Publicado
Lipid vesicles: applications, principal components and methods used in their formulations: A review
Vesículas lipídicas: aplicaciones, principales componentes y métodos utilizados en sus formulaciones. Una revisión
Vesículas Lipídicas: Aplicações, Principais componentes e métodos utilizados em suas formulações
DOI:
https://doi.org/10.15446/abc.v25n2.74830Palabras clave:
Component, formulation, liposomes, nanomedicine, niosomes (en)Componentes, formulaciones, limosas, nanomedicina, niosomas (es)
Lipossomas, niosomas, componentes, métodos, formulações (pt)
Descargas
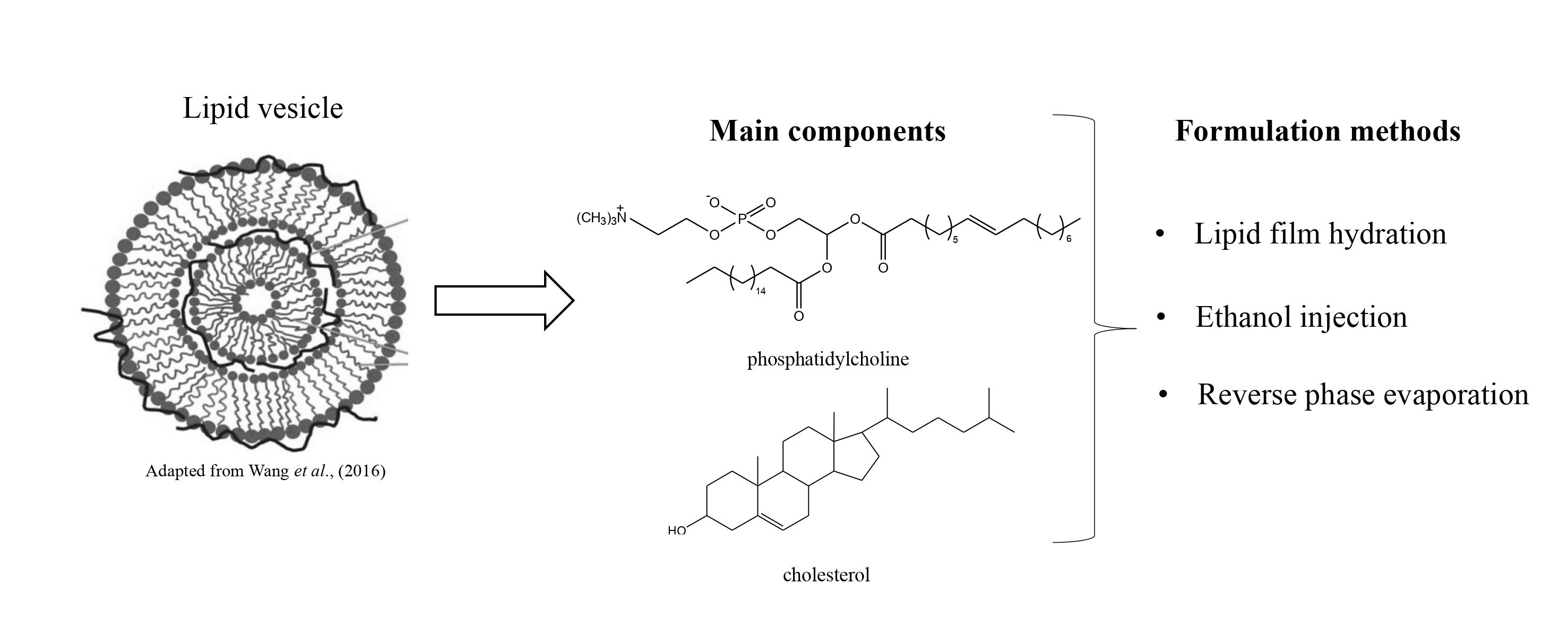
Liposomes and niosomes are currently the most studied lipid vesicles in the nanomedicine field. The system formed by a phospholipid bilayer in aqueous medium allows these vesicles to carry both hydrophilic and lipophilic compounds, providing an increase in solubility of drugs lready used in conventional therapy. The focus on the development of these vesicles should be directed to determining the ideal composition, with low toxicity, biocompatibility and which remains stable for long periods. These characteristics are related to the components used for formulation and the substances that will be encapsulated. Another important point relates to the methods used during formulation, which are important in determining the type of vesicle formed, whether these be large or small, unilamellar or multilamellar. Because of the deliberate actions applied in the development of these vesicles, this review sought to gather updated information regarding the different methods used, including their main components while considering the behavior of each of them when used in different formulations. Also, data showing the importance of formulations in the medical field evidencing studies performed with liposome and niosome vesicles as promising in this area, and others, were included. The approach allows a better understanding of the participation of components in formulations such as cholesterol and non-ionic surfactants, as well as the basis for choosing the ideal components and methods for future research in the development of these vesicles.
