Maintenance of red-tail coral snake (Micrurus mipartitus) in captivity and evaluation of individual venom variability
Mantenimiento en cautiverio de la coral rabo de ají (Micrurus mipartitus) y evaluación en la variabilidad individual de su veneno
DOI:
https://doi.org/10.15446/abc.v21n3.51651Palabras clave:
antivenom, force-feeding, mortality. (en)alimentación forzada, antiveneno, mortalidad. (es)
Descargas
Red-tail coral snake (Micrurus mipartitus) is a long and thin bicolor coral snake widely distributed in Colombia and is the coral that causes the majority of accidents in the Andean region, so it is important to keep this species in captivity for anti-venom production and research. However, maintaining this species in captivity is very difficult because it refuses to feed, in addition to the high mortality rate due to maladaptation syndrome. In this study a force feeding diet, diverse substrates for maintenance and a milking technique were evaluated. Additionally, individual variability of the venom was determined by High Performance Liquid Chromatography (HPLC), Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Coagulant, Anticoagulant and Hemolytic activities. The results of this study demonstrate that it was possible to increase the survival rate of this species in captivity and to determine some of the important factors in the maintenance. As to the individual variability of the venom, we found differences in number and intensity of peaks recovered by chromatography and also displayed variations in some of its biological activities.
La coral “rabo de ají” es una coral bicolor larga y delgada. Esta especie está ampliamente distribuida en Colombia y es la coral que causa el mayor número de accidentes en la región Andina, por esto es importante mantener esta especie en cautiverio con fines de producción de antivenenos e investigación. No obstante, el mantenimiento de esta especie en cautiverio es difícil, debido a que se rehúsan a alimentarse voluntariamente y a que presentan alta mortalidad por el denominado síndrome de mal adaptación. En este estudio se evaluaron varios sustratos para el mantenimiento, además de una dieta forzada y una técnica de ordeño. Adicionalmente, se evaluó la variabilidad individual del veneno a través de cromatografía liquida de alta eficiencia (HPLC), electroforesis (SDS-PAGE) y las actividades coagulante, anticoagulante y hemolítica indirecta. Los resultados de este estudio demostraron que fue posible incrementar la sobrevivencia de esta especie en cautiverio, así como determinar algunos factores importantes en su mantenimiento. A partir de la evaluación del veneno se encontraron diferencias en el número y en la intensidad de picos en la cromatografía, así como en algunas de sus actividades biológicas.
DOI: https://doi.org/10.15446/abc.v21n3.51651
MAINTENANCE OF RED-TAIL CORAL SNAKE (Micrurus mipartitus) IN CAPTIVITY AND EVALUATION OF INDIVIDUAL VENOM VARIABILITY
Mantenimiento en cautiverio de la coral rabo de ají (Micrurus mipartitus) y evaluación en la variabilidad individual de su veneno
Ana María HENAO DUQUE1; Vitelbina NÚÑEZ RANGEL1,2.
1 Programa de Ofidismo/Escorpionismo, Facultad de Ciencias Farmacéuticas y Alimentarias. Universidad de Antioquia UdeA.Carrera 50A n°. 63-65. Medellín, Colombia.
2 Escuela de Microbiología. Universidad de Antioquia UdeA; Calle 70 n°. 52-21, Medellín, Colombia.
For correspondence. anam.henao@udea.edu.co
Received: 8th July 2015, Returned for revision: 30th November 2015, Accepted:17th January 2016.
Associate Editor: Martha Lucia Ramírez.
Citation/Citar este artículo como: Henao Duque AM, Núñez Rangel V. Maintenance of red-tail coral snake (Micrurus mipartitus) in captivity and evaluation of individual venom variability. Acta biol. Colomb. 2016;21(3):593-600. DOI: https://doi.org/10.15446/abc.v21n3.51651
ABSTRACT
Red-tail coral snake (Micrurus mipartitus) is a long and thin bicolor coral snake widely distributed in Colombia and is the coral that causes the majority of accidents in the Andean region, so it is important to keep this species in captivity for anti-venom production and research. However, maintaining this species in captivity is very difficult because it refuses to feed, in addition to the high mortality rate due to maladaptation syndrome. In this study a force feeding diet, diverse substrates for maintenance and a milking technique were evaluated. Additionally, individual variability of the venom was determined by High Performance Liquid Chromatography (HPLC), Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Coagulant, Anticoagulant and Hemolytic activities. The results of this study demonstrate that it was possible to increase the survival rate of this species in captivity and to determine some of the important factors in the maintenance. As to the individual variability of the venom, we found differences in number and intensity of peaks recovered by chromatography and also displayed variations in some of its biological activities.
Keywords: antivenom, force-feeding, mortality.
RESUMEN
La coral "rabo de ají" es una coral bicolor larga y delgada. Esta especie está ampliamente distribuida en Colombia y es la coral que causa el mayor número de accidentes en la región Andina, por esto es importante mantener esta especie en cautiverio con fines de producción de antivenenos e investigación. No obstante, el mantenimiento de esta especie en cautiverio es difícil, debido a que se rehúsan a alimentarse voluntariamente y a que presentan alta mortalidad por el denominado síndrome de mal adaptación. En este estudio se evaluaron varios sustratos para el mantenimiento, además de una dieta forzada y una técnica de ordeño. Adicionalmente, se evaluó la variabilidad individual del veneno a través de cromatografía liquida de alta eficiencia (HPLC), electroforesis (SDS-PAGE) y las actividades coagulante, anticoagulante y hemolítica indirecta. Los resultados de este estudio demostraron que fue posible incrementar la sobrevivencia de esta especie en cautiverio, así como determinar algunos factores importantes en su mantenimiento. A partir de la evaluación del veneno se encontraron diferencias en el número y en la intensidad de picos en la cromatografía, así como en algunas de sus actividades biológicas.
Palabras clave: alimentación forzada, antiveneno, mortalidad.
