Susceptibility of Corythaica cyathicollis Costa to a native isolation of Beauveria bassiana (Vuillemin)
Keywords:
eggplant, lace bug, entomopathogenic fungus, biological control, pathogenicity, lethal concentrations. (es)Downloads
The research aimed to evaluate a native isolation of B. bassiana under laboratory conditions as a biological alternative control for the eggplant lace bug (C. cyathicollis). The experiments were conducted in the Pathology and Entomology Laboratories at the Universidad de Córdoba, Monteria (Colombia). Initially, the pathogenicity of B. bassiana on adult insects was studied using a concentration of 1·107 spores/mL, in which mortality and intrinsic mortality were determined, in addition, the presented symptomatology was described. Subsequently, the concentrations 1·100 (control treatment), 1·103, 1·105, 1·106, 1·107 and 1·108 spores/mL were evaluated to determine the lethal concentrations of CL50 and CL90 through Probit analysis. The results obtained showed that the native isolation was pathogenic since post-inoculation was observed, between the fifth and sixth days, with 50% mortality (and 87.7% at 12 days), in addition, the intrinsic mortality was 92.4%. The symptomatology showed loss of mobility and lack of appetite between 11 and 12 hours post-inoculation, approximately, and the presence of mycelium 2 days after death. The percentages of mortality for the evaluated concentrations were 0, 46.6, 73.3, 83.3, 90.0 and 96.6% respectively, and the lethal concentrations of CL50 and CL90 were 1.8·103 and 6.5·106 spores/mL respectively. These results indicate the potential of entomopathogenic fungus as an alternative that could be articulated into integrated pest management.
CROP PROTECTION
Susceptibility of Corythaica cyathicollis Costa to a native isolation of Beauveria bassiana (Vuillemin)
Susceptibilidad de Corythaica cyathicollis Costa a un aislamiento nativo de Beauveria bassiana (Vuillemin)
Ender M. Correa1 , Orlando J. Marsiglia2 and Claudio R. Fernández1
1Entomology Group University of Cordoba (GREUC in Spanish), Faculty of Agricultural Sciences, Universidad de Córdoba. Montería (Colombia). claudiofernandezherrera@gmail.com2Technical assistant Uraba area. Antioquia (Colombia). Received for publication: 13 April, 2010. Accepted for publication: 29 June, 2012.
ABSTRACT
The research aimed to evaluate a native isolation of B. bassiana under laboratory conditions as a biological alternative control for the eggplant lace bug (C. cyathicollis). The experiments were conducted in the Pathology and Entomology Laboratories at the Universidad de Córdoba, Monteria (Colombia). Initially, the pathogenicity of B. bassiana on adult insects was studied using a concentration of 1·107 spores/mL, in which mortality and intrinsic mortality were determined, in addition, the presented symptomatology was described. Subsequently, the concentrations 1·100 (control treatment), 1·103, 1·105, 1·106, 1·107 and 1·108 spores/mL were evaluated to determine the lethal concentrations of CL50 and CL90 through Probit analysis. The results obtained showed that the native isolation was pathogenic since post-inoculation was observed, between the fifth and sixth days, with 50% mortality (and 87.7% at 12 days), in addition, the intrinsic mortality was 92.4%. The symptomatology showed loss of mobility and lack of appetite between 11 and 12 hours post-inoculation, approximately, and the presence of mycelium 2 days after death. The percentages of mortality for the evaluated concentrations were 0, 46.6, 73.3, 83.3, 90.0 and 96.6% respectively, and the lethal concentrations of CL50 and CL90 were 1.8·103 and 6.5·106 spores/mL respectively. These results indicate the potential of entomopathogenic fungus as an alternative that could be articulated into integrated pest management.
