Publicado
Lack of expression of hepatitis C virus core protein in human monocyte-erived dendritic cells using recombinant semliki forest virus
Ausencia de expresión de la proteína Core del virus de la hepatitis C en células dendríticas derivadas de monocitos humanos utilizando virus del Bosque de Semliki recombinante
DOI:
https://doi.org/10.15446/abc.v24n3.79368Palabras clave:
Dendritic cells, HCV, SFV, viral vector (en)Células dendríticas, SFV, VHC, vector viral (es)
Descargas
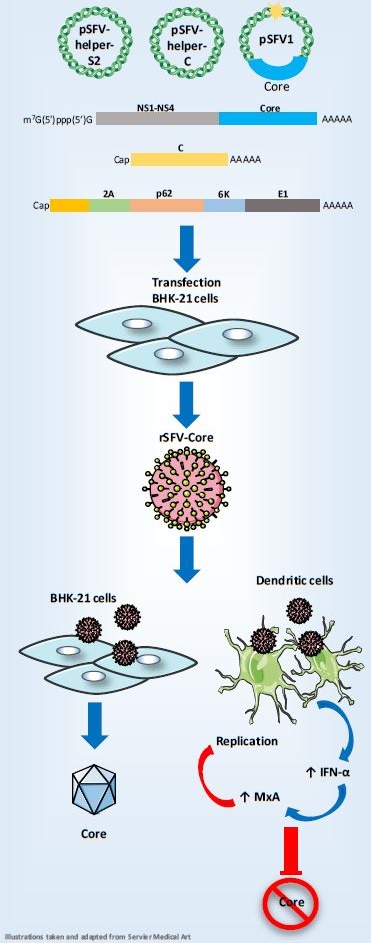
Hepatitis C Virus belongs to the Flaviviridae family. One proposed mechanism of HCV persistence in the ability to infect hematopoietic cells, including Dendritic cells (DCs). HCV infection of DCs could impair their functions that represent one of the mechanisms, thus hampering viral clearance by the host immune system. Among HCV-encoded proteins, the highly conserved Core protein has been suggested to be responsible for the immunomodulatory properties of this Hepacivirus. Recombinant viral vectors expressing the HCV Core protein and allowing its transduction and therefore the expression of the protein into DCs could be useful tools for the analysis of the properties of the Core protein. Vaccinia Virus and retrovirus have been used to transduce human DCs. Likewise, gene transfer into DCs using Semliki Forest Virus has been reported. This study aimed to express the HCV Core protein in human monocyte-derived DCs using an SFV vector, in which the subgenomic RNA encoding the structural proteins was replaced by the HCV Core sequence and then analyze the effects of its expression on DCs functions.
El virus de la Hepatitis C (VHC) pertenece a la familia Flaviviridae. Uno de los mecanismos propuestos de la persistencia del VHC es la capacidad de infectar células hematopoyéticas, incluidas las células dendríticas (DCs). La infección por VHC de DCs podría alterar sus funciones y corresponde a uno de los mecanismos que impiden el aclaramiento de la infección por VHC por el sistema inmunitario del hospedero. Entre las proteínas codificadas por el VHC, se ha sugerido que la proteína Core, altamente conservada, es responsable de las propiedades inmunomoduladoras de este Hepacivirus. Los vectores virales recombinantes que expresan la proteína Core y permiten su transducción a DCs podrían ser herramientas útiles para el análisis de las propiedades de esta proteína. El virus Vaccinia y el retrovirus se han utilizado para la transducción de DCs humanas. Del mismo modo, la transducción de DCs usando el virus del bosque de Semliki ha sido reportada. El objetivo de este estudio fue expresar la proteína Core de VHC en DCs derivadas de monocitos humanos utilizando un vector de SFV, en el que el ARN subgenómico que codifica las proteínas estructurales fue reemplazado por la secuencia Core del VHC y evaluar los efectos de su expresión en las funciones de DCs.
Referencias
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. Doi: https://doi.org/10.1038/32588
Berglund P, Fleeton MN, Smerdou C, Liljeström P. Immunization with recombinant Semliki Forest virus induces protection against influenza challenge in mice. Vaccine. 1999;17(5):497–507.
Brown M, Davies DH, Skinner MA, Bowen G, Hollingsworth SJ, Mufti GJ, et al. Antigen gene transfer to cultured human dendritic cells using recombinant avipoxvirus vectors. Cancer Gene Ther. 1999;6(3):238–245. Doi: https://doi.org/10.1038/sj.cgt.7700014
Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189(5):821–829. Doi: https://doi.org/10.1084/jem.189.5.821
Di Nicola M, Siena S, Bregni M, Longoni P, Magni M, Milanesi M, et al. Gene transfer into human dendritic antigen-presenting cells by vaccinia virus and adenovirus vectors. Cancer Gene Ther. 1998;5(6):350–356.