Los liposomas y los niosomas son actualmente las vesículas lipídicas más estudiadas en el área de la nanomedicina. El sistema formado por bicapas de fosfolipídos en medio acuoso, permite que estas vesículas puedan cargar compuestos tanto hidrofílicos como lipofílicos, proporcionando el aumento de solubilidad de las drogas ya utilizadas en la terapia convencional. El foco del desarrollo de estas vesículas debe estar direccionado para la determinación de una composición ideal, con baja toxicidad, biocompatibilidad y estabilidad por un largo período. Estas características estan relacionadas a los componentes utilizados para la formulación y las substancias que serán encapsuladas. Otro punto importante está relacionado con los métodos utilizados para la formulación, que son importantes en la determinación del tipo de vesícula formada, sea ella grande o pequeña, unilamelar o multilamelar. Teniendo en cuenta los estrictos criterios de acciones aplicadas en el desarrollo de estas vesículas, esta revisión buscó reunir información actualizada sobre los diversos métodos utilizados, incluyendo sus principales componentes, considerando el comportamento de cada uno de ellos, cuando son utilizados en diferentes formulaciones. Además, se incluyeron datos que muestran la importancia de las formulaciones en el área médica, evidenciando estudios realizados con las vesículas liposomas e niosomas como promisorias en esta y en otras áreas. Este abordaje permite una mejor comprensión de la participación de componentes como el colesterol y los surfactantes no-ionicos en las formulaciones y también puede servir de base para la selección de los componentes y métodos ideales para futuras investigaciones en el desarrollo de esas vesículas.
Referencias
Abaee A, Madadlou A. Niosome-loaded cold-set whey protein hydrogels. Food Chem. 2016; 196: 106-113. DOI: 10.1016/j.foodchem.2015.09.037.
Abdel-Hafez SM, Hathout RM, Sammour AO. Curcumin-loaded ultra deformable nanovesicles as a potential delivery system for breast cancer therapy. Colloids Surf B Biointerfaces. 2018; 167: 63-72. DOI: 10.1016/j.colsurfb.2018.03.051.
Alam MDS, Ahad A, Abidin L, Aqil M, Mir SR, Mujeeb M. Embelin-loaded oral niosomes ameliorate streptozotocin-induced diabetes in Wistar rats. Biomed Pharmacother. 2018; 97: 1514–1520. DOI: https://doi.org/10.1016/j.biopha.2017.11.073.
Alomrani AH, Al-Agamy MH, Badran MM. In vitro skin penetration and antimycotic activity of itraconazole loaded niosomes: Various non-ionic surfactants. J Drug Deliv Sci Technol. 2015; 28: 37-45. DOI: https://doi.org/10.1016/j.jddst.2015.04.009.
Andreu V, Arruebo M. Current progress and challenges of nanoparticle-based therapeutics in pain management. J Control Release. 2018; 269: 189-213. DOI: https://doi.org/10.1016/j.jconrel.2017.11.018.
Araújo RS, Silveira ALM, Souza ELS, Freire RH, Souza CM, Reis DC, Costa BRC, Sugimoto MA, Silveira JN, Martins FS, Cassali GD, Leite JIA, Sousa LP, Ferreira AVM, Oliveira MC, Cardoso VN. Intestinal toxicity evaluation of long-circulating and pH-sensitive liposomes loaded with cisplatin. Eur J Pharm Sc. 2017; 106: 142-151. DOI: 10.1016/j.ejps.2017.05.046.
Arriaga LR, Rodríguez-García R, Moleiro LH, Prévost S, Montero IL, Hellweg T, Monroy F. Dissipative dynamics of fluid lipid membranes enriched in cholesterol. Adv Colloid Interface Sci. 2017; 247: 514-520. DOI: https://doi.org/10.1016/j.cis.2017.07.007.
Assis LM, Zavareze ER, Prentice-Hernández C, Souza‑soares LA. Characteristics of nanoparticles and their potential applications in foods. Braz J Food Technol. 2012; 15: 99-109. DOI: http://dx.doi.org/10.1590/S1981-67232012005000004.
Attia N, Mashal M, Grijalvo S, Eritja R, Zárate J, Puras G, Pedraz JL. Stem cell-based gene delivery mediated by cationic niosomes for bone regeneration. Nanomedicine: NBM. 2018; 14: 521-531. DOI: 10.1016/j.nano.2017.11.005.
Babazadeh A, Ghanbarzadeh B, Hamishehkar H. Phosphatidylcholine-rutin complex as a potential nanocarrier for food applications. J Funct Foods. 2017; 33: 134-141. DOI: https://doi.org/10.1016/j.jff.2017.03.038.
Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965; 13: 238-252. DOI: https://doi.org/10.1016/S0022-2836(65)80093-6.
Bansal S, Aggarwal G, Chandel P, Harikumar SL. Design and development of cefdinir niosomes for oral delivery. J Pharm Bioallied Sci. 2013; 5: 318-326. DOI: 10.4103/0975-7406.120080.
Barroso MF, Luna MA, Moyano F, Delerue-Matos C, Correa NM, Molina PG. Study of lipid peroxidation and ascorbic acid protective role in large unilamellar vesicles from a new electrochemical performance. Bioelectrochemistry. 2018; 120: 120-126. DOI: https://doi.org/10.1016/j.bioelechem.2017.12.003.