Key words: eggplant, lace bug, entomopathogenic fungus, biological control, pathogenicity, lethal concentrations
RESUMEN
La investigación tuvo como objetivo evaluar bajo condiciones de laboratorio un aislamiento nativo de B. bassiana como alternativa de control biológico al chinche de encaje de la berenjena (C. cyathicollis). Los experimentos se desarrollaron en instalaciones de los Laboratorios de Fitopatología y Entomología de la Universidad de Córdoba, Monteria (Colombia). Inicialmente se estudió la patogenicidad de B. bassiana sobre adultos del insecto, usando una concentración de 1·107 esporas/ mL, en éstos se determinó la mortalidad, mortalidad intrínseca y se describió la sintomatología presentada. Posteriormente, se evaluaron las concentraciones 1·100 (testigo), 1·103, 1.105, 1·106, 1·107 y 1·108 esporas/mL, con las cuales se determinaron las concentraciones letales CL50 y CL90 a través del análisis Probit. Los resultados obtenidos mostraron que el aislamiento nativo fue patogénico al presentar entre el quinto y sexto día postinoculación, mortalidad del 50% y a los 12 días del 87,7%, además, la mortalidad intrínseca para esta lectura fue del 92,4%. La sintomatología mostrada fue pérdida de movilidad e inapetencia aproximadamente entre las 11 y 12 horas postinoculación y aparición de micelio a los 2 días después de muerto. Los porcentajes de mortalidad para las concentraciones evaluadas fueron de 0, 46,6; 73,3; 83,3; 90.0 y 96,6% respectivamente y las concentraciones letales CL50 y CL90 fueron de 1,8·103 y 6,5·106 esporas/mL respectivamente. Estos resultados indican el potencial del entomopatógeno como alternativa para ser articulada en el manejo integrado de la plaga.
Palabras clave: berenjena, chinche de encaje, entomopatógeno, control biológico, patogenicidad, concentraciones letales.
Introduction
In Colombian departments such as Córdoba and Sucre, eggplant is one of the main vegetable cultivations that are part of the rural economy. In this country, the Plan Hortícola Nacional (PHN) prioritizes eggplant cultivation since it is one of the vegetables which receives more benefits as an alternative of agricultural diversification and a prospectus of export (Acopaflor, 2007), by being one of the ten most consumed vegetables worldwide (MA DR, 2006) and providing health benefits (Gonçalves et al., 2006; González et al., 2007; Duran et al., 2007), especially with its antioxidant contribution (Whitaker and Stommel, 2003; Marion, 2004; Luthria and Mukhopadhyay, 2006; Sadilova et al., 2006; San José et al., 2007; Raigon et al., 2008). In addition, the Colombian Caribbean has competitive advantages for eggplant cultivation, such as its climatic factors, as well as the physical and chemical characteristics of the land, which allow for its production year round.
In Córdoba and Sucre, agricultural treatment problems are seen as the principal limits of eggplant cultivation, especially land pathogens associated with the illness know as "wilt" and arthropods like Bemisia tabaci (Gennadius), Tetranychus sp., Leptinotarsa decemlineata (Say) and Corythaica cyathicollis Costa (Aramendiz et al., 2008). The latter one, apart from attacking eggplant (Solanum melongena L.), has been associated with solanaceas species like S. atropurpureum Schrank, S. fastigiatum Willd., S. granuloso-leprosum Dunal, S. mauritianum Scopoli (Pedrosa-Macedo et al., 2003; Olckers et al., 2002) and cultivated species like S. lycopersicum L., S. tuberosum L. (Kogan, 1960) and Solanum gilo Raddi (Torres, 1995).
In the Colombian departments previously mentioned, the dry periods generally favor a high population of C. cyathicollis in eggplant cultivation (Torres, 2003). The mechanical damage is caused by nymphs as well lace bug adults when they suck up the leaf sap, which is initially presented as chlorotic spot joining, eventually causing a yellowish color, drying, and detachment of the leaves. This process also influences production in a significant manner; the loss of leaves favors damage in the fruits known as sunburn, which deteriorates quality (Kogan, 1960; Salgado and Regino, 2001).
In eggplant cultivations in Brazil, some management practices like fertilization and covering levels have been evaluated to control C. cyathicollis (Da Silva, 2006; Da Silva et al., 2008). In vegetable cultivation in Colombia, particularly for tomatoes, pest management based exclusively on chemical control has led to an important increase in production costs, a negative impact on the environment and a potential toxicity risk for workers and consumers (Guerrero, 2003; Fuentes and Barreto, 2006).
In contrast to this outlook, national and international specialized markets constantly emphasize products obtained under Good Agricultural Practices (GAP), as well as in an agro-ecological manner, especially in vegetables characterized by a low cooking times or no cooking requirement at all. An alternative to this problem is organic control through the use of natural enemies like entomopathogenic fungi. The most common fungi are Paecilomyces, Hirsutella, Metarhizium y Beauveria (France et al., 1999). The latter one has shown good results in controlling other tropical pests (Mena et al., 2003; Lucero et al., 2004; Pariona et al., 2007; Cova et al., 2009), meaning it should be considered as a potential agent for mycoinsecticides development (Alves, 1986).