Dietz AB, Vuk-Pavlović S. High efficiency adenovirus-mediated gene transfer to human dendritic cells. Blood. 1998;91(2):392–398.
Faradji A, Bohbot A, Schmitt-Goguel M, Siffert JC, Dumont S, Wiesel ML, et al. Large scale isolation of human blood monocytes by continuous flow centrifugation leukapheresis and counterflow centrifugation elutriation for adoptive cellular immunotherapy in cancer patients. J Immunol Methods. 1994;174(1–2):297–309.
Fernández-Ponce C, Dominguez-Villar M, Muñoz-Miranda JP, Arbulo-Echevarria MM, Litrán R, Aguado E, et al. Immune modulation by the hepatitis C virus core protein. J Viral Hepat. 2017;24(5):350–356. Doi: https://doi.org/10.1111/jvh.12675
Frese M, Kochs G, Feldmann H, Hertkorn C, Haller O. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J Virol. 1996;70(2):915–923.
Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman RM. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol. 1998;72(4):2733–2737.
Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, et al. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186(6):801–812.
Hahn CS, Cho YG, Kang B-S, Lester IM, Hahn YS. The HCV Core Protein Acts as a Positive Regulator of Fas-Mediated Apoptosis in a Human Lymphoblastoid T Cell Line. Virology. 2000;276(1):127–137. Doi: https://doi.org/10.1006/viro.2000.0541
Henao LF, Cortés F, Navas MC. Semliki Forest Virus: a viral vector with multiple applications. Colombia Médica. 2007;38(2):159–169.
Johnston LJ, Halliday GM, King NJ. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J Invest Dermatol. 2000;114(3):560–568. Doi: https://doi.org/10.1046/j.1523-1747.2000.00904.x
Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162(9):5584–5591.
Knight SC. Mechanisms of retrovirally induced immunosuppression acting via dendritic cells. Adv Exp Med Biol. 1995;378:423–427. Doi: https://doi.org/10.1007/978-1-4615-1971-3_95
Kolykhalov AA, Agapov EV, Blight KJ, Mihalik K, Feinstone SM, Rice CM. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277(5325):570–574. Doi: https://doi.org/10.1126/science.277.5325.570
Landis H, Simon-Jödicke A, Klöti A, Di Paolo C, Schnorr JJ, Schneider-Schaulies S, et al. Human MxA protein confers resistance to Semliki Forest virus and inhibits the amplification of a Semliki Forest virus-based replicon in the absence of viral structural proteins. J Virol. 1998;72(2):1516–1522.
Large MK, Kittlesen DJ, Hahn YS. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J Immunol. 1999;162(2):931–938.
Laskus T, Radkowski M, Piasek A, Nowicki M, Horban A, Cianciara J, et al. Hepatitis C virus in lymphoid cells of patients coinfected with human immunodeficiency virus type 1: evidence of active replication in monocytes/macrophages and lymphocytes. J Infect Dis. 2000;181(2):442–448. Doi: https://doi.org/10.1086/315283
Lebon P, Ponsot G, Aicardi J, Goutières F, Arthuis M. Early intrathecal synthesis of interferon in herpes encephalitis. Biomedicine. 1979;31(9–10):267–271.
Liljeström P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (NY). 1991;9(12):1356–1361.
Liljeström P, Garoff H. Expression of proteins using Semliki Forest virus vectors. Curr Protoc Mol Biol. 2001;Chapter 16:Unit16.20. Doi: https://doi.org/10.1002/0471142727.mb1620s29
Macatonia SE, Cruickshank JK, Rudge P, Knight SC. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses. 1992;8(9):1699–1706. Doi: https://doi.org/10.1089/aid.1992.8.1699
Makino M, Wakamatsu S, Shimokubo S, Arima N, Baba M. Production of functionally deficient dendritic cells from HTLV-I-infected monocytes: implications for the dendritic cell defect in adult T cell leukemia. Virology. 2000;274(1):140–148. Doi: https://doi.org/10.1006/viro.2000.0445
Matsumoto M, Hsieh TY, Zhu N, VanArsdale T, Hwang SB, Jeng KS, et al. Hepatitis C virus core protein interacts with the cytoplasmic tail of lymphotoxin-beta receptor. J Virol. 1997;71(2):1301–1309.
