Publicado
METODOLOGÍAS PARA EL ESTUDIO DE LAS INTERACCIONES PROTEICAS Y APLICACIONES EN EL CASO DE LA RELACIÓN PLANTA-BACTERIA
Methodologies Employed in the Study of Protein Interactions and Applications in the Case of Plant-bacteria Relationship
DOI:
https://doi.org/10.15446/abc.v29n1.98597Palabras clave:
anticuerpos, bio-moléculas, inmunidad, patógeno, proteína fusión (es)antibody, immunity, pathogen, fusion protein, biomolecules (en)
Descargas
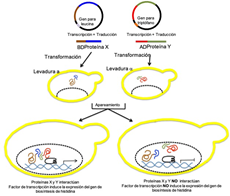
La maquinaría que permite el correcto funcionamiento celular involucra principalmente proteínas. Para cumplir con sus actividades, las proteínas establecen interacciones entre ellas. Para su estudio se han empleado principalmente las técnicas de doble híbrido de levaduras, co-immunoprecipitación, GST pull-down, localización celular, BiFC y FRET. En esta revisión se presenta una descripción de estas metodologías. Además se presenta, a manera de caso de estudio, una breve descripción de cómo la aplicación de estas metodologías ha permitido ahondar en el conocimiento de los mecanismos que se activan durante la relación que establecen las plantas con las bacterias fitopatógenas.
Proteins are the molecular machinery that allows the correct functioning of the cells. To achieve these functions, proteins establish interaction between them. There are several molecular techniques to understand the interactions, such as yeast-two-hybrid, coimmunoprecipitation, GST pull-down, cell localization, BiFC, and FRET. This review presents a general description of these technologies and a brief explanation of how their application expanded the knowledge of the mechanisms activated during the interactions between plants and their bacterial pathogens.
Referencias
Alberts, B. (1998). The cell as a collection of protein machines: Preparing the next generation of molecular biologists. Cell, 92(3):291–294. https://doi.org/10.1016/S0092-8674(00)80922-8
Axtell, M. J. y Staskawicz, B. J. (2003). Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell, 112(3): 369–377. https://doi.org/10.1016/s0092-8674(03)00036-9
Barberini, M. L. y Muschietti, J. P. (2017). Coimmunoprecipitation of Plant Receptor Kinases. Methods in Molecular Biology (Clifton, N.J.), 1621:109–112. https://doi.org/10.1007/978-1-4939-7063-6_10
Ben Rejeb, I., Pastor, V. y Mauch-Mani, B. (2014). Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants, 3:458–475. https://doi.org/10.3390/plants3040458
Bolte, S. y Cordelières, F. P. (2006). A guided tour into subcellular colocalization analysis in light microscopy. Journal of Microscopy, 224(3):213–232. https://doi.org/10.1111/j.1365-2818.2006.01706.x
Boutrot, F. y Zipfel, C. (2017). Function, Discovery and Exploitation of Plant Pattern Recognition Receptors for Broad-Spectrum Disease Resistance. Annual Review of Phytopathology, 55:257–286. https://doi.org/10.1146/annurev-phyto-080614-120106.
Clontech. (2009). Matchmaker ® Gold Yeast Two-Hybrid System User Manual. Consultado 20/11/2021. Disponible en: https://www.takarabio.com/documents/User%20Manual/Matchmaker%20Gold%20Yeast%20Two/Matchmaker%20Gold%20Yeast%20Two-Hybrid%20System%20User%20Manual.pdf
Cook, D. E., Mesarich, C. H. y Thomma, B. P. H. J. (2015). Understanding plant immunity as a surveillance system to detect invasion. Annual Review of Phytopathology, 53:541–563. https://doi.org/10.1146/annurev-phyto-080614-120114
Chalfie, M. (1995). Green fluorescent protein. Photochemistry and Photobiology, 62(4):651–656. https://doi.org/10.1111/j.1751-1097.1995.tb08712.x
Chinchilla, D., Zipfel, C., Robatzek, S., Kemmerling, B., Nürnberger, T., Jones, J. D. G., Felix, G. y Boller, T. (2007). A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature, 448(7152):497–500. https://doi.org/10.1038/nature05999
Chisholm, S. T., Coaker, G., Day, B. y Staskawicz, B. J. (2006). Host-microbe interactions: shaping the evolution of the plant immune response. Cell, 124(4):803–814. https://doi.org/10.1016/j.cell.2006.02.008
Feng, F., Yang, F., Rong, W., Wu, X., Zhang, J., Chen, S., He, C. y Zhou, J.M. (2012). A Xanthomonas uridine 5’-monophosphate transferase inhibits plant immune kinases. Nature, 485(7396):114–118. https://doi.org/10.1038/nature10962