Basiri L, Rajabzadeh G, Bostan A. Physicochemical properties and release behavior of Span 60/Tween 60 niosomes as vehicle for a-Tocopherol delivery. LWT- Food Sci Technol. 2017; 84, 471-478. DOI: https://doi.org/10.1016/j.lwt.2017.06.009.
Batzri S, Korn ED. Single bilayer liposomes prepared without sonication. BBA – Biomembranes. 1973; 298: 1015-1019. DOI: https://doi.org/10.1016/0005-2736(73)90408-2.
Bhattacharjee S. DLS and zeta potential – What they are and what they are not?. J. Controlled Release. 2016; 235: 337–351. DOI: 10.1016/j.jconrel.2016.06.017.
Bnyan R, Khan I, Ehtezazi T, Saleem I, Gordon S, O'Neill F, Roberts M. Surfactant Effects on Lipid-Based Vesicles Properties. J. Pharm. Sci. 2018; 1. DOI: 10.1016/j.xphs.2018.01.005.
Bruxel F, Laux M, Wild LB, Fraga M, Koester LS, Teixeira HF. Nanoemulsions as parenteral drug delivery systems. Quím nova. 2012; 35: 1827-1840. DOI: http://dx.doi.org/10.1590/S0100-40422012000900023.
Cagno M, Styskala J, Hlaváč J, Brandl M, Bauer-Brandl A, Skalko-Basnet N. Liposomal solubilization of new 3-hydroxy-quinolinone derivatives with promising anticancer activity: a screening method to identify maximum incorporation capacity. J Liposome Res. 2011; 21: 272-278. DOI: 10.3109/08982104.2010.550265.
Chabanon M, Rangamani P. Solubilization kinetics determines the pulsatory dynamics of lipid vesicles exposed to surfactante. BBA – Biomembranes. 2018; 1-20. DOI: https://doi.org/10.1101/225946.
Charcosset C, Juban A, Valour JP, Urbaniak S, Fessi H. Preparation of liposomes at large scale using theethanol injection method: Effect of scale-up and injection devices. chemical engineering research and design.
Chem Eng Res Des. 2015; 94: 508–515. DOI: 10.1016/j.cherd.2014.09.008
Chorilli M, Leonardi GR, Oliveira AG, Scarpa MV. Lipossomas em formulações dermocosméticas. Infarma. 2004; 16: 75-79.
Chorilli M, Rimério TC, Oliveira AG, Scarpa MV. Study of liposomes stability containing soy phosphatidylcholine and hydrogenated soy phosphatydylcholine adding or not cholesterol by turbidity method. Lat Am J Pharm. 2006; 26: 31-37.
Cuomo F, Cofelice M, Venditti F, Ceglie A, Miguel M, Lindman B, Lopez F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf B Biointerfaces. 2017; 1: 1-6. DOI: 10.1016/j.colsurfb.2017.11.047.
Disalvo EA, Bouchet AM. Electrophoretic mobility and zeta potential of liposomes due to arginine andpolyarginine adsorption. Colloids Surf A Physicochem Eng Asp. 2014; 440: 170– 174. DOI: https://doi.org/10.1016/j.colsurfa.2012.09.012.
Eloy JO, Souza MC, Petrilli R, Barcellos JPA, Lee R, Marchetti JM. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf B Biointerfaces. 2014; 123: 345–363. DOOI: 10.1016/j.colsurfb.2014.09.029.
Fathima SJ, Fathima I, Abhishek V, Khanum F. Phosphatidylcholine, an edible carrier for nanoencapsulation of unstable thiamine. Food chem. 2016; 197: 562-570. DOI: 10.1016/j.foodchem.2015.11.005.
Fontes MAP, Vaz GC, Cardoso TZD, Oliveira MF, Campagnole-Santos MJ, Santos RAS, Sharma NM, Patel KP, Frézard F. GABA-containing liposomes: neuroscience applications and translational perspectives for targeting neurological diseases. Nanomedicine. 2018; 14: 781-788. DOI: 10.1016/j.nano.2017.12.007.
Freixeiro P, Pensado A, Allen L, Humphries H, Taylor S, Seijo B, Ferreirós C, Gorringe A, Sánchez S, Sánchez A. Solid sorbitan esters nanoparticles are efficient and low-cost vehicles for subunit vaccines: Proof of concept with Neisseria meningitidis protein Mip. J Drug Deliv Sci Technol. 2017; 42: 299-306. DOI: https://doi.org/10.1016/j.jddst.2017.04.031.
Fricker G, Kromp T, Wendel A, Blume A, Zirkel J, Rebmann H, Setzer C, Quinkert RO, Martin F, Müller-Goymann C. Phospholipids and Lipid-Based Formulations in Oral Drug Delivery. Pharm Res. 2010; 27: 1469–1486. DOI: 10.1007/s11095-010-0130-x.