INTRODUCTION
The genus Micrurus (Duméril, Bibron & Duméril, 1854) is distributed from the southern United States to Central and South America as far as northern Argentina (Campbell and Lamar, 2004). There are about 70 species living from level sea to 3000 m above sea level. Its diet is poorly understood, but it shows preference for small, limbless animals with elongated appearance such as snakes, lizards, some fish and amphisbaenians (Jackson and Franz, 1981; Roze, 1996; Da Silva and Aird, 2001; Campbell and Lamar, 2004).
Colombia is the third American country with the greatest biodiversity of coral snakes. Twenty-eight species of coral snakes have been reported (Sarmiento Acuña, 2012) showing a wide distribution: the western, central and eastern Andes ranges, including the valleys and the eastern slopes of the Cordillera Oriental; the Sierra Nevada to the north and Gorgona Island in the Pacific. They dwell in the mountain forests up to 2700 m and are commonly found near human settlements (Schmidt, 1955; Roze, 1996; Campbell and Lamar, 2004).
Accidents caused by coral snake bites in Colombia do not exceed 3 % (Otero et al., 2001; Castrillón-Estrada et al., 2007; Heredia, 2009; Sarmiento Acuña, 2012), causing neurotoxic symptoms due to the molecular low weight neurotoxins that bind to endplate receptors generating flaccid paralysis, which cause respiratory arrest (Castrillón-Estrada et al., 2007; Sarmiento Acuña, 2012). Although myotoxic and indirect hemolytic activity in M. mipartitus venom in vitro studies had been demonstrated, it had not been clinical evidenced (Otero et al., 1992, Renjifo et al., 2012). Bites caused by coral snakes are generally classified as severe and their treatment involves the administration of a specific antivenom because it is known that cross-neutralization between the venoms of the Micrurus species is of low magnitude, forcing produced anti-venoms with high neutralizing capacity that can be used against venoms for most species of coral snakes (Rey-Suárez et al., 2011).
Micrurus mipartitus (Duméril, Bibron & Duméril, 1854) is a long and thin coral snake with black, white (or yellowish) rings on body and a red parietal band and two - four red rings on tail. In northwestern South America is commonly known as "rabo de ají" o "rabo de candela" (Campbell and Lamar, 2004). One important aspect while keeping this species in captivity is the production of anti-venoms and researching its toxins. However, it is known that the maintenance of many species of Micrurus in captivity is difficult, largely due to the lack of knowledge of its natural habits in the wild and the consequential difficulty of capture (Ciscotto et al., 2011) Additionally, the feeding process in captivity is difficult because a maladaptation syndrome (Roze, 1996; Chacón et al., 2012; Renjifo et al., 2012).
The known natural diet of M. mipartitus consists of snakes and lizards, including Atractus werneri, Lepidoblepharis sanctaemartae and some individuals of Amphisbaenians (Campbell and Lamar, 2004). However, individuals who have been in captivity have never voluntarily fed on young individuals of Mastigodryas sp., Liophis sp., Atractus sp. or Typhlops sp. adults (dead or alive). Additionally, the coral snakes had never lived more than six months in captivity in our institution and the known life expectancy for Micrurus specimens rarely exceeds one year (Chacón et al., 2012). Therefore, it is necessary to evaluate alternatives to standardize protocols for the maintenance of these species in captivity. The scope of this study is to evaluate different approaches on captive maintenance and characterize the individual variability of venom in adult individuals of Micrurus mipartitus, since there is little knowledge on the venom composition and action for this species and the importance of producing specific antivenoms (Rey-Suárez et al., 2011; Rey-Suárez et al., 2012, Renjifo et al., 2012).
MATERIALS AND METHODS
Animals and captivity
Animals from the Southwestern region of Antioquia were received and housed in the Serpentarium of Universidad de Antioquia - UdeA (Research permission AMVA No. 0000504, May 5th, 2009). Twelve adults of M. mipartitus (six females and six males) were housed individually in plastic boxes (43 cm long x 31.3 cm width, 18 cm high). Boxes were labeled with identification tag (ID) and water ad libitum was provided. Each animal was assigned an ID (Table 1). All quarantine protocols established by Serpentarium were followed. Recommendations of the Ethics Committee for Animal Experimentation (CEEA) from Universidad de Antioquia were followed. Weight, total length and sex were determined, they were also dewormed with Fenbendazol as unique doses (Carpenter, 2005). As part of the protocol, the clinical status and weight were monitored every ten days before feeding. The animals were kept under the environmental conditions of Medellín city; temperature 16-28 °C and 50-65 % relative humidity (IDEAM, 2005).
Diet
The feeding process was based on the force-feeding technique described by Fowler (1981). The animal was positioned vertically and a lubricated 8 gauge Nelaton® probe was inserted as far as the first quarter of the animal. The food was introduced slowly and then the probe was gently extracted. The animal's belly was massaged while maintaining it in upright position for a few minutes to avoid regurgitation. A diet for critical care A/D Hill's Prescription diet® and water 1:1 (Carpenter, 2005) supplemented with 1/8 teaspoon Esencial® (Holliday Laboratories) was used. The administered volume was determined for each animal; 2 mL for every 15 g snake weight and 3.5 to 4 mL for a 30-40 g snake weight at every ten-day interval. In cases in which an animal was found sick or had weight loss, force-feeding was performed every five days until the animal regained its previous weight. After this happened it was returned to the ten day-frequency used for the healthy animals.
Substratum
To determinate what type of substratum offered the best conditions of humidity, temperature and refuge, we evaluated three types of substrates: vermiculite, a coconut substrate for reptiles (Eco earth®, ZooMed) and corrugated cardboard with a shelter made of the same material. Each substrate was evaluated for four animals; they were randomly assigned to the type of substrate. Using a thermohygrometer kit (Cole Parmer 37951-00, Vernon Hills, Illinois) twice daily, humidity and temperature inside boxes with different substrates were monitored.
Milking
The animals were milked at an every 20-day interval. They were divided in two groups. The first group was induced to bite on a vial covered with Parafilm®. The second group was induced to bite on a vial covered with Dacron. Venom was collected and identified individually in two consecutive milkings to analyze individual variation in this species. Collected venom was immediately lyophilized and stored at -70 °C.