Finley, R. L. y Mairiang, D. (2018). Two-Hybrid Systems to Measure Protein–Protein Interactions. In eLS, John Wiley & Sons, Ltd (Ed.). https://doi.org/10.1002/9780470015902.a0005980.pub3
Förster, T. (2012). Energy migration and fluorescence. Journal of Biomedical Optics, 17(1):011002. https://doi.org/10.1117/1.JBO.17.1.011002
Göhre, V., Spallek, T., Häweker, H., Mersmann, S., Mentzel, T., Boller, T., de Torres, M., Mansfield, J. W. y Robatzek, S. (2008). Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Current Biology: CB, 18(23):1824–1832. https://doi.org/10.1016/j.cub.2008.10.063
Halter, T., Imkampe, J., Mazzotta, S., Wierzba, M., Postel, S., Bücherl, C., Kiefer, C., Stahl, M., Chinchilla, D., Wang, X., Nürnberger, T., Zipfel, C., Clouse, S., Borst, J. W., Boeren, S., de Vries, S. C., Tax, F. y Kemmerling, B. (2014). The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Current Biology: CB, 24(2):134–143. https://doi.org/10.1016/j.cub.2013.11.047
Imkampe, J., Halter, T., Huang, S., Schulze, S., Mazzotta, S., Schmidt, N., Manstretta, R., Postel, S., Wierzba, M., Yang, Y., van Dongen, W. M. A. M., Stahl, M., Zipfel, C., Goshe, M. B., Clouse, S., de Vries, S. C., Tax, F., Wang, X. y Kemmerling, B. (2017). The Arabidopsis Leucine-Rich Repeat Receptor Kinase BIR3 Negatively Regulates BAK1 Receptor Complex Formation and Stabilizes BAK1. The Plant Cell, 29(9):2285–2303. https://doi.org/10.1105/tpc.17.00376
Jones, J. D. G. y Dangl, J. L. (2006). The plant immune system. Nature, 444(7117):323–329. https://doi.org/10.1038/nature05286
Kaboord, B. y Perr, M. (2008). Isolation of proteins and protein complexes by immunoprecipitation. Methods in Molecular Biology (Clifton, N.J.), 424:349–364. https://doi.org/10.1007/978-1-60327-064-9_27
Kadota, Y., Sklenar, J., Derbyshire, P., Stransfeld, L., Asai, S., Ntoukakis, V., Jones, J. D., Shirasu, K., Menke, F., Jones, A. y Zipfel, C. (2014). Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Molecular Cell, 54(1):43–55. https://doi.org/10.1016/j.molcel.2014.02.021
Kim, S.Y. y Hakoshima, T. (2019). GST Pull-Down Assay to Measure Complex Formations. Methods in Molecular Biology (Clifton, N.J.), 1893:273–280. https://doi.org/10.1007/978-1-4939-8910-2_20
Kodama, Y. y Hu, C.D. (2012). Bimolecular fluorescence complementation (BiFC): a 5-year update and future perspectives. BioTechniques, 53(5):285–298. https://doi.org/10.2144/000113943
Kourelis, J. y van der Hoorn, R. A. L. (2018). Defended to the Nines: 25 Years of Resistance Gene Cloning Identifies Nine Mechanisms for R Protein Function. The Plant Cell, 30(2):285–299. https://doi.org/10.1105/tpc.17.00579
Lalonde, S., Ehrhardt, D. W., Loqué, D., Chen, J., Rhee, S. Y. y Frommer, W. B. (2008). Molecular and cellular approaches for the detection of protein–protein interactions: Latest techniques and current limitations. The Plant Journal, 53:610–635. https://doi.org/10.1111/j.1365-313X.2007.03332.x
Lampugnani, E. R., Wink, R. H., Persson, S. y Somssich, M. (2018). The Toolbox to Study Protein–Protein Interactions in Plants. Critical Reviews in Plant Sciences, 37(4):308–334. https://doi.org/10.1080/07352689.2018.1500136
Lewis, J. D., Lee, A. H.Y., Hassan, J. A., Wan, J., Hurley, B., Jhingree, J. R., Wang, P. W., Lo, T., Youn, J.-Y., Guttman, D. S. y Desveaux, D. (2013). The Arabidopsis ZED1 pseudokinase is required for ZAR1-mediated immunity induced by the Pseudomonas syringae type III effector HopZ1a. PNAS, 110(46):18722–18727. https://doi.org/10.1073/pnas.1315520110
Li, L., Kim, P., Yu, L., Cai, G., Chen, S., Alfano, J. R. y Zhou, J.M. (2016). Activation-Dependent Destruction of a Coreceptor by a Pseudomonas syringae Effector Dampens Plant Immunity. Cell Host y Microbe, 20(4):504–514. https://doi.org/10.1016/j.chom.2016.09.007
Lu, D., Wu, S., Gao, X., Zhang, Y., Shan, L. y He, P. (2010). A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. PNAS, 107(1):496–501. https://doi.org/10.1073/pnas.0909705107
Lu, D., Lin, W., Gao, X., Wu, S., Cheng, C., Avila, J., Heese, A., Devarenne, T. P., He, P. y Shan, L. (2011). Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innateimmunity. Science, 332(6036):1439–1442. https://doi.org/10.1126/science.1204903