Fuse T, Tagami T, Tane M, Ozeki T. Effective light-triggered contents release from helper lipid-incorporated liposomes co-encapsulating gemcitabine and a water-soluble photosensitizer. Int. J. Pharm. 2018; 540: 50-56. DOI: 10.1016/j.ijpharm.2018.01.040.
Gálvez P, Ruiz A, Clares B. El futuro de la medicina clínica hacia nuevas terapias: terapia celular, génica y nanomedicina the future of new therapies in clinical medicine. Med Clín. 2011; 137: 645-649. DOI: https://doi.org/10.1016/j.medcli.2010.12.005.
Garcia FM. Avanços da nanomedicina: a nova fronteira da medicina – artigo de atualização. Rev Ciên Saúde Nova Esperan. 2014; 12: 110-117.
Giri TK, Mukherjee P, Barman TK, Maity S. Nano-encapsulation of capsaicin on lipid vesicle and evaluation of their hepatocellular protective effect. Int J Biol Macromol. 2016; 88: 236-243. DOI: 10.1016/j.ijbiomac.2016.03.056.
Gunes A, Guler E, Um RN, Demir B, Barlas FB, Yavuz M, Coskunol H, Timur S. Niosomes of Nerium oleander extracts: In vitro assessment of bioactive nanovesicular structures. J Drug Deliv. Sci. Techno. 2017; 37: 158-165. DOI: https://doi.org/10.1016/j.jddst.2016.12.013.
Gutiérrez G, Matos M, Barrero P, Pando D, Iglesias O, Pazos C. Iron-entrapped niosomes and their potential application for yogurt fortification. LWT - food sci technol. 2016; 74: 550-556. DOI: https://doi.org/10.1016/j.lwt.2016.08.025.
Hayashi K, Shimanouchi T, Kato K, Miyazaki T, Nakamura A, Umakoshi H. Span 80 vesicles have a more fluid, flexible and “wet” surface than phospholipid liposomes. Colloids Surf B Biointerfaces. 2011; 87: 28-35. DOI: https://doi.org/10.1016/j.colsurfb.2011.04.029.
Hong SS, Kim SH, Lim SJ. Effects of triglycerides on the hydrophobic drug loading capacity of saturated phosphatidylcholine-based liposomes. Int J Pharm. 2015; 483: 142-150. DOI: 10.1016/j.ijpharm.2015.02.013.
Hua W, Liu T. Preparation and properties of highly stable innocuous niosome in Span 80/PEG 400/H2O system. Colloids Surf A Physicochem Eng Asp. 2007; 302: 377-382. DOI: https://doi.org/10.1016/j.colsurfa.2007.02.068.
Ibarguren M, Bomans PHH, Ruiz-Mirazo K, Frederik PM, Alonso A, Goni FM. Thermally-induced aggregation and fusion of protein-free lipid vesicles. Colloids Surf B Biointerfaces. 2015; 136: 545-552. DOI: 10.1016/j.colsurfb.2015.09.047.
Imam SS, Ahad A, Aqil M, Akhtar M, Sultana Y, Ali A. Formulation by design based risperidone nano soft lipid vesicle as a new strategy for enhanced transdermal drug delivery: In-vitro characterization, and in-vivo appraisal. Mater. Sci Eng. 2017; 75: 1198-1205. DOI: 10.1016/j.msec.2017.02.149.
Junyaprasert VB, Singhsa P, Suksiriworapong J, Chantasart D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int J Pharm. 2012; 423: 303-311. DOI: 10.1016/j.ijpharm.2011.11.032.
Kaddah S, Khreich N, Kaddah F, Charcosset C. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem Toxicol. 2018; 113: 40-48. DOI: 10.1016/j.fct.2018.01.017.
Kanaani L, Tabrizi MM, Khiyavi AA, Javadi I. Improvement the Efficacy of Cisplatin by Niosome Nanoparticles Against Human Breast Cancer Cell Line BT-20 : an In Vitro Study. Asian Pac J Cancer Biolo. 2017; 2: 25-26. DOI: 10.22034/APJCB.2017.2.2.25.
Kaur G, Mehta SK. Developments of Polysorbate (Tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. Int. J. Pharm. 2017; 529: 134-160. DOI: 10.1016/j.ijpharm.2017.06.059.
Khan MI, Madni A, Ahmad S, Mahmood MA, Rehman M, Ashfaq M. Formulation design and characterization of a non-ionic surfactante based vesicular system for the sustained delivery of a new chondroprotective agente. Braz J Pharm Sci. 2015; 51: 607-615. DOI: http://dx.doi.org/10.1590/S1984-82502015000300012
Khan MI, Madni A, Peltonen L. Development and in-vitro characterization of sorbitan monolaurate and poloxamer 184 based niosomes for oral delivery of diacerein. Eur J Pharm Sci. 2016; 95: 88-95. DOI: 10.1016/j.ejps.2016.09.002.
Kitayama H, Takechi Y, Tamai N, Matsuki H, Yomota C, Saito H. Thermotropic Phase Behavior of Hydrogenated Soybean Phosphatidylcholine–Cholesterol Binary Liposome Membrane. Pharm Soci Japan. 2014; 62: 58-63.