In vitro individual venom characterization and biological activities
Venom from seven adult coral snakes (four females and three males) was evaluated through high performance liquid chromatography (HPLC) using the method described by Rey-Suárez et al., (2011); thus 2 mg of each venom (except one snake of which only 1.6 mg was obtained) were diluted in 200 μL of 0.1 % trifluoroacetic acid (TFA, A solution), centrifuged at 7000 g for seven minutes and loaded on a Pinnacle DB C18 column (5 μm 250 x 4.6 mm) using a Shimadzu chromatograph. Elution was performed at 1 mL/min by applying a gradient solution B (acetonitrile, containing 0.1 % TFA) as follows: 5 % B for five minutes, 5-15 % B over ten min, 15-45 B over 60 min, and 45-70 % B over ten min. Absorbance was monitored at 215 nm. Each fraction was manually collected into vials and was subsequently dried in a vacuum centrifuge (Vacufuge plus, Eppendorf) for further characterization.
The higher peaks obtained of HPLC were from two venoms with similar chromatogram profiles to describe Rey-Suárez et al., (2011) and two venoms with different chromatogram were evaluated through 15 % polyacrylamide gel electrophoresis in sodium dodecyl-sulfate (SDS-PAGE) in non-reducing conditions. Protein bands were visualized using Coomassie brilliant blue R-250 staining.
The coagulant, anticoagulant and indirect hemolytic activities were evaluated to six whole venoms. The coagulant activity was tested by adding 20 μg (in 50 μL of phosphate-buffered saline; PBS; 0.12 M NaCl, 40 mM sodium phosphate, pH 7.2) to 200 μL of citrated human plasma, previously incubated at 37 °C. The clotting time was determined in a coagulometer (Human Clot Junior ®). The anticoagulant activity of venoms was tested by preincubating 20 µg (in 50 μL PBS) with 500 µL of citrated human plasma for ten minutes at 37 °C in the double boiler. Finally 50 µL of 0.25 M CaCl2 was added and the clotting time was measured.
Indirect hemolytic activity was performed using the erythrocyte-egg yolk method (Gutiérrez et al., 1988). Venom (2 μg/16 μL PBS) was added to agarose-yolk-erythrocyte gels. The plates were incubated at 37 °C for twenty hours and the hemolysis halo was measured individually. Assays were done in triplicate. PBS and Bothrops asper (Garman, 1883) venom were used as negative and positive control respectively.
Statistical Analysis
An analysis of variance (ANOVA) for repeated measurements with Bonferroni correction, a t-test for the mean difference, a chi square to evaluate association and non-parametric Mann Whitney test in IBM SPSS Statistics Version 20 (N° 9988-003-2014, Licensed Material-Property of IBM Corp) was performed. A value of p ≤ 0.05 was considered statistically significant.
RESULTS
Captive conditions
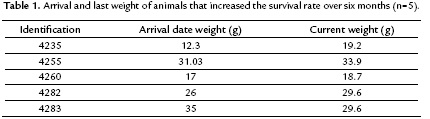
Forty-one percent (n=5) of the animals that were received during the study (12 months) increased the rates of survival to six months in captivity (UdeA Serpentarium record). In addition, tree of them survived more than a year, one of which is still alive, with greater survival to 33 months.
Mortality rate was 58.3 %. Major causes of death were drowning (8.3 %, n=1); asphyxia (16.6 %, n=2); septicemia (16.6 %, n=2); maladaptation syndrome (41.6 %, n=5) and unknown cause (16.6 %, n=2). The frequency of the pathologies found in captivity was: parasitism 16.6 % (n=2) [Kalicephalus spp. (n=2) and Trichomonas spp. (n=1)]; rostral abrasion 41.6 % (n=5); dysecdysis 33.3 % (n=4); weight loss at least once in captivity 58.3 % (n=7) and dermatitis 58.3 % (n=7). Topical therapies for sick animals were used, because its low weight difficult the dosage of many drugs. Also literature reports about the toxicity of some drugs in this species are scarse.
Diet evaluation
Nine of 12 animals were able to maintain or increase their weight during the time they remained in the study. As shown in table 1, four of the five animals that increased the survival rate over six months, increased their weight in captivity in relation to the weight of arrival date. Furthermore, it was concluded there was not variation in the weight of the animals administered with food (p= 0.561). Although weight gain of the animals was observed, it was not statistically significant.
Substratum evaluation
The average temperature was similar for three substrates evaluated (Table 2). Respect to the humidity, Eco earth® substrate for reptiles showed highest average 84.89 % (79.07-90.71 %) following by vermiculite 80.75 % (72.40-89.10 %). Although the cardboard substrate had the lowest average humidity 71.05 % (65.28-76.82 %) this is within the relative humidity range described for M. mipartitus (Campbell and Lamar, 2004).
When we compared substratum type with the presence of any disease, it was concluded that there was no association between dysecdysis and substratum (p=0.687); however, substratum and dermatitis were correlated (p=0.012). All animals that had contact with Eco earth® substrate (n=4) developed dermatitis. Also, 75 % (n=3) of the animals that were in contact with vermiculite developed this pathology, contrasted with zero individual in the cardboard substrate.
The presentation of rostral abrasion, a common problem in captive reptiles and stress-related entity, was independent of the type of substrate used (p=0.254).
Milking
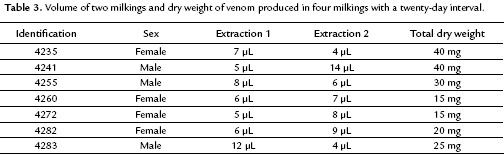
At the end of one year of monitoring, the Parafilm® membrane was demonstrated less traumatic because the snakes did not develop oral lacerations or secondary stomatitis. Also this membrane allowed for the proper collecting of venom that was released by the snake. In contrast, the Dacron membrane did not collect venom because the fangs of M. mipartitus are very small and did not pierce this membrane. As shown in table 3, some variation in the amount of venom collected in two milking sessions was observed. The volume of venom range was 4 to 14 µL. Also, there were no sexual differences in the venom volume collected (p=0.074). Thus the mean volume for females was 13 µL (11.4-14.6 µL) and for males was 16.3 µL (13.8-18.8 µL).
