Publicado
Transición epitelio-mesénquima inducida por virus
Epithelial-to-Mesenchymal Transition Induced by Virus
TRANSIÇÃO EPITÉLIO-MESÉNQUIMA INDUZIDA PELO VÍRUS
DOI:
https://doi.org/10.15446/abc.v26n1.79358Palabras clave:
EMT, microRNAs, Patogénesis viral, Plasticidad celular, Relación Virus-Hospedero (es)Cellular plasticity, EMT, microRNAs, Viral pathogenesis, Virus-Host relationship (en)
Descargas
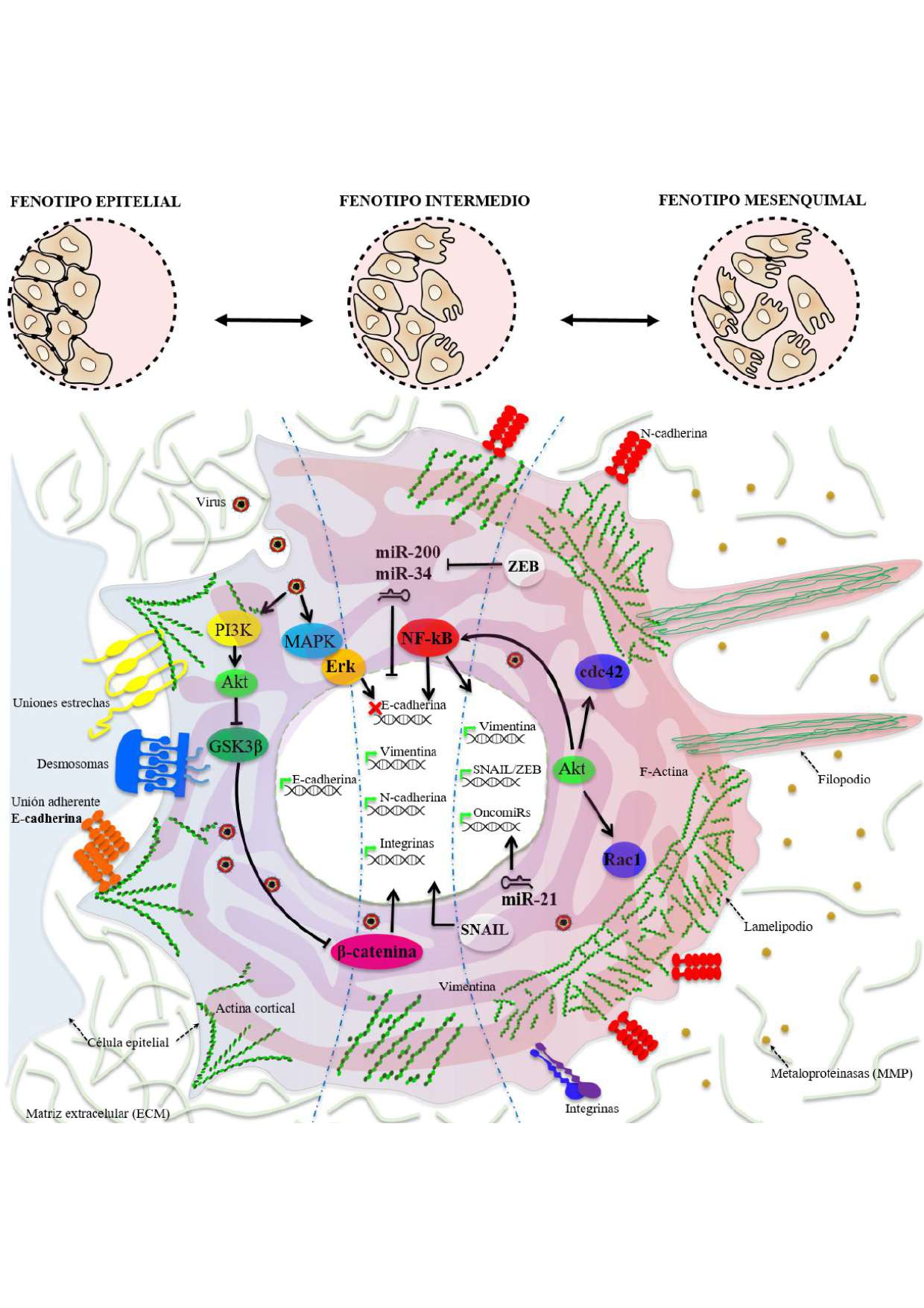
La Transición Epitelio-Mesénquima (EMT) es un proceso de dediferenciación altamente conservado en vertebrados. Este ocurre en células epiteliales con la activación progresiva de la pérdida de la polaridad, la adquisición de motilidad individual y la capacidad invasiva a otros tejidos. La EMT es un proceso normal durante el desarrollo; no obstante, en condiciones patológicas está relacionada con la inducción de metástasis, lo cual representa una vía alterna al desarrollo de procesos oncogénicos tempranos. Aunque la EMT es activada principalmente por factores de crecimiento, también se puede desencadenar por infecciones de patógenos intracelulares mediante la activación de rutas moleculares inductoras de este proceso. Por lo tanto, una infección bacteriana o viral pueda generar predisposición al desarrollo de tumores. Nuestro interés está enfocado principalmente encaracterizar la relación virus-hospedero, y en el caso de los virus, varios ya se han descrito como inductores de la EMT. En este artículo de revisión se describenelfenómeno de la plasticidad celular y la ocurrencia detallada del proceso de EMT, los patógenos virales reportados como inductores, los mecanismos moleculares usados para ello y las vías de regulación mediante miRNAs. Por último, se discute cómo esta relación virus-hospedero puede explicar la patogénesis de la enfermedad causada por Dengue virus, favoreciendo la identificación de blancos moleculares para terapia, estrategia conocida como Antivirales dirigidos a blancos celulares o HTA (Host-targeting antivirals).