Kolbina M, Bodmeier R, Körber M. Saturated phosphatidylcholine as matrix former for oral extended release dosage forms. Eur J Pharm Sci. 2017; 108: 86-92. DOI: 10.1016/j.ejps.2017.07.017.
Kuo AT, Tu CL, Yang YM, Chang CH. Enhanced physical stability of positively charged catanionic vesicles: Role of cholesterol-adjusted molecular packing. J Taiwan Inst Chem E. 2018; 000: 1-7. DOI: https://doi.org/10.1016/j.jtice.2018.02.013.
Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007; 65: 259-269. DOI: 10.1016/j.ejpb.2006.11.009.
Liang R, Chen L, Yokoyama W, Williams PA, Zhong F. Niosomes Consisting of Tween-60 and Cholesterol Improve the Chemical Stability and Antioxidant Activity of (−)-Epigallocatechin Gallate under Intestinal Tract Conditions. J Agric Food Chem. 2016; 64: 9180–9188. DOI: 10.1021/acs.jafc.6b04147.
Lim SB, Banerjee A, Önyüksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J Control Release. 2012; 163: 34-45. DOI: 10.1016/j.jconrel.2012.06.002.
Lim WH, Lawrence MJ. Influence of surfactant and lipid chain length on the solubilisation of phosphatidylcholine vesicles by micelles comprised of polyoxyethylene sorbitan monoesters. Colloids Surf A Physicochem Eng Asp. 2004; 250: 449-447. DOI: https://doi.org/10.1016/j.colsurfa.2004.06.042.
Marquardt D, Kucerka N, Wassall SR, Harroun TA, Katsaras J. Cholesterol’s location in lipid bilayers. Chem. Phys. Lipids. 2016; 199: 17-25. DOI: 10.1016/j.chemphyslip.2016.04.001.
Matsuki H, Kato K, Okamoto H, Yoshida S, Goto M, Tamai N, Kaneshina S. Ligand partitioning into lipid bilayer membranes under high pressure: Implication of variation in phase-transition temperatures. Chem Phys Lipids. 2017; 209: 9-18. DOI: 10.1016/j.chemphyslip.2017.10.002.
Mertins O, Sebben M, Schneider PH, Pohlmann AR, Silveira NP. Characterization of soybean phosphatidylcholine purity by 1H and 31P NMR. Quím nova. 2008; 31: 1856-1859. DOI: http://dx.doi.org/10.1590/S0100-40422008000700043.
Mitchell NJ, Seaton P, Pokorny A. Branched phospholipids render lipid vesicles more susceptible to membrane-active peptides. Biochim Biophys Acta. 2016; 1858: 988–994. DOI: 10.1016/j.bbamem.2015.10.014.
Moghddam SRM, Ahad A, Aqil M, Imam SS, Sultana Y. Formulation and optimization of niosomes for topical diacerein delivery using 3-factor, 3-level Box-Behnken design for the management of psoriasis. Mater Sci Eng. 2016; 69: 789–797. DOI: 10.1016/j.msec.2016.07.043.
Morini MA, Sierra MB, Pedroni VI, Alarcon LM, Appignanesi GA, Disalvo EA. Influence of temperature, anions and size distribution on the zeta potential of DMPC, DPPC and DMPE lipid vesicles. Colloids Surf B Biointerfaces. 2015; 131: 54-58. DOI: 10.1016/j.colsurfb.2015.03.054.
Müller LK, Landfester K. Natural liposomes and synthetic polymeric structures for biomedical applications. Biochem Biophys Res Commun. 2015; 468: 411-418. DOI: 10.1016/j.bbrc.2015.08.088.
Ojeda E, Agirre M, Villate-Beitia I, Mashal M, Puras G, Zarate J, Pedraz JL. Elaboration and Physicochemical Characterization of Niosome-Based Nioplexes for Gene Delivery Purposes. Methods Mol Bio. 2016; 1445: 63-75. DOI: 10.1007/978-1-4939-3718-9_5.
Pando D, Matos M, Gutiérrez G, Pazos C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf B Biointerfaces. 2015; 128: 398-404. 10.1016/j.colsurfb.2015.02.037.
Perrier DL, Rems L, Boukany PE. Lipid vesicles in pulsed electric fields: Fundamental principles of the membrane response and its biomedical applications. Adv Colloid Interface Sci. 2017; 249: 248-271. DOI: 10.1016/j.cis.2017.04.016.
Pleguezuelos-Villa M, Mir-Palomo S, Díez-Sales O, Buso MAOV, Sauri AR, Nácher A. Novos lipossomas ultra-formuláveis de Naringina para terapia anti-inflamatória. Colloids Surf B Biointerfaces. 2018; 162: 265-270. DOI: https://doi.org/10.1016/j.colsurfb.2017.11.068.
Poh S, Chelvam V, Ayala-López W, Putt KS, Low PS. Selective liposome targeting of folate receptor positive immune cells in inflammatory diseases. Nanomedicine. 2018; 14: 1033-1043. DOI: https://doi.org/10.1016/j.nano.2018.01.009.