Ma, W., Wang, Y. y McDowell, J. (2018). Focus on Effector-Triggered Susceptibility. Molecular Plant-Microbe Interactions : MPMI, 31(1):5. https://doi.org/10.1094/MPMI-11-17-0275-LE
Mackey, D., Holt, B. F.,Wiig, A. y Dangl, J. L. (2002). RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell, 108(6):743–754. https://doi.org/10.1016/s0092-8674(02)00661-x
Mackey, D., Belkhadir, Y., Alonso, J. M., Ecker, J. R. y Dangl, J. L. (2003). Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell, 112(3):379–389. https://doi.org/10.1016/s0092-8674(03)00040-0
Mishra, B., Kumar, N. yMukhtar, M. (2021). Network biology to uncover functional and structural properties of the plant immune system. Current Opinion in Plant Biology, 62. https://doi.org/10.1016/j.pbi.2021.102057
Paiano, A., Margiotta, A., De Luca, M. y Bucci, C. (2019). Yeast Two-Hybrid Assay to Identify Interacting Proteins. Current Protocols in Protein Science, 95(1). https://doi.org/10.1002/cpps.70
Qin, J., Zhou, X., Sun, L., Wang, K., Yang, F., Liao, H., Rong, W., Yin, J., Chen, H., Chen, X. y Zhang, J. (2018). The Xanthomonas effector XopK harbours E3 ubiquitin-ligase activity that is required for virulence. The New Phytologist, 220(1):219–231. https://doi.org/10.1111/nph.15287
Rodríguez-Negrete, E., Bejarano, E. R. y Castillo, A. G. (2014). Using the yeast two-hybrid system to identify proteinprotein interactions. Methods in Molecular Biology (Clifton,N.J.), 1072:241–258. https://doi.org/10.1007/978-1-62703-631-3_18
Shaner, N. C., Patterson, G. H. y Davidson, M. W. (2007). Advances in fluorescent protein technology. Journal of Cell Science, 120(24):4247–4260. https://doi.org/10.1242/jcs.005801
Speth, C., Toledo-Filho, L. A. A. y Laubinger, S. (2014). Immunoprecipitation-based analysis of protein-protein interactions. Methods in Molecular Biology), 1158:175–185. https://doi.org/10.1007/978-1-4939-0700-7_11
Struk, S., Jacobs, A., Martín-Fontecha, E., Gevaert, K., Cubas, P.y Goormachtig, S. (2019). Exploring the protein–protein interaction landscape in plants. Plant Cell Environ, 42(2):387– 409. https://doi.org/10.1111/pce.13433
Sun, Y., Zhu, Y. X., Balint-Kurti, P. J.y Wang, G. F. (2020). Fine-Tuning Immunity: Players and Regulators for Plant NLRs. Trends in plant science, 25(7):695–713. https://doi.org/10.1016/j.tplants.2020.02.008
van der Hoorn, R. A. L. y Kamoun, S. (2008). From Guard to Decoy: a new model for perception of plant pathogen effectors. The Plant Cell, 20(8):2009–2017. https://doi.org/10.1105/tpc.108.060194
Walter, M., Chaban, C., Schütze, K., Batistic, O., Weckermann, K., Näke, C., Blazevic, D., Grefen, C., Schumacher, K., Oecking, C., Harter, K. y Kudla, J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. The Plant Journal: For Cell and Molecular Biology, 40(3):428–438. https://doi.org/10.1111/j.1365-313X.2004.02219.x
Wang, G., Roux, B., Feng, F., Guy, E., Li, L., Li, N., Zhang, X., Lautier, M., Jardinaud, M.-F., Chabannes, M., Arlat, M., Chen, S., He, C., Noël, L. D. y Zhou, J.M. (2015). The Decoy Substrate of a Pathogen Effector and a Pseudokinase Specify Pathogen-Induced Modified-Self Recognition and Immunity in Plants. Cell Host y Microbe, 18(3):285–295. https://doi.org/10.1016/j.chom.2015.08.004
Watson, J., Baker, T., Bell, S., Gann, A., Levine, M., Losick, R. (2004). Molecular Biology of the Gene. 4th Edición. Editorial Pearson.
Xing, S., Wallmeroth, N., Berendzen, K. W. y Grefen, C. (2016). Techniques for the analysis of protein–protein interactions in vivo. Plant Physiology, 171:727–758. https://doi.org/10.1104/pp.16.00470
Yuan, M., Ngou, B. P. K., Ding, P.y Xin, X. F. (2021). PTI-ETI crosstalk: an integrative view of plant immunity, Current Opinion in Plant Biology, 62. https://doi.org/10.1016/j.pbi.2021.102030
Zhong, G., Zhu, Q., Li, Y., Liu, Y. y Wang, H. (2017). Once for All: A Novel Robust System for Co-expression of Multiple Chimeric Fluorescent Fusion Proteins in Plants. Frontiers in Plant Science, 8. https://doi.org/10.3389/FPLS.2017.01071
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).



