A transição epitélio-mesenquimal (EMT) é um processo de desdiferenciação altamente conservado em vertebrados. Isso ocorre em células epiteliais que, após certos estímulos, ativam a perda de polaridade, a aquisição da motilidade individual e a capacidade invasiva de outros tecidos. A EMT é um processo normal durante o desenvolvimento, no entanto, em condições patológicas está relacionada à indução de metástase, representando um caminho alternativo para o desenvolvimento de processos oncogênicos precoces. Embora a EMT seja principalmente ativada por fatores de crescimento, ela também pode ser desencadeada por infecções por patógenos intracelulares, ativando vias moleculares que induzem esse processo. Portanto, uma infecção bacteriana ou viral pode gerar predisposição ao desenvolvimento de tumores. Nosso interesse é principalmente focado em caracterizar a relação vírus-hospedeiro e, no caso de vírus, vários já foram descritos como indutores de EMT. Neste artigo de revisão, são descritos o fenômeno da plasticidade celular e a ocorrência detalhada do processo de EMT. Também descrito em detalhe a ocorrência deste processo, os agentes patogénicos virais relatados como indutores, os mecanismos moleculares utilizados para os mesmos, e por vias reguladoras miARNs. Finalmente, discute-se como esta relação vírus-hospedeiro podem explicar a patogénese da doença causada pelo vírus do dengue, favorecendo a identificação dos alvos moleculares para a terapêutica, conhecida como antivirais segmentação alvos celulares ou estratégia HBP (antivirais-direccionamento hospedeiras).