Quinn PJ. The effect of tocopherol on the structure and permeability of phosphatidylcholine liposomes. J Controlled Release. 2012; 160: 158-163. 10.1016/j.jconrel.2011.12.029.
Rahnfeld L, Thamm J, Steiniger F, Hoogevest PV, Luciani P. Study on the in situ aggregation of liposomes with negatively chargedphospholipids for use as injectable depot formulation. Colloids Surf B Biointerfaces. 2018; 1-8. DOI: 10.1016/j.colsurfb.2018.02.023.
Ravaghi M, Razavi SH, Mousavi SM, Sinico C, Fadda AM. Stabilization of natural canthaxanthin produced by Dietzia natronolimnaea HS-1 by encapsulation in niosomes. LWT - Food Sci Technol. 2016; 73: 498-504. DOI: https://doi.org/10.1016/j.lwt.2016.06.027.
Raval A, Bahadur P, Raval A. Effect of nonionic surfactants in release media on accelerated in-vitro release profile of sirolimus eluting stents with biodegradable polymeric coating. J Pharmaceut Anal. 2018; 8: 45-54. DOI: https://doi.org/10.1016/j.jpha.2017.06.002.
Ribeiro MENP, Moura CL, Vieira MGS, Gramosa NV, Chaibundit C, Mattos MC, Attwood D, Yeates SG, Nixon SK, Ricardo NPS. Solubilisation capacity of Brij surfactants. Int. J. Pharm. 2012; 436: 631-635. DOI: 10.1016/j.ijpharm.2012.07.032.
Rudolphi-Skórska E, Filek M, Zembala M. The Effects of the Structure and Composition of the Hydrophobic Parts of Phosphatidylcholine-Containing Systems on Phosphatidylcholine Oxidation by Ozone. J Membr Biol. 2017; 250: 493–505. DOI: 10.1007/s00232-017-9976-8.
Saha S, Verma RJ. Molecular interactions of active constituents of essential oils in zwitterionic lipid bilayers. Chem. Phys. Lipids. 2018; 1: 1-38. DOI: 10.1016/j.chemphyslip.2018.03.008.
Samed N, Sharma V, Sundaramurthy A. Hydrogen Bonded Niosomes for Encapsulation and Release of Hydrophilic and Hydrophobic Anti-Diabetic Drugs: An Efficient System for Oral Anti-Diabetic Formulation. Appl Surf Sci. 2017; 423: 1-24. DOI: https://doi.org/10.1016/j.apsusc.2017.11.055.
Santos NC, Castanho MARB. Liposomes: has the magic bullet hit the target?. Quim Nova. 2002; 25: 1181-1185. DOI: http://dx.doi.org/10.1590/S0100-40422002000700019.
Shehata T, Kimura T, Higaki K, Ogawara HI. In-vivo disposition characteristics of PEG niosome and its interaction with serum proteins. Int J Pharm. 2016; 512: 322-328. DOI: 10.1016/j.ijpharm.2016.08.058.
Silva JDF, Silva YP, Piatnicki CMS, Böckel WJ, Mendonça CRB. Microemulsions: components, characteristics, potentialities in food chemistry and other Applications. Quím nova. 2015; 38: 1196- 1206. DOI: http://dx.doi.org/10.5935/0100-4042.20150135
Singh S. Liposome encapsulation of doxorubicin and celecoxib in combination inhibits progression of human skin cancer cells. Int J Nanomedicine. 2018; 13: 11-13. DOI: https://doi.org/10.2147/IJN.S124701.
Sohrabi S, Haeri A, Mahboubi A, Mortazavi A, Dadashzadeh S. Chitosan gel-embedded moxifloxacin niosomes: An efficient antimicrobial hybrid system for burn infection. Int. J Biol Macromol. 2016; 85: 625-633. DOI: 10.1016/j.ijbiomac.2016.01.013.
Somjid S, Krongsuk S, Johns JR. Cholesterol concentration effect on the bilayer properties and phase formation of niosome bilayers: A molecular dynamics simulation study. J Mol Liq. 2018; 256: 591-598. DOI: https://doi.org/10.1016/j.molliq.2018.02.077.
Sun Y, Dai C, Yin M, Lu J, Hu H, Chen D. Hepatocellular carcinoma-targeted effect of configurations and groups of glycyrrhetinic acid by evaluation of its derivative-modified liposomes. Int J Nanomedicine. 2018; 13: 1621–1632. DOI: https://doi.org/10.2147/IJN.S153944.
Suzuki A, Kuroiwa T, Horikoshi K, Kanazawa A, Ichikawa S. Freeze-dryable lipid vesicles with size tunability and high encapsulation efficiency prepared by the multiple emulsification-solvent evaporation method. Colloids Surf B Biointerfaces. 2017; 159: 412-418. DOI: 10.1016/j.colsurfb.2017.07.092.
Tagami T, Ernsting MJ, Li SD. Optimization of a novel and improved thermosensitive liposome formulated with DPPC and a Brij surfactant using a robust in vitro system. J Controlled Release. 2011; 154: 290-297. DOI: 10.1016/j.jconrel.2011.05.020.