Navas M-C, Fuchs A, Schvoerer E, Bohbot A, Aubertin A-M, Stoll-Keller F. Dendritic cell susceptibility to hepatitis C virus genotype 1 infection. J Med Virol. 2002;67(2):152–161. Doi: https://doi.org/10.1002/jmv.2204
Osterroth F, Garbe A, Fisch P, Veelken H. Stimulation of cytotoxic T cells against idiotype immunoglobulin of malignant lymphoma with protein-pulsed or idiotype-transduced dendritic cells. Blood. 2000;95(4):1342–1349.
Pavlovic J, Zürcher T, Haller O, Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990;64(7):3370–3375.
Ríos-Ocampo WA, Daemen T, Buist-Homan M, Faber KN, Navas M-C, Moshage H. Hepatitis C virus core or NS3/4A protein expression preconditions hepatocytes against oxidative stress and endoplasmic reticulum stress. Redox Report. 2019;24(1):17–26. Doi: https://doi.org/10.1080/13510002.2019.1596431
Sallusto F, Lanzavecchia A. Pillars Article: Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994. 179:1109-1118. J Immunol. 2018;200(3):887–896.
Schlender J, Schnorr JJ, Spielhoffer P, Cathomen T, Cattaneo R, Billeter MA, et al. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc Natl Acad Sci USA. 1996;93(23):13194–13199. Doi: https://doi.org/10.1073/pnas.93.23.13194
Schneider-Schaulies S, Schneider-Schaulies J, Schuster A, Bayer M, Pavlovic J, ter Meulen V. Cell type-specific MxA-mediated inhibition of measles virus transcription in human brain cells. J Virol. 1994;68(11):6910–6917.
Specht JM, Wang G, Do MT, Lam JS, Royal RE, Reeves ME, et al. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J Exp Med. 1997;186(8):1213–1221. Doi: https://doi.org/10.1084/jem.186.8.1213
Vidalin O, Fournillier A, Renard N, Chen M, Depla E, Boucreux D, et al. Use of conventional or replicating nucleic acid-based vaccines and recombinant Semliki forest virus-derived particles for the induction of immune responses against hepatitis C virus core and E2 antigens. Virology. 2000;276(2):259–270. Doi: https://doi.org/10.1006/viro.2000.0566
Waisman A, Lukas D, Clausen BE, Yogev N. Dendritic cells as gatekeepers of tolerance. Semin Immunopathol. 2017;39(2):153–163. Doi: https://doi.org/10.1007/s00281-016-0583-z
World Health Organization (WHO). Hepatitis C [Internet]. 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, et al. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6(7):816–820. Doi: https://doi.org/10.1038/77553
Yasui K, Wakita T, Tsukiyama-Kohara K, Funahashi SI, Ichikawa M, Kajita T, et al. The native form and maturation process of hepatitis C virus core protein. J Virol. 1998;72(7):6048–6055.
Yi Z, Chen J, Kozlowski M, Yuan Z. Innate detection of hepatitis B and C virus and viral inhibition of the response. Cell Microbiol. 2015;17(9):1295–1303. Doi: https://doi.org/10.1111/cmi.12489
Zehender G, Meroni L, De Maddalena C, Varchetta S, Monti G, Galli M. Detection of hepatitis C virus RNA in CD19 peripheral blood mononuclear cells of chronically infected patients. J Infect Dis. 1997;176(5):1209–1214. Doi: https://doi.org/10.1086/514114
Zhao H, De BP, Das T, Banerjee AK. Inhibition of human parainfluenza virus-3 replication by interferon and human MxA. Virology. 1996;220(2):330–338. Doi: https://doi.org/10.1006/viro.1996.0321
Zhou X, Berglund P, Rhodes G, Parker SE, Jondal M, Liljeström P. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine. 1994;12(16):1510–1514.
Zhou X, Berglund P, Zhao H, Liljeström P, Jondal M. Generation of cytotoxic and humoral immune responses by nonreplicative recombinant Semliki Forest virus. Proc Natl Acad Sci USA. 1995;92(7):3009–3013. Doi: https://doi.org/10.1073/pnas.92.7.3009
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Catalina Mira, Jesús Orlando Yepes, Luis Felipe Henao, Melissa Montoya Guzmán, Maria-Cristina Navas. (2020). EXPRESIÓN DE LA PROTEÍNA CORE DEL VIRUS DE LA HEPATITIS C EN CÉLULAS HEPG2 USANDO EL VIRUS DEL BOSQUE DE SEMLIKI. Acta Biológica Colombiana, 26(1), p.72. https://doi.org/10.15446/abc.v26n1.79365.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2019 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).




