Individual venom characterization and biological activities in vitro
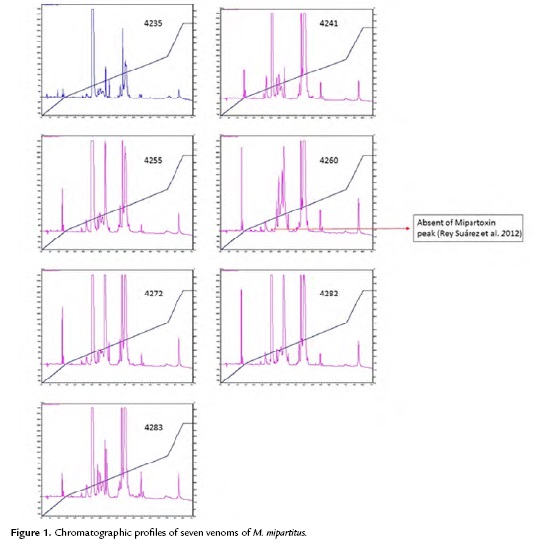
In the HPLC, 18 to 21 fractions were collected for each venom sample. The venom chromatographic profiles obtained from each specimen were similar in relationship to the number of peaks (Fig. 1). However, a marked difference in peak intensity of some specimens was observed. The peaks were distributed mainly in the region corresponding to three finger type toxins and phospholipases regions, according to the description by Rey-Suárez et al. (2011) for this species. The number of peaks in the region of three-finger type toxins ranged from six to nine; it agreed with gel electrophoresis that showed protein bands with molecular mass of ~10 kDa. Some specimens, however, evidenced some differences, for example specimens 4260, 4283 and 4235 showed less peaks in this region. Particularly, snake number 4260 did not evidence the peak eluting at ~30 min.
The number of peaks corresponding to phospholipase region ranged from five to eight. It agreed with gel electrophoresis that showed protein bands with molecular mass of ~15 kDa. Snake 4271 showed the lowest number of peaks in this region, while snakes 4241, 4260 and 4235 had the highest number of peaks.
In the SDS-PAGE the fractions eluted between ~30 and 39 min showed the bands of ~10 kDa molecular mass, which is characteristic of three-finger type toxins. Peaks collected from ~40 min, the region of phospholipases, showed bands with molecular masses ranging from 10 to 20 kDa. Peaks collected between ~60 and 70 min corresponded to 15 and 35 kDa protein bands for snake 4241. Snakes 4282, 4260 and 4283 showed protein bands with 15 and 25 kDa molecular masses. The last peak eluting at ~82 min corresponded to a protein band between 50 and 60 kDa for snakes 4241, 4283 and 4282. The last peak for snake 4260 corresponds to a protein band of ~ 35 kDa.
Venom of M. mipartitus specimens no induced coagulation of plasma up to 30 min., but all showed some anticoagulant activity with some differences (p=0.023) (Table 4). Thus, all venoms increased clotting time between 0.23 to 4.35 minutes compared to control. Snake 4260 had the highest anticoagulant activity, increasing clotting time up to 4.35 min compared to control. Furthermore, hemolytic activity of venom from specimen 4260 was statistically significantly higher than that of other animals; 4260 vs. 4272 (p=0.002), 4260 vs. 4282 (p=0.002), 4260 vs. 4283 (p=0.003), 4260 vs. 4241 (p=0.002) and 4260 vs. 4255 (p=0.002).
Additionally, all venoms caused hemolysis, however venoms from specimens 4260 and 4241 developed the highest hemolytic halos. No significant differences between the hemolysis halos were statistically observed (p=0.229).
DISCUSSION
Although the idea of feeding snakes in captivity is based on providing their natural prey (Nist, 1994), it is very common that coral snake kept in captivity refuse the food offered to them (Roze, 1996). Inadequate temperature maintenance has also been associated with anorexia in captive snakes (Kauffeld, 1953). However, the average temperature in Medellin (23 °C) is suitable for the requirements of M. mipartitus (Campbell, 1973; Roze, 1996; Campbell and Lamar, 2004).
A feeding alternative in captivity is to provide linear fragments of fish or beef impregnated with the smell of natural prey, and offer food after the ecdysis process (Kauffeld, 1953). Also, a frozen fish diet (small and thin size species) that in some cases commercially available was described by Nist (1964), and fillets of Tilapia (Oreochromis sp.) fish were used by Chacón et al. (2012). Prior to feeding the fish was thawed for subsequent feedings to the snake. Chacón et al. (2012) found that a fish-based diet promoted a significantly higher survival time of M. nigrocinctus in captivity. However, a dietary regimen similar to the one described in this study was unsuccessfully used in the UdeA Serpentarium. Nevertheless, this technique was considered impractical and non-viable as permanent diet in captivity because of the high levels of thiaminase frozen fish can have, thus inducing thiamin deficiency if used solely, which causes typical neurological symptoms (Mader, 2006). However, Chacón et al. (2012) kept coral snakes fed solely on fish diet until 168 ±21 weeks.
When there is no possibility to provide a natural diet, conditioning to feeding on dead prey, a force-feeding is recommended (Nist, 1964). Therefore, due to the difficulty of obtaining natural prey and voluntary feeding at UdeA Serpentarium, it was decided to use a forced diet as previously recommended by Fix and Minton (1976) and Fowler (1981). A diet based on A/D® Hill's with mineral and vitamin supplement was used in this work and provided satisfactory results. This diet was chosen because have highly digestible ingredients and extra calories, preserving lean body mass and also have a soft consistency that facilitates tube feeding. This diet was described by Carpenter (2005) as alternative diet for anorexic carnivore's reptiles and it was recommended by a colleague with experience in maintaining of American coral snakes. Table 1 shows that the supplied diet allowed to maintain and/or increase the weight of the animals in captivity, one important aspect is to keep coral snakes for more than six months in captivity in the UdeA Serpentarium.