Currently, vegetable and fruit cultivation continues to grow significantly, and customers are searching for the best purveyors, who have the capacity to provide them with products all year long. There is great opportunity in mass production and export of vegetables, hence, producers who meet the technological requirements to satisfy the markets will make the most of the commercial and competitive advantages (Acopaflor, 2007).
With the aim of contributing to the search for management alternatives compatible with food safety terms, sustainability and competitiveness in eggplant cultivation, this research had the goal of evaluating the potential of a native isolation of B. bassiana as a biological control for the eggplant lace bug (C. cyathicollis) under laboratory conditions.
Materials and methods
The research was conducted in the Phytopathology and Entomology Laboratories at the Universidad de Córdoba, Monteria (Colombia) under controlled conditions with a temperature of 26°C and 85% relative humidity. The host was the C. cyathicollis (Costa) species, with biological states obtained from offspring developed in eggplant plants available in the Entomology Laboratory of the Faculty of Agricultural Science. The C. cyathicollis population was started with the adults and nymphs harvested from commercial eggplant cultivations. The source of the entomopathogenic fungus came from an isolation of the C. cyathicollis species collected from the field (native isolation). The inoculum was obtained through the methodology suggested by Antia et al. (1992) and Vélez et al. (1997). The generic identification of the entomopathogenic fungus was conducted through the taxonomical key proposed by Barnet and Hunter (1998) for imperfect fungi.
Pathogenicity test
The pathogenic of the native isolation of B. bassiana was executed on C. cyathicollis adults 5 d after emergence, all of them were active and healthy, guaranteeing the homogeneity of the biological material, using the 1·107 spores/ mL concentration of the entomopathogenic fungus. The main solution of the native isolation was prepared with 10 g of rice boiled with the B. bassiana inoculum in 100 mL of sterile distilled water, plus two drops of spores dispersant (Tween®). The solution concentration was calculated at 10-3 dilution through cell counting in a Neubauer chamber.
Subsequently, it was adjusted to the 1·107 spores/mL concentration. The utilized control treatment was (1·100 spores/ mL) sterile distilled water plus the dispersant, in order to avoid the adverse effects of these components on insects that may influence the B. bassiana pathogenic action.
The infection with B. bassiana was made by direct spraying with an atomizer at an approximate distance of 10 cm on C. cyathicollis adults using a volume of 5 mL for the concentrations 1·100 (control treatment) and 1·107 spores/mL. Three populations of thirty of insects were used for each concentration. Each population was insolated in plastic trays and fed with eggplant leaves, which were changed daily for 11 d.
The symptoms of the illness caused by B. bassiana were described through daily observations of the changes and behaviors shown by the C. cyathicollis adults (lack of appetite, loss of mobility and coloration). The dead insects were placed in a moist chamber to elicit mycelium emission and fungal sporulation on the bodies. The evaluated variables were mortality, through the daily counting of post-infection dead adults until the 12th d, and intrinsic mortality (number of dead insects with the presence of the fungus), determined by counting the total number of dead insects that presented the signs and symptoms of the illness caused by B. bassiana until the 12th reading.
Determination of lethal concentrations CL 50 and CL 90
The concentrations 1·103, 1·105, 1·106, 1·107 and 1·108 spores/ mL and a control treatment 1·100 (ADE + dispersant) were evaluated, which were obtained through the procedure described in the pathogenicity test. Each concentration constituted a treatment which was distributed in a random design with three repetitions; 60 adults were infected by the treatments. Mortality counts were done until the 12th d. With the mortality data, the fifty (CL50) and ninety (CL90) lethal concentrations were calculated through Probit analysis (Finney, 1972; Raymons, 1985).