Tahara K, Kobayashi M, Yoshida S, Onodera R, Inoue N, Takeuchi H. Effects of cationic liposomes with stearylamine against virus infection. Int J Pharm. 2018; 1: 1-30. DOI: 10.1016/j.ijpharm.2018.04.001.
Thomas AH, Catalá A, Vignoni M. Soybean phosphatidylcholine liposomes as model membranes to study lipid peroxidation photoinduced by pterin. Biochim Biophys Acta. 2016; 1858: 139–1450. DOI: 10.1016/j.bbamem.2015.11.002.
Toniazzo T, Peres MS, Ramos AP, Pinho SC. Encapsulation of quercetin in liposomes by ethanol injection and physicochemical characterization of dispersions and lyophilized vesicles. Food Biosci. 2017; 19: 17-25. DOI: https://doi.org/10.1016/j.fbio.2017.05.003.
Wang JP, Pan Y, Li P, Zhao GJ. Photophysical investigation of methyl 2-hydroxy-3-naphthoate (MHN23) in different self-organized supramolecular assemblies of micelles and niosomes formed by nonionic surfactante. J Alloys Compd. 2016; 686: 656-661. DOI: https://doi.org/10.1016/j.jallcom.2016.05.283.
Wang WX, Feng SS, Zheng CH. A comparison between conventional liposome and drug-cyclodextrin complex in liposome system. Int J Pharm. 2016; 513: 387–392. DOI: 10.1016/j.ijpharm.2016.09.043.
Wang X, Gao Y. Effects of length and unsaturation of the alkyl chain on the hydrophobic binding of curcumin with Tween micelles. Food Chem. 2018; 246: 242-248. DOI: https://doi.org/10.1016/j.foodchem.2017.11.024.
Yeom S, Shin BS, Han S. An electron spin resonance study of non-ionic surfactant vesicles (niosomes). Chem Phys Lipids. 2014; 181: 83-89. DOI: 10.1016/j.chemphyslip.2014.03.004.
Yingyuad P, Sinthuvanich C, Leepasert T, Thongyoo P, Boonrungsiman S. Preparation, characterization and in vitro evaluation of calothrixin B liposomes. J Drug Deliv Sci Technol. 2018; 44: 491-497. DOI: https://doi.org/10.1016/j.jddst.2018.02.010.
Zhao M, Zhao M, Fu C, Yu Y, Fu A. Targeted therapy of intracranial glioma model mice with curcumin nanoliposomes. Int J Nanomedicine. 2018; 13: 1601–1610. DOI: 10.2147/IJN.S157019.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Jorge Ederson Gonçalves Santana, Cícera Datiane de Morais Oliveira-Tintino, Gabriel Gonçalves Alencar, Gustavo Miguel Siqueira, Daniel Sampaio Alves, Talysson Felismino Moura, Saulo Relison Tintino, Irwin Rose Alencar de Menezes, João Pedro Viana Rodrigues, Vanessa Barbosa Pinheiro Gonçalves, Roberto Nicolete, Talha Bin Emran, Clara Mariana Gonçalves Lima, Sheikh F. Ahmad, Henrique Douglas Melo Coutinho, Teresinha Gonçalves da Silva. (2023). Comparative Antibacterial and Efflux Pump Inhibitory Activity of Isolated Nerolidol, Farnesol, and α-Bisabolol Sesquiterpenes and Their Liposomal Nanoformulations. Molecules, 28(22), p.7649. https://doi.org/10.3390/molecules28227649.
2. Karuppiah Nagaraj. (2025). Nanoparticle and Gene Therapy Strategies for Site‐Specific Pain Management: A Review. Advanced Therapeutics, 8(12) https://doi.org/10.1002/adtp.202500356.
3. Phuvamin Suriyaamporn, Chaiyakarn Pornpitchanarong, Thapakorn Charoenying, Koranat Dechsri, Tanasait Ngawhirunpat, Praneet Opanasopit, Boonnada Pamornpathomkul. (2025). Artificial intelligence-driven hydrogel microneedle patches integrating 5-fluorouracil inclusion complex-loaded flexible pegylated liposomes for enhanced non-melanoma skin cancer treatment. International Journal of Pharmaceutics, 669, p.125072. https://doi.org/10.1016/j.ijpharm.2024.125072.
4. Sara Pozos-Nonato, Clara Luisa Domínguez-Delgado, Kenia Areli Campos-Santander, Allyson Amelia Benavides, Sandy María Pacheco-Ortin, Rosa Isabel Higuera-Piedrahita, Guillermo Resendiz-González, Eva María Molina-Trinidad. (2023). Novel Nanotechnological Strategies for Skin Anti-aging. Current Pharmaceutical Biotechnology, 24(11), p.1397. https://doi.org/10.2174/1389201024666221223095315.
5. Melissa Garcia-Carrasco, Itzel F. Parra-Aguilar, Erick P. Gutiérrez-Grijalva, Angel Licea-Claverie, J. Basilio Heredia. (2022). Food, Medical, and Environmental Applications of Nanomaterials. , p.473. https://doi.org/10.1016/B978-0-12-822858-6.00017-0.