Referencias
Ahodantin J, Lekbaby B, Bou Nader M, Soussan P, Kremsdorf D. Hepatitis B virus X protein enhances the development of liver fibrosis and the expression of genes associated with epithelial-mesenchymal transitions and tumor progenitor cells. Carcinogenesis. 2019; 41(3):358–367. Doi:https://doi.org/10.1093/carcin/bgz109
Álvarez-Díaz DA, Gutiérrez-Díaz AA, Orozco-García E, Puerta-Gonzáles A, Bermúdez-Santana CI, Gallego-Gómez JC. Dengue virus potentially promotes migratory responses on endothelial cells by enhancing pro-migratory soluble factors and miRNAs. Virus Res. 2019;259:68–76. Doi:https://doi.org/10.1016/j.virusres.2018.10.018
ATCC. American Type Culture Collection: The Global Bioresource Center. ATCC. 1925. Disponible en: www.atcc.org/Products/All/CRL-3243.aspx. Citado:10 Abr 2018
Barriere G, Fici P, Gallerani G, Fabbri F, Rigaud M. Epithelial Mesenchymal Transition: A double-edged sword. Clin Transl Med. 2015;4(14):1–6. Doi:https://doi.org/10.1186/s40169-015-0055-4
Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, et al. Plasticity of the differentiated state. Science. 1985;230:758–766. Doi:https://doi.org/10.1126/science.2414846
Bloom W. Cellular differentiation and tissue culture. Physiol Rev. 1937;(149),589-617. Doi:https://doi.org/10.1152/physrev.1937.17.4.589
Bose SK, Meyer K, Di Bisceglie AM, Ray RB, Ray R. Hepatitis C virus induces epithelial-mesenchymal transition in primary human hepatocytes. J Virol. 2012;86(24):13621–13628. Doi:https://doi.org/10.1128/JVI.02016-12
Bullock MD, Sayan AE, Packham GK, Mirnezami AH. MicroRNAs: Critical regulators of epithelial to mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in cancer progression. Biol Cell. 2012;104(1):3–12. Doi:https://doi.org/10.1111/boc.201100115
Chargui A, Sanda M, Brest P, Hofman P, Vouret-Craviari V. Epithelial to mesenchymal transition in microbial pathogenesis. In: Hamilton G, editor. Cytokeratins Tools in Oncology. Niza, Francia: IntechOpen; 2011. p. 35-54. Doi:https://doi.org/10.5772/34135
Chen X, Bode AM, Dong Z, Cao Y. The epithelial-mesenchymal transition (EMT) is regulated by oncoviruses in cancer. FASEB Journal, 2016;30(9):3001-3010. Doi:https://doi.org/10.1096/fj.201600388R
Chen YS, Mathias RA, Mathivanan S, Kapp EA, Moritz RL, Zhu HJ, et al. Proteomics profiling of madin-darby canine kidney plasma membranes reveals Wnt-5a involvement during oncogenic H-Ras/TGFβ-mediated epithelial-mesenchymal transition. Mol Cell Proteomics. 2011;10:1-15. Doi:https://doi.org/10.1074/mcp.M110.001131
Clarke TB, Francella N, Huegel A, Weiser JN. Invasive bacterial pathogens exploit TLR-mediated downregulation of tight junction components to facilitate translocation across the epithelium. Cell Host Microbe. 2011;9(5)404–414. Doi:https://doi.org/10.1016/j.chom.2011.04.012
Cuartas-López AM, Hernández-Cuellar CE, Gallego-Gómez JC. Disentangling the role of PI3K/Akt, Rho GTPase and the actin cytoskeleton on dengue virus infection. Virus Res. 2018;256:153–165. Doi:https://doi.org/10.1016/j.virusres.2018.08.013
Cullen BR. Viral and cellular messenger RNA targets of viral microRNAs. Nature. 2009;457(7228):421-425. Doi:https://doi.org/10.1038/nature07757
Cursons J, Pillman KA, Scheer KG, Gregory PA, Foroutan M, Hediyeh-Zadeh S, et al. Combinatorial targeting by microRNAs co-ordinates post-transcriptional control of EMT. Cell Systems. 2018;7(1),77–91. Doi:https://doi.org/10.1016/j.cels.2018.05.019
de Oliveira DE, Müller-Coan BG, Pagano JS. Viral carcinogenesis beyond malignant transformation: EBV in the progression of human cancers. Trends Microbiol. 2016;24(8):649–664. Doi:https://doi.org/10.1016/j.tim.2016.03.008