Results and discussion
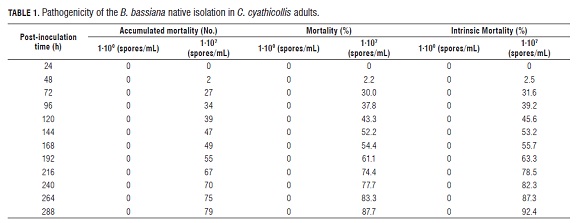
Pathogenicity of the native isolation of B. bassiana The native isolation was pathogenic and showed high levels of virulence, between the fifth and sixth d post-inoculation, with 50% mortality (and 87.7% at 12 d) in adults. In addition, the intrinsic mortality for this reading was 92.4%, which confirms that the insect deaths were caused by the entomopathogenic attack (Tab.1). The high virulence might be due to the natural specificity between the inoculum and the host, since the native fungus isolation was applied to the host were it was isolated; which coincides with the views of authors like Roberts and Humbre (1984); Torres and López (1997); France et al. (1999) who recommend the utilization of native isolations extracted from the insect, since these stocks have suffered natural selection processes and coevolution with the pathogenic action. Moreover, Alves (1986) pointed out that the pathogenicity is one of the entomopathogenic microorganism genetic characteristics, which makes it go straight through the insect and cause the illness, as long as the virulence is of the degree of pathogenicity of isolations toward a specific host.
Symptomatology of infection in C. cyathicollis The symptoms of infection produced by B. bassiana in C. cyathicollis in adults begin with the changing color of affected individuals, from black to reddish in the abdominal part, and the lack of appetite between 11 and 12 h post-inoculation, approximately. Once the insect is dead, it suffers dehydration, turns rigid; and 2 d after that, myce- lium appears between the abdominal sutures. The external emission of fungus mycelium is observed the third day after the insect's death, initiating between the abdominal sutures, thorax, and head of the adult. Consequently, the insect is adhered to the surface where it dies. Then, mycelium continues with the legs, wings, antennas, and finally it covers the entire body of the insect (Fig.1).
These symptoms match the ones described in Schistocerca piceifrons peruviana (Pariona et al., 2007), as far as the invasion of the entomopathogenic in the hemocele, producing paralysis in the insect, which may explain the immobility and rigidity observed in C. cyathicollis adults.
In other tingidae like the lace bug of avocado (Pseudacysta perseae), B. bassiana, under laboratory conditions, produced a high level of mortality with mycelium presence starting with the fourth d of application. Furthermore, symptom appearance and verification through sporulation was clear (Almaguel et al., 1997).
Average and ninety lethal concentrations of native isolation of B. bassiana
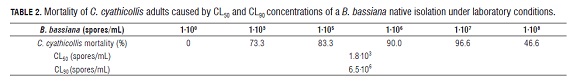
The mortality caused by the native isolation of B. bassiana in e ach treatment 1·103, 1·105, 1·106, 1·107, 1·108 spores/mL) for 7 d post-inoculation was 46.6, 73.3, 83.3, 90.0, and 96.6% respectively, while in the control treatment there was no C. cyathicollis mortality of C. cyathicollis. The CL50 and CL90 concentrations, obtained through mortality data in each treatment, were 1.8·103 and 6.5·106 spores/mL respectively, according to the Probit analysis (Tab.2).
These results indicate that there is a direct relationship between the spores' concentration in the native isolation of B. bassiana and the mortality of C. cyathicollis adults under the study conditions. Even though the B. bassiana fungus causes high mortality in the lace bug C. cyathicollis under laboratory conditions, it is not always reflected in the field due to the fungi structures and, particularly, to the dissection or inhibition spores may suffer by other microorganisms when they are exposed to the environment; among other factors such as humidity, solar radiation and temperature.
Conclusions
The native isolation of Beauveria bassiana presented as pathogenic in adults of the Corythaica cyathicollis species under laboratory conditions, causing an intrinsic mortality of 92.4% at 12 d post-inoculation for the concentration 1·107 spores/mL.
The average lethal concentration (CL50) and the ninety lethal concentration (CL90) of the native B. bassiana isolation against C. cyathicollis under laboratory conditions were 1.8·103 and 6.5·106 spores/mL respectively.
C. cyathicollis adults with symptoms of the infection produced by B. bassiana under field conditions can be recognized by the change in abdominal coloration, from black to reddish, which presents before the external emission of the fungus mycelium.
Literature cited
Acopaflor. 2007. Plan para incrementar la producción y consumo de hortalizas. Rev. Acopaflor 14, 32-39.
Almaguel, L., E. Blanco, P. Suárez, P. de La Torre, I. Cáceres, C. Nieves, M. Márquez, and L. Blanco. 1997. Control de la chinche del aguacate (Pseudacysta perseae (Heidemann)) en ciudad de la Habana. Fitosanidad 3(2), 69-74.