6. Weijie Liu, Yixuan Wang, Xu Zhao. (2026). Efficient non-viral siRNA carriers for triple-negative breast cancer: advances in 2020 − 2025. Materials & Design, 262, p.115506. https://doi.org/10.1016/j.matdes.2026.115506.
7. Isabela Santos Lopes, Norma Lucía Buriticá Zuluaga, Iolanda Midea Cuccovia, Marcia Regina Franzolin, Beatriz Fuzinato dos Santos, Felipe Wodtke, Mariana P. Darbem, Alcindo A. Dos Santos, Lilia Coronato Courrol. (2024). Interactions between gamma-aminobutyric capped silver nanoparticles and large unilamellar vesicles (LUVs) and their antimicrobial activities. Journal of Drug Delivery Science and Technology, 101, p.106165. https://doi.org/10.1016/j.jddst.2024.106165.
8. Mohit Kumar, Puja Keshwania, Shruti Chopra, Syed Mahmood, Amit Bhatia. (2023). Therapeutic Potential of Nanocarrier-Mediated Delivery of Phytoconstituents for Wound Healing: Their Current Status and Future Perspective. AAPS PharmSciTech, 24(6) https://doi.org/10.1208/s12249-023-02616-6.
9. Naiyer Shahzad, Abdullah R. Alzahrani, Ibrahim Abdel Aziz Ibrahim, Imran Shahid, Ibrahim M. Alanazi, Alaa Hisham Falemban, Mohammad Tarique Imam, Nehal Mohsin, Mohd Fahami Nur Azlina, Palanisamy Arulselvan. (2024). Therapeutic strategy of biological macromolecules based natural bioactive compounds of diabetes mellitus and future perspectives: A systematic review. Heliyon, 10(2), p.e24207. https://doi.org/10.1016/j.heliyon.2024.e24207.
10. Hammad Alam, Vartika Srivastava, Aijaz Ahmad. (2022). Nanotechnology for Infectious Diseases. , p.345. https://doi.org/10.1007/978-981-16-9190-4_16.
11. Akshay Kumar, Rajesh Gautam, Vir Vikram. (2025). Nanovesicular Carriers in Medicine: The Rise of Spanlastics for Targeted Drug Delivery in Dermatological Therapy. Micro and Nanosystems, 17(3), p.182. https://doi.org/10.2174/0118764029343409241217043736.
12. Zdravka Slavkova, Denitsa Yancheva, Julia Genova. (2024). Phase behaviour and structural properties of SOPC model lipid system in a sucrose solution. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 304, p.123287. https://doi.org/10.1016/j.saa.2023.123287.
13. Bhavana Raj, Harika Sapa, Shona S Shaji, Kaladhar Kamalasanan. (2025). Biomimetic niosomal versus liposomal nanoparticle-based aspirin injection for treating stroke and myocardial infarction. Journal of Biomaterials Applications, 39(8), p.952. https://doi.org/10.1177/08853282241307908.
14. Alaa S. Eita, Amna M.A. Makky, Asem Anter, Islam A. Khalil. (2022). Atorvastatin-loaded emulsomes foam as a topical antifungal formulation. International Journal of Pharmaceutics: X, 4, p.100140. https://doi.org/10.1016/j.ijpx.2022.100140.
15. Samruddhi Kulkarni, Bala Prabhakar, Pravin Shende. (2022). Stabilization of lipid vesicles: Upcoming strategic insights for product development. Journal of Molecular Liquids, 348, p.118430. https://doi.org/10.1016/j.molliq.2021.118430.
16. Husna Zolkepli, Riyanto Teguh Widodo, Syed Mahmood, Norazlinaliza Salim, Khalijah Awang, Noraini Ahmad, Rozana Othman. (2022). A Review on the Delivery of Plant-Based Antidiabetic Agents Using Nanocarriers: Current Status and Their Role in Combatting Hyperglycaemia. Polymers, 14(15), p.2991. https://doi.org/10.3390/polym14152991.
17. Nermin M. Sheta, Shady M. Abd El-Halim, Sally A. Fahim, Marwa Sharaky, Alaadin E. El-Haddad, Mohamed A. Mamdouh, Sara M. Soliman. (2024). Xanthone-loaded niosomes as innovative potential delivery platform: Formulation, characterization, and cytotoxic effect on human cancer cell lines. Journal of Drug Delivery Science and Technology, 102, p.106384. https://doi.org/10.1016/j.jddst.2024.106384.
18. María Celina Sánchez-Cerviño, Codrin Paul Fuioaga, Leonard Ionut Atanase, Gustavo A. Abraham, Guadalupe Rivero. (2023). Electrohydrodynamic Techniques for the Manufacture and/or Immobilization of Vesicles. Polymers, 15(4), p.795. https://doi.org/10.3390/polym15040795.
19. Sunita Dahiya, Rajiv Dahiya. (2022). Nanocosmeceuticals. , p.169. https://doi.org/10.1016/B978-0-323-91077-4.00002-8.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2020 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).



