Den Boon JA, Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu Rev Microbiol. 2010;64:241–256. Doi:https://doi.org/10.1146/annurev.micro.112408.134012
Dong P, Xiong Y, Watari H, Hanley SJ, Konno Y, Ihira K, et al. miR-137 and miR-34a directly target SNAIL and inhibit EMT, invasion and sphere-forming ability of ovarian cancer cells. J Exp Clin Cancer Res. 2016;35(1):1–9. Doi:https://doi.org/10.1186/s13046-016-0415-y
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69-84. Doi:https://doi.org/10.1038/s41580-018-0080-4
Dorey K, Amaya E. FGF signalling: Diverse roles during early vertebrate embryogenesis. Development. 2010;137(22):3731–3742. Doi:https://doi.org/10.1242/dev.037689
Garcia AI, Buisson M, Bertrand P, Rimokh R, Rouleau E, Lopez BS, et al. Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. EMBO Mol Med. 2011;3(5):279–290. Doi:https://doi.org/10.1002/emmm.201100136
Gasperini P, Espigol-Frigole G, McCormick PJ, Salvucci O, Maric D, Uldrick TS, et al. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through notch-dependent signaling. Cancer Res. 2012;72(5):1157–1169. Doi:https://doi.org/10.1158/0008-5472
Gaur N, Tikla T, Kaul R. Kaposi sarcoma-associated herpes virus (KSHV) latent protein LANA modulates cellular genes associated with epithelial-to-mesenchymal transition. Arch Virol. 2019;164(1):91-104. Doi:https://doi.org/10.1007/s00705-018-4060-y
Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):1–17. Doi:https://doi.org/10.1126/scisignal.2005189
Gumbiner B. Generation and maintenance of epithelial cell polarity. Curr Opin Cell Biol. 1990;2(5):881–887. Doi:https://doi.org/10.1016/0955-0674(90)90087-U
Hall K, Ran S. Regulation of tumor angiogenesis by the local environment. Front Biosci. 2010;15:195-212. Doi:https://doi.org/10.2741/3615
Hao J, Zhang Y, Deng M, Ye R, Zhao S, Wang Y, et al. MicroRNA control of epithelial-mesenchymal transition in cancer stem cells. Int J Cancer. 2014;135(5):1019–1027. Doi:https://doi.org/10.1002/ijc.28761
Hay ED. An overview of epithelia-mesenchymal transformation. Acta Anat (Basel). 1995;154(1):8-20. Doi:https://doi.org/10.1159/000147748
Hay ED. EMT Concept and Examples from the Vertebrate Embryo. In: Savagner P, editor. Rise and Fall of Epithelial Phenotype: Concepts of epithelial-mesenchymal transition. New York: Plenum Publishers; 2005. 11 p.
He M, Zhang W, Bakken T, Schutten M, Toth Z, Jung JU, et al. Cancer angiogenesis induced by Kaposi Sarcoma-associated herpesvirus is mediated by EZH2. Cancer Res. 2012;72(14):3582–92. Doi:https://doi.org/10.1158/0008-5472.CAN-11-2876
Hofman P, Vouret-Craviari V. Microbes-induced EMT at the crossroad of inflammation and cancer. Gut Microbes. 2012;3(3):176-185. Doi:https://doi.org/10.4161/gmic.20288
Hu B, Xie S, Hu Y, Chen W, Chen X, Zheng Y, et al. Hepatitis C virus NS4B protein induces epithelial-mesenchymal transition by upregulation of SNAIL. Virol J. 2017;14(1):1–9. Doi:https://doi.org/10.1186/s12985-017-0737-1
Hu Z, Dong J, Wang LE, Ma H, Liu J, Zhao Y et al. Serum microRNA profiling and breast cancer risk: the use of miR-484/191 as endogenous controls. Carcinogenesis. 2012;33(4):828–834. Doi:https://doi.org/10.1093/carcin/bgs030
International Society of Differentiation. 2019. Disponible en: www.isdifferentiation.org/?page_id=10#history. Citado: 18 Abr 2019.
Jhan MK, Tsai TT, Chen CL, Tsai CC, Cheng YL, Lee YC, et al. Dengue virus infection increases microglial cell migration. Sci Rep. 2017;7(1),1–11. Doi:https://doi.org/10.1038/s41598-017-00182-z
Junqueira LC, Carneiro J. Histología básica: texto y atlas. 12 ed. Bogotá: Editorial Médica Panamericana S.A.; 2015. 556 p.
Kardong KV. Life History. In: Vertebrates: Comparative Anatomy, Function, Evolution 6 ed. Washington: McGraw Hill; 2011. p. 161–211.