Alves, S. 1986. Fungos entomopatogénicos. Control microbiano de insectos. Editorial Manole, Sao Paulo.
Aramendiz, H, J. Cadena, and E. Correa. 2008. Línea base del sistema de producción de berenjena en los departamentos de Córdoba y Sucre. Corpoica; Universidad de Córdoba, Cereté, Colombia.
Barnet, L. and B. Hunter. 1998. Illustrate general of imperfect fungi. 4th ed. APS Press, St Paul, MN .
Cova, L., J. Scorza, D. García, L. Cañizález, C. Guedez, M. Maffey, and M. Medina. 2009. Patogenicidad in vitro de Beauveria brongniartii (Sacc.) Petch en Musca domestica (L.) como posible estrategia de control biológico en áreas ganaderas. Zootec. Trop. 27(2), 113-120.
Da Silva, S. 2006. Efeito da adubação, coberturas vivas do solo e do controle na população de Corythaica cyathicollis (Costa, 1864) (Hemiptera, Tingidae) em plantas de Solanum, melongena (linneaus, 1767) (solanaceae) cultivadas em sistema convensional e orgânico. Ph.D. thesis. Universidad Federal Rural de Rio de Janeiro (UFRRJ), Rio de Janeiro, Brazil.
Da Silva, S., A. De Carvalho, and F. Teixeira. 2008. Efeito da adubação na população de Corythaica cyathicollis em berinjela, em função do período de coleta. Rev. Biotemas 21(1), 47-51.
Duran, M., N. Contreras, and H. Valencia. 2007. Obtención de la oleorresina de la berenjena (Solanum melongena L.) y su posible uso industrial. Scientia et Technica 13(33), 457-458.
Finney, J. 1972. Probit analysis. 3rd ed. Cambridge University Press, London.
France, A., M. Gerding, A. Sandoval, S. Espinosa, and E. Vivanco. 1999. Patología de insectos. Serie Quilamapu No. 122. INIA Quilamapu, Chillán, Chile.
Fuentes, L.S. and C. Barreto. 2006. El manejo de las plagas en el cultivo de tomate en Colombia. In: Memorias Primer Congreso Colombiano de Horticultura. Sociedad Colombiana de Ciencias Hortícolas-SCCH, Bogota.
Gonçalves, M., M. Melo, J. Borba, X. Nunes, and J. Barbosa. 2006. Berinjela (Solanum melongena L.) - mito ou realidade no combate as dislipidemias? Rev. Bras. Farmacogn. 16(2), 252-257.
González, J., Y. Montes, and M. Domínguez. 2007. Breve reseña de la especie Solanum melongena L. Rev. Cubana Plantas Med. 12(3), 1-13.
Guerrero, J. 2003. Estudio de residuos de plaguicidas en frutas y hortalizas en áreas específicas de Colombia. Agron. Colomb. 21(3), 198-209.
Kogan, M. 1960. Corythaica cyathicollis (Costa, 1864), aspectos sistemáticos, biológicos y económicos (Hemiptera, Tingidae). Mem. Inst. Oswaldo Cruz 58(1), 59-88.
MADR, Ministerio de Agricultura y Desarrollo Rural. 2006. Acuerdo de competitividad de la cadena de hortalizas. In: http://www.antioquia.gov.co/antioquia-v1/organismos/agricultura/hortofruticola/hortofruticola/acuerdo%20de%20competitividad.pdf; consulted: July, 2012.
Marion, R. 2004. La berenjena contiene niveles altos de un compuesto antioxidante. In: Usda, http://www.ars.usda.gov/is/espanol/pr/2004/040108.es.htm; consulted: July, 2012.
Mena, J., A. Peña, and R. González. 2003. CL50 y variación de la patogenicidad en aislamientos de Beauveria bassiana y Metarhizium anisopliae evaluados en poblaciones de Premnotrypes vorax. Rev. Corpoica 4(1), 49-54.
Lucero, A.M., L.A. Peña, and T. Bacca. 2004. Evaluación de la actividad biocontroladora de Beauveria bassiana y Metarhizium anisopliae sobre larvas de Ancognatha scarabaeiodes (Coleoptera: Scarabaeidae). Rev. Corpoica 5(1), 43-48.
Luthria, D. and S. Mukhopadhyay. 2006. Influence of sample preparation on assay of phenolic acids from eggplant. J. Agric