One coral snake is still alive, reaching 33 months in captivity and it is the first report for Colombian M. mipartitus.
The complications that can result from forced feeding, such as trauma or esophageal and/or stomach rupture and regurgitation are known (Fowler, 1981). In this study we used a lubricated ten gauge Nelaton® probe, which is the right size for this coral snake to avoid trauma to the lung and esophagus, and also to prevent the folding of it. Only regurgitation was observed during the first feeding. Trauma or rupture was not observed during necropsies performed on snakes that died during the study. The pathologies that occurred in captivity and gastrointestinal parasites observed in snakes quarantined agree with those described by Mader (2006) and Pasmans et al. (2008).
All evaluated substrates offered appropriate conditions of temperature and humidity for the species (T: 26 °C, H: 70-85 %) according to the report by Campbell (1973), Roze (1996) and Campbell and Lamar (2004). Coral snakes maintained on cardboard remained more active while animals that were in the Eco earth® and vermiculite substrates remained calmer because these substrates allowed coral snakes to make caves and hideouts. This agrees with the natural behavior of this species; they spend a lot of time under the shelter (Jackson and Franz, 1981; Campbell and Lamar, 2004). However, it is important to note that the substrates that retained more moisture required frequent maintenance and strict disinfection. Furthermore, they were more susceptible to environmental fungi colonization.
According to what was described by Fix and Minton (1976), the technique of milking with Parafilm® membrane yielded a good amount of venom released by the snake, and not cause trauma to the snake mouth. The results of venom evaluation and the volume of venom range (4 to 14 µL) were consistent with what was previously described by Rey-Suárez et al. (2011). However, several venoms showed some variations in chromatographic and electrophoretic profiles, related to the number of peaks eluting in the region of three-finger type toxins, which varied between six and nine and in the region of Phospholipase A2 from five and eight. Specimens that showed fewer peaks corresponding to the region of three-finger type toxins showed the highest number of peaks in the region of Phospholipases A2 (4235 and 4260 specimens).
In addition there was a particular case in the venom of snake 4260, in which the peak eluting at ~30 min was not observed. According to Rey-Suárez et al. (2012) this peak corresponds to the "Mipartoxin", a three-finger type toxin that showed high lethality. It would be important to determine if the venom lethality of this specimen may be lower in relation to the other specimens. Regarding to electrophoresis, the first fractions collected (before 28 min) did not contain proteins, as no bands were evident, which is based on what was described by Rey-Suárez et al. (2011).
The indirect hemolytic and anticoagulant activity evaluated is related to the presence of Phospholipases A2 in the venoms (Kini, 1997). In this study all venoms showed their effect. Nevertheless, venoms from specimens 4241 and 4260 had a higher hemolytic activity, and they had the greater number of peaks in the chromatogram region of phospholipases. In the same way, all venoms showed anticoagulant activity, but specimen 4260 presented the highest clotting time to 261 seconds related to control. In the same way Kopper et al. (2013), reported differences in the PLA2 enzyme activity of venoms from 13 M. tener tener specimen.
The intra-specific variations of venoms have been related to animal factors including phylogeny, geographic origin, age, sex and favorite prey (Da Silva and Aird, 2001). In this study venom variability between sexes was not found (results are not shown). This agrees with what was described by Kopper et al. (2013) with M. tener tener in another study. The inter-specific variability in the venom has been described in 49 Micrurus spp. It has been related to prey preference since all venoms tested were more toxic to natural prey than to those which were not (Da Silva and Aird, 2001).
Gutiérrez et al. (1992), found inter-specific variability in the myotoxic activity of venoms of eleven species of Micrurus. In addition, Da Silva and Aird (2001) considered that the coral snakes may be intra-population, inter-sub specific and inter-specific variability in the composition of their venoms. Another study that evaluated M. frontalis, M. surinamensis, M. lemniscatus and M. nigrocinctus venoms found variations in the anticoagulant and phospholipase A2 activity (Cecchini et al., 2005). This venom variability is an important factor to low cross-reactivity of polyvalent antivenoms of coral snakes (Ciscotto et al., 2011).
Individual variability in snake's venoms has been described in other genus such as Crotalus durissus cumanensis (Aguilar et al., 2007), Echis spp. (Barlow et al., 2009) and Sistrurus spp. (Gibbs et al., 2009). A recent study that evaluated the individual venom variability of a Texas coral snake (M. tener tener) concluded that there is an inverse relationship between volume and total protein concentration, although not found variations in the electrophoretic profiles of individuals (Kopper et al., 2013). In the same study was found that phospholipase A2 activity differed greatly among individuals. However, a correlation with gender in the venom volume or total protein concentration was not found, but females always produced less volume of venom (Kopper et al., 2013).
CONCLUSION
The results of this study support that the diet used prolonged the lifetime of M. mipartitus in captivity. Also, allowed to progress in the standardization of the maintenance of this species. However, further studies are required to improve their living conditions in captivity in order to reduce the mortality of this species and maladaptation syndrome. On the other hand, we expect to establish a venom bank that allows to produce antivenom and to improve the prognosis of people who are bitten by this species.
ACKNOWLEDGEMENTS
We thank Jack Facente, Agritoxins Labs (Florida, USA) for openly sharing his experience in maintenance American Micrurus species in captivity and his advices and sending materials about diet and milking technique. We thank the staff of Gabriel Peláez Montoya Hospital and the Serpentarium of Universidad de Antioquia-UdeA. To Jorge Enrique Asprilla, snake caretaker for his assistance in the management and maintenance of these animals and Camilo Patiño for their technical assistance in the chromatography. This work was funded by grants obtained from Programa de Sostenibilidad, Universidad de Antioquia (2014-2015), "Programa de Jóvenes Investigadores" COLCIENCIAS and COLCIENCIAS Project 111556933661.