Karim SM, Liu L, Le TD, Li J. Identification of miRNA-mRNA regulatory modules by exploring collective group relationships. BMC Genomics. 2016;17(1):72–84. Doi:https://doi.org/10.1186/s12864-015-2300-z
Kindrachuk J, Wahl-Jensen V, Safronetz D, Trost B, Hoenen T, Arsenault R, et al. Ebola virus modulates transforming growth factor b signaling and cellular markers of mesenchyme-like transition in hepatocytes. J Virol. 2014;88:9877–92. Doi:https://doi.org/10.1128/JVI.01410-14
Kraft A, Rubin BP. Changing cells: An analysis of the concept of plasticity in the context of cellular differentiation. BioSocieties. 2016;11(4):497–525. Doi:https://doi.org/10.1057/s41292-016-0027-y
Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGFβ-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci. 2012;125:1259–1273. Doi:https://doi.org/10.1242/jcs.095299
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. Doi:https://doi.org/10.1038/nrm3758
Leidinger P, Backes C, Dahmke IN, Galata V, Huwer H, Stehle I, et al. What makes a blood cell based miRNA expression pattern disease specific? A miRNome analysis of blood cell subsets in lung cancer patients and healthy controls. Oncotarget. 2014;5:9484–9497. Doi:https://doi.org/10.18632/oncotarget.2419
Lin C, Zong J, Lin W, Wang M, Xu Y, Zhou R, et al. EBV-miR-BART8-3p induces epithelial-mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma cells through activating NF-κB and Erk1/2 pathways. J Exp Clin Cancer Res, 2018; 37(1)283. Doi:https://doi.org/10.1186/s13046-018-0953-6
Luria SE, Darnell JE, Baltimore D, Campbell A. General virology. New York: Wiley. 1953. 578 p.
Mayor R, Etienne-Manneville S. The front and rear of collective cell migration. Nat Rev Mol Cell Biol. 2016;17(2):97-109. Doi:https://doi.org/10.1038/nrm.2015.14
Moustakas A, Heldin P. TGFβ and matrix-regulated epithelial to mesenchymal transition. Biochim Biophys Acta. 2014;1840(8):2621–2634. Doi:https://doi.org/10.1016/j.bbagen.2014.02.004
National Academy of Sciences. 2016. Disponible en: www.nasonline.org/member-directory/members/3000435.html. Citado: 18 Abr 2019.
Nieto MA. The Ins and Outs of the Epithelial to Mesenchymal Transition in Health and Disease. Annu Rev Cell DevBiol. 2011;27:347–376. Doi:10.1146/annurev-cellbio-092910-154036. Doi:https://doi.org/10.1146/annurev-cellbio-092910-154036
Nieto MA. Context-specific roles of EMT programmes in cancer cell dissemination. Nat Cell Biol. 2017;19(5):416–418. Doi:https://doi.org/10.1038/ncb3520
Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21–45. Doi:https://doi.org/10.1016/j.cell.2016.06.028
Ospina-Bedoya M, Campillo-Pedroza N, Franco-Salazar JP, Gallego-Gómez JC. Computational Identification of Dengue Virus MicroRNA-Like Structures and their Cellular Targets. Bioinform Biol Insights. 2014;8:169–176. Doi:https://doi.org/10.4137/BBI.S13649
Park GB, Kim D, Kim YS, Kim S, Lee HK, Yang JW, et al. The Epstein-barr virus causes epithelial-mesenchymal transition in human corneal epithelial cells via Syk/Src and Akt/Erk signaling pathways. Invest Ophthalmol Vis Sci. 2014;55(3): 1770–1779. Doi:https://doi.org/10.1167/iovs.13-12988
Qi X, Zhang L, Lu X. New insights into the epithelial-to-mesenchymal transition in cancer. Trends Pharmacol Sci. 2016;37(4):246-248. Doi:https://doi.org/10.1016/j.tips.2016.01.002
Qin Z, He W, Tang J, Ye Q, Dang W, Lu Y, et al. MicroRNAs provide feedback regulation of transition induced by growth factors. J Cell Physiol. 2016;231(1):120–129. Doi:https://doi.org/10.1002/jcp.25060
