonal Micropropagation of Cymbidium erythrostylum Rolfe. International Journal of Plant Biology, 14(1), p.28. https://doi.org/10.3390/ijpb14010003.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Acta Agronómica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Política sobre Derechos de autor:Los autores que publican en la revista se acogen al código de licencia creative commons 4.0 de atribución, no comercial, sin derivados.
Es decir, que aún siendo la Revista Acta Agronómica de acceso libre, los usuarios pueden descargar la información contenida en ella, pero deben darle atribución o reconocimiento de propiedad intelectual, deben usarlo tal como está, sin derivación alguna y no debe ser usado con fines comerciales.




