REFERENCES
Aguilar I, Guerrero B, Salazar AM, Girón ME, Pérez JH, Sánchez EE, Rodríguez-Acosta A. Individual venom variability in the South American rattle snake Crotalus durissus cumanensis. Toxicon. 2007;50(2):214-224. Doi: 10.1016/j.toxicon.2007.03.012.
Barlow A, Pook CE, Harrison RA, Wüster W. Evolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc R Soc B. 2009;276(1666):2443-2449. Doi: 10.1098/rspb.2009.0048.
Campbell J, Lamar W. The Venomous Reptiles of the Western Hemisphere. Vol. I. Ithaca: Cornell University Press; 2004. p. 200-202.
Campbell JA. A Captive Hatching of Micrurus fulvius tenere (Serpentes, Elapidae). J Herpetol. 1973;7(3):312-315. Doi: 10.2307/1563021.
Carpenter JW. Exotic Animal Formulary. 3rd ed. St. Louis: SAUNDERS Elsevier; 2005. p. 86.
Castrillón-Estrada DF, Acosta Vélez JF, Hernández-Ruiz EA, Alonso Palacio LM. Envenenamiento ofídico. Salud. 2007;23:96-111.
Ciscotto PH, Rates B, Silva DA, Richardson M, Silva LP, Andrade H, et al. Venomic analysis and evaluation of antivenom cross-reactivity of South American Micrurus species. J Proteomics. 2011;74(9):1810-1825. Doi: 10.1016/j.jprot.2011.07.011.
Chacón D, Rodríguez S, Arias J, Solano G, Bonilla F, Gómez A. Maintaining Coral Snakes (Micrurus nigrocinctus, Serpentes: Elapidae) for venom production on an alternative fish-based diet. Toxicon. 2012;60(3):249-253. Doi: 10.1016/j.toxicon.2012.04.332.
Cecchini AL, Marcussi S, Silveira LB, Borja-Oliveira CR, Rodrigues-Simionid L, Amara L, et al. Biological and enzymatic activities of Micrurus sp. (Coral) snake venoms. Comp Biochem Physiol A Mol Integr Physiol. 2005;140(1):125-134. Doi: 10.1016/j.cbpb.2004.11.012.
Da Silva NJ, Aird SD. Prey specificity, comparative lethality and compositional differences of coral snake venoms. Comp Biochem Physiol C Toxicol Pharmacol. 2001;128(3):425-456. Doi: 10.1016/S1532-0456(00)00215-5.
Fix JD, and Minton SA Jr. Venom extraction and yields from the North American coral snake, Micrurus fulvius. Toxicon. 1976;14(2):143-145. Doi: 10.1016/0041-0101(76)90106-9.
Fowler ME. Force-feeding techniques in wild animals. J Zoo Wildlife Med. 1981;12(1):3-10.
Gibbs HL, Mackessy SP. Functional basis of a molecular adaptation: Prey-specific toxic effects of venom from Sistrurus rattlesnakes. Toxicon. 2009;53(6):672-679. Doi: 10.1016/j.toxicon.2009.01.034.
Gutiérrez JM, Ávila C, Rojas E, Cerdas L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 1988;26(4):411-413. Doi: 10.1016/0041-0101(88)90010-4.
Gutiérrez JM, Rojas G, Da Silva NJ Jr., Nunez J. Experimental myonecrosis induced by the venoms of South American Micrurus coral snakes. Toxicon. 1992;30(10):1299-1302.
Heredia, D. Comportamiento del accidente ofídico en Colombia. Informe epidemiológico Accidente Ofídico. Instituto Nacional de Salud. [Cited: 14 September 2015]. Avaliable in: http://www.ins.gov.co/lineas-de-accion/Subdireccion-vigilancia/sivigila/Protocolos%20SIVIGILA/PRO%20Accidente%20Ofidico.pdf.
IDEAM. Atlas climatológico de Colombia. Bogotá: Imprenta Nacional de Colombia. 2005. p. 117-121.
Jackson DR, Franz R. Ecology of the Eastern Coral Snake (Micrurus fulvius) in Northern Peninsular Florida. Herpetologica. 1981;37(4):213-228.
Kauffeld CF. Methods of Feeding Captive Snakes. Herpetologica. 1953;9(3):129-131.
Kini MR. Venom Phospholipase A2 enzymes: Structure, function and mechanism. New York: John Wiley & Sons Ltd; 1997. p. 1-29.
Kopper RA, Harper GR, Zimmerman S, Hook J. Comparison of total protein and phospholipase A2 levels in individual coral snake venoms. Toxicon. 2013;76:59-62. Doi: 10.1016/j.toxicon.2013.09.011.
Mader DR. Reptile Medicine and Surgery. 2nd ed. London: SAUNDERS; 2006. p. 243.
Nist JF. The Ideal Food for Force Feeding Snakes. Am Biol Teach. 1964;26(7):498.
Otero R, Callejas ME, Gutiérrez J, Lotero GJ, Rodríguez O, Villa NH, et al. Necesidades reales de antivenenos en Colombia. Características de los productos y del mercado. Rev Epidemiol Antioq. 2001;26:49-59.
Otero R, Osorio RG, Valderrama R, Giraldo CA. Efectos farmacológicos y enzimáticos de los venenos de serpientes de Antioquia y Chocó (Colombia). Toxicon. 1992;30(5-6):611-620. Doi: 10.1016/0041-0101(92)90855-Y.
Pasmans F, Blahak S, Martel A, Pantchev N. Introducing reptiles into a captive collection: The role of the veterinarian. Vet J. 2008;175(1):53-68. Doi: 10.1016/j.tvjl.2006.12.009.
Renjifo C, Smith EN, Wayne CH, Renjifo JM, Sánchez A, Acosta R, et al. Neuromuscular activity of the venoms of the Colombian coral snakes Micrurus dissoleucus and Micrurus mipartitus: An evolutionary Perspective. Toxicon. 2012;59(1):132-142. Doi: 10.1016/j.toxicon.2011.10.017.