Rawal P, Siddiqui H, Hassan M, Chaudhary MC, Tripathi DM, Nain V, et al. Endothelial cell-derived TGF-β Promotes Epithelial-Mesenchymal Transition via CD133 in HBx-infected Hepatoma cells. Front Oncol. 2019; 9, 308. Doi:https://doi.org/10.3389/fonc.2019.00308
Ren Y, Zhou X, Yang JJ, Liu X, Zhao XH, Wang QX, et al. AC1MMYR2 impairs high dose paclitaxel-induced tumor metastasis by targeting miR-21/CDK5 axis. Cancer Lett. 2015;362(2):174–182. Doi:https://doi.org/10.1016/j.canlet.2015.03.038
Roa-Linares VC, Gallego-Gómez JC. La pérdida de función de la quinasa dependiente de ciclina 5 (CDK5) altera el citoesqueleto y reduce la infección in vitro por DENV-2. Acta biol. Colomb. 2019;24(3):474-485. Doi:http://dx.doi.org/10.15446/abc.v24n3.79347
Rodriguez-Boulan E, Kreitzer G, Müsch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6(3):233–247. Doi:https://doi.org/10.1038/nrm1593
Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245(4919):718–725. Doi:https://doi.org/10.1126/science.2672330
Sayce AC, Miller JL, Zitzmann N. Targeting a host process as an antiviral approach against dengue virus. Trends Microbiol. 2010;18(7):323-330. Doi:https://doi.org/10.1016/j.tim.2010.04.003
Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans. 2009;37(Pt4):918–925. Doi:https://doi.org/10.1042/BST0370918
Shi CS, Huang NN, Kehrl JH. Regulator of G-protein signaling 3 isoform 1 (PDZ-RGS3) enhances canonical Wnt signaling and promotes epithelial mesenchymal transition. J Biol Chem. 2012;287(40):33480-87. Doi:https://doi.org/10.1074/jbc.M112.361873
Shih W, Yamada S. N-cadherin as a key regulator of collective cell migration in a 3D environment. Cell Adh Migr. 2012;6(6):513-517. Doi:https://doi.org/10.4161/cam.21766
Skalsky RL, Corcoran DL, Gottwein E, Frank CL, Kang D, Hafner M et al. The viral and cellular microRNA targetome in lymphoblastoid cell lines. PLoS Pathog. 2012;8(1):1–21. Doi:https://doi.org/10.1371/journal.ppat.1002484
Stewart C. Differentiation. Science Direct. International Society of Differentiation; 1973 ISSN:0301-4681 Supports open access. 95 p. Disponible en: www.sciencedirect.com/journal/differentiation. Citado: 18 Abr 2019.
Talbot LJ, Bhattacharya SD, Kuo PC. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol. 2012;3(2):117–136.
Tang YT, Huang YY, Li JH, Qin SH, Xu Y, An TX, et al. Alterations in exosomal miRNA profile upon epithelial-mesenchymal transition in human lung cancer cell lines. BMC Genomics. 2018;19(1):802. Doi:https://doi.org/10.1186/s12864-018-5143-6
Tata PR, Rajagopal J. Cellular plasticity: 1712 to the present day. Curr Opin Cell Biol. 2016;43:46–54. Doi:https://doi.org/10.1016/j.ceb.2016.07.005
Thomas M, Banks L. Upsetting the balance: When viruses manipulate cell polarity control. J Mol Biol. 2018;430(19):3481–3503. Doi:https://doi.org/10.1016/j.jmb.2018.04.016
Tiwari I, Yoon MH, Park BJ, Jang KL. Hepatitis C virus core protein induces epithelial-mesenchymal transition in human hepatocytes by upregulating E12/E47 levels. Cancer Lett. 2015;362(1):131–138. Doi:https://doi.org/10.1016/j.canlet.2015.03.032
Tiwari N, Gheldof A, Tatari M, Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol. 2012;22(3):194–207. Doi:https://doi.org/10.1016/j.semcancer.2012.02.013
Trembley A. Mémoires pour servir à l’histoire d’un genre de polypes d’eau douce, à bras en forme de cornes. 1744. 324 p. Biodiversity Heritage Library (BHL). Disponible en: www.biodiversitylibrary.org/item/130183#page/1/mode/1up. Citado: 06 Sep 2019. DOI: https://doi.org/10.5962/bhl.title.64073
Vecchione A, Belletti B, Lovat F, Volinia S, Chiappetta G, Giglio S, et al. A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis. Proc Natl Acad Sci U S A. 2013;110(24):9845–9850. Doi:https://doi.org/10.1073/pnas.1305472110
Waddington CH. The strategy of the genes. United Kingdom: Routledge; 1957. 274 p. DOI: https://doi.org/10.1001/jama.1957.72980210019019b
Wang JY, Gao YB, Zhang N, Zou DW, Wang P, Zhu ZY, et al. miR-21 overexpression enhances TGFβ1-induced epithelial-to-mesenchymal transition by target SMAD7 and aggravates renal damage in diabetic nephropathy. Mol Cell Endocrinol. 2014;392(1-2):163–172. Doi:https://doi.org/10.1016/j.mce.2014.05.018
Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang KM, De W. Prognostic significance of serum miRNA-21 expression in human non-small cell lung cancer. J Surg Oncol. 2011;104(7):847–851. Doi:https://doi.org/10.1002/jso.22008
Welch-Reardon KM, Wu N, Hughes CC. A role for partial endothelial-mesenchymal transitions in angiogenesis? Arterioscler Thromb Vasc Biol. 2015;35(2):303-8. Doi:https://doi.org/10.1161/ATVBAHA.114.303220
WHO. World Health Organization. Special Programme for Research and Training in Tropical Diseases (TDR). Dengue guidelines for diagnosis, treatment, prevention and control. Geneva 2009. 211 p.
Wong IY, Javaid S, Wong EA, Perk S, Haber DA, Toner M, et al. Collective and individual migration following the epithelial–mesenchymal transition. Nat Mater. 2014;13(11):1063–1071. Doi:https://doi.org/10.1038/NMAT4062
Xia L, Dai L, Yu Q, Yang Q. Persistent transmissible gastroenteritis virus infection enhances enterotoxigenic Escherichia coli k88 adhesion by promoting epithelial-mesenchymal transition in intestinal epithelial cells. J Virol, 2017;91(21):31. Doi:https://doi.org/10.1128/JVI.01256-17
Ye X, Zhang HM, Qiu Y, Hanson PJ, Hemida MG, Wei W, et al. Coxsackievirus-induced miR-21 disrupts cardiomyocyte interactions via the downregulation of intercalated disk components. PLoS Pathog. 2014;10(4):1-19. Doi:https://doi.org/10.1371/journal.ppat.1004070
Zaravinos A. The regulatory role of microRNAs in EMT and cancer. J Oncol. 2015; 865816:1–13. Doi:https://doi.org/10.1155/2015/865816
Zearo S, Kim E, Zhu Y, Zhao JT, Sidhu SB, Robinson BG, et al. MicroRNA-484 is more highly expressed in serum of early breast cancer patients compared to healthy volunteers. BMC Cancer. 2014;14:1–7. Doi:https://doi.org/10.1186/1471-2407-14-200
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Clara Isabel Bermudez-Santana, Juan Carlos Gallego-Gómez. (2024). Toward a Categorization of Virus-ncRNA Interactions in the World of RNA to Disentangle the Tiny Secrets of Dengue Virus. Viruses, 16(5), p.804. https://doi.org/10.3390/v16050804.
2. Jenny Paola Alfaro-García, Carlos Alberto Orozco-Castaño, Julián Andrés Sánchez-Rendón, Herley Fernando Casanova-Yépes, Miguel Vicente-Manzanares, Juan Carlos Gallego-Gómez. (2025). Characterization of the Temporal Dynamics of the Endothelial–Mesenchymal-like Transition Induced by Soluble Factors from Dengue Virus Infection in Microvascular Endothelial Cells. International Journal of Molecular Sciences, 26(5), p.2139. https://doi.org/10.3390/ijms26052139.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2020 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).



