Rey-Suárez P, Núñez V, Gutiérrez JM, Lomonte B. Proteomic and biological characterization of the venom of the redtail coral snake, Micrurus mipartitus (Elapidae), from Colombia and Costa Rica. J Proteomics. 2011;75(2):655-667. Doi: 10.1016/j.jprot.2011.09.003.
Rey-Suárez P, Stuani-Floriano R, Rostelato-Ferreira S, Saldarriaga-Córdoba M, Núñez V, Rodrigues-Simioni L, et al. Mipartoxin-I, a novel three-finger toxin, is the major neurotoxic component in the venom of the redtail coral snake Micrurus mipartitus (Elapidae). Toxicon. 2012;60(5):851-863. Doi: 10.1016/j.toxicon.2012.05.023.
Roze AJ. Coral snakes of the Americas: biology, identification and venoms. Malabar: Kreiger Publishing; 1996. p. 195-199.
Sarmiento Acuña K. Aspectos biomédicos del accidente ofídico. Universitas Medica. 2012;53(1):68-82.
Schmidt KP. Coral Snakes of the genus Micrurus in Colombia. Fieldiana Zool. 1955;34:337-359.
Referencias
Aguilar I, Guerrero B, Salazar AM, Girón ME, Pérez JH, Sánchez EE, Rodríguez-Acosta A. Individual venom variability in the South American rattle snake Crotalus durissus cumanensis. Toxicon. 2007;50(2):214-224. Doi:10.1016/j.toxicon.2007.03.012
Barlow A, Pook CE, Harrison RA, Wüster W. Evolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc R Soc B. 2009;276(1666):2443-2449. Doi:10.1098/rspb.2009.0048
Campbell J, Lamar W. The Venomous Reptiles of the Western Hemisphere. Vol. I. Ithaca: Cornell University Press; 2004. p. 200-202.
Campbell JA. A Captive Hatching of Micrurus fulvius tenere (Serpentes, Elapidae). J Herpetol. 1973;7(3):312-315. Doi:10.2307/1563021
Carpenter JW. Exotic Animal Formulary. 3rd ed. St. Louis: SAUNDERS Elsevier; 2005. p. 86.
Castrillón-Estrada DF, Acosta Vélez JF, Hernández-Ruiz EA, Alonso Palacio LM. Envenenamiento ofídico. Salud. 2007;23:96-111.
Ciscotto PH, Rates B, Silva DA, Richardson M, Silva LP, Andrade H, et al. Venomic analysis and evaluation of antivenom cross-reactivity of South American Micrurus species. J Proteomics. 2011;74(9):1810-1825. Doi:10.1016/j.jprot.2011.07.011
Chacón D, Rodríguez S, Arias J, Solano G, Bonilla F, Gómez A. Maintaining Coral Snakes (Micrurus nigrocinctus, Serpentes: Elapidae) for venom production on an alternative fish-based diet. Toxicon. 2012;60(3):249-253. Doi:10.1016/j.toxicon.2012.04.332
Cecchini AL, Marcussi S, Silveira LB, Borja-Oliveira CR, Rodrigues-Simionid L, Amara L, et al. Biological and enzymatic activities of Micrurus sp. (Coral) snake venoms. Comp Biochem Physiol A Mol Integr Physiol. 2005;140(1):125-134. Doi:10.1016/j.cbpb.2004.11.012
Da Silva NJ, Aird SD. Prey specificity, comparative lethality and compositional differences of coral snake venoms. Comp Biochem Physiol C Toxicol Pharmacol. 2001;128(3):425-456. Doi:10.1016/S1532-0456(00)00215-5
Fix JD, and Minton SA Jr. Venom extraction and yields from the North American coral snake, Micrurus fulvius. Toxicon. 1976;14(2):143-145. Doi:10.1016/0041-0101(76)90106-9
Fowler ME. Force-feeding techniques in wild animals. J Zoo Wildlife Med. 1981;12(1):3-10.
Gibbs HL, Mackessy SP. Functional basis of a molecular adaptation: Prey-specific toxic effects of venom from Sistrurus rattlesnakes. Toxicon. 2009;53(6):672-679. Doi:10.1016/j.toxicon.2009.01.034
Gutiérrez JM, Ávila C, Rojas E, Cerdas L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 1988;26(4):411-413. Doi:10.1016/0041-0101(88)90010-4
Gutiérrez JM, Rojas G, Da Silva NJ Jr., Nunez J. Experimental myonecrosis induced by the venoms of South American Micrurus coral snakes. Toxicon. 1992;30(10):1299-1302.
Heredia, D. Comportamiento del accidente ofídico en Colombia. Informe epidemiológico Accidente Ofídico. Instituto Nacional de Salud. [Cited: 14 September 2015].
Avaliable in: http://www.ins.gov.co/lineas-de-accion/Subdireccion-vigilancia/sivigila/Protocolos%20SIVIGILA/PRO%20Accidente%20Ofidico.pdf.
IDEAM. Atlas climatológico de Colombia. Bogotá: Imprenta Nacional de Colombia. 2005. p. 117-121.
Jackson DR, Franz R. Ecology of the Eastern Coral Snake (Micrurus fulvius) in Northern Peninsular Florida. Herpetologica. 1981;37(4):213-228.
Kauffeld CF. Methods of Feeding Captive Snakes. Herpetologica. 1953;9(3):129-131.
Kini MR. Venom Phospholipase A2 enzymes: Structure, function and mechanism. New York: John Wiley & Sons Ltd; 1997. p. 1-29.
Kopper RA, Harper GR, Zimmerman S, Hook J. Comparison of total protein and phospholipase A2 levels in individual coral snake venoms. Toxicon. 2013;76:59-62. Doi:10.1016/j.toxicon.2013.09.011
Mader DR. Reptile Medicine and Surgery. 2nd ed. London: SAUNDERS; 2006. p. 243.
Nist JF. The Ideal Food for Force Feeding Snakes. Am Biol Teach. 1964;26(7):498.
Otero R, Callejas ME, Gutiérrez J, Lotero GJ, Rodríguez O, Villa NH, et al. Necesidades reales de antivenenos en Colombia. Características de los productos y del mercado. Rev Epidemiol Antioq. 2001;26:49-59.
Otero R, Osorio RG, Valderrama R, Giraldo CA. Efectos farmacológicos y enzimáticos de los venenos de serpientes de Antioquia y Chocó (Colombia). Toxicon. 1992;30(5-6):611-620. Doi:10.1016/0041-0101(92)90855-Y
Pasmans F, Blahak S, Martel A, Pantchev N. Introducing reptiles into a captive collection: The role of the veterinarian. Vet J. 2008;175(1):53-68. Doi:10.1016/j.tvjl.2006.12.009
Renjifo C, Smith EN, Wayne CH, Renjifo JM, Sánchez A, Acosta R, et al. Neuromuscular activity of the venoms of the Colombian coral snakes Micrurus dissoleucus and Micrurus mipartitus: An evolutionary Perspective. Toxicon. 2012;59(1):132-142. Doi:10.1016/j.toxicon.2011.10.017
Rey-Suárez P, Núñez V, Gutiérrez JM, Lomonte B. Proteomic and biological characterization of the venom of the redtail coral snake, Micrurus mipartitus (Elapidae), from Colombia and Costa Rica. J Proteomics. 2011;75(2):655-667. Doi:10.1016/j.jprot.2011.09.003
Rey-Suárez P, Stuani-Floriano R, Rostelato-Ferreira S, Saldarriaga-Córdoba M, Núñez V, Rodrigues-Simioni L, et al. Mipartoxin-I, a novel three-finger toxin, is the major neurotoxic component in the venom of the redtail coral snake Micrurus mipartitus (Elapidae). Toxicon. 2012;60(5):851-863. Doi:10.1016/j.toxicon.2012.05.023
Roze AJ. Coral snakes of the Americas: biology, identification and venoms. Malabar: Kreiger Publishing; 1996. p. 195-199.
Sarmiento Acuña K. Aspectos biomédicos del accidente ofídico. Universitas Medica. 2012;53(1):68-82.
Schmidt KP. Coral Snakes of the genus Micrurus in Colombia. Fieldiana Zool. 1955;34:337-359.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Janeth Alejandra Bolívar-Barbosa, Ariadna Lorena Rodríguez-Vargas. (2020). Actividad neurotóxica del veneno de serpientes del género Micrurus y métodos para su análisis. Revisión de la literatura. Revista de la Facultad de Medicina, 68(3) https://doi.org/10.15446/revfacmed.v68n3.75992.
2. Bruno Lomonte, Paola Rey-Suárez, Julián Fernández, Mahmood Sasa, Davinia Pla, Nancy Vargas, Melisa Bénard-Valle, Libia Sanz, Carlos Corrêa-Netto, Vitelbina Núñez, Alberto Alape-Girón, Alejandro Alagón, José María Gutiérrez, Juan J. Calvete. (2016). Venoms of Micrurus coral snakes: Evolutionary trends in compositional patterns emerging from proteomic analyses. Toxicon, 122, p.7. https://doi.org/10.1016/j.toxicon.2016.09.008.
3. Danilo Chacón, Aarón Gómez, Greivin Corrales. (2020). Ex situ management of a Coral Snake (Serpentes: Elapidae): the interesting case of Micrurus ruatanus. Caribbean Journal of Science, 50(1), p.86. https://doi.org/10.18475/cjos.v50i1.a11.
4. Ariadna Rodríguez-Vargas, Adrián Marcelo Franco-Vásquez, Janeth Alejandra Bolívar-Barbosa, Nohora Vega, Edgar Reyes-Montaño, Roberto Arreguín-Espinosa, Alejandro Carbajal-Saucedo, Teddy Angarita-Sierra, Francisco Ruiz-Gómez. (2023). Unveiling the Venom Composition of the Colombian Coral Snakes Micrurus helleri, M. medemi, and M. sangilensis. Toxins, 15(11), p.622. https://doi.org/10.3390/toxins15110622.
5. Luz Elena Romero Giraldo, Sergio Pulido, Mario Andrés Berrío, María Fernanda Flórez, Paola Rey-Suárez, Vitelbina Núñez-Rangel, Mónica Saldarriaga Córdoba, Jaime Andrés Pereañez. (2024). Immunogenic potential and neutralizing ability of a heterologous version of the most abundant three-finger toxin from the coral snake Micrurus mipartitus. Journal of Venomous Animals and Toxins including Tropical Diseases, 30 https://doi.org/10.1590/1678-9199-jvatitd-2023-0074.
6. Karen de Morais-Zani, Caroline Serino-Silva, Nathália da Costa Galizio, Lídia Jorge Tasima, Josias Falararo Pagotto, Marisa Maria Teixeira da Rocha, José Roberto Marcelino, Sávio Stefanini Sant'Anna, Alexandre Keiji Tashima, Anita Mitico Tanaka-Azevedo, Kathleen Fernandes Grego. (2018). Does the administration of pilocarpine prior to venom milking influence the composition of Micrurus corallinus venom?. Journal of Proteomics, 174, p.17. https://doi.org/10.1016/j.jprot.2017.12.010.
7. Paola Rey-Suárez, Jeisson Gómez-Robles, Julián Fernández, Bruno Lomonte, Mahmood Sasa, Mónica Saldarriaga-Cordoba, Jaime Andrés Pereañez, Omayra Aguilera, Vitelbina Núñez-Rangel. (2025). Assessment of venom variation and phylogenetic relationships of Micrurus dumerilii from three different regions of Colombia. Biochimie, 235, p.93. https://doi.org/10.1016/j.biochi.2025.06.003.
8. Juan Diego Piedrahita, Ana Cardona-Ruda, Jaime Andrés Pereañez, Paola Rey-Suárez. (2024). In-depth immunorecognition and neutralization analyses of Micrurus mipartitus and M. dumerilii venoms and toxins by a commercial antivenom. Biochimie, 216, p.120. https://doi.org/10.1016/j.biochi.2023.10.009.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2016 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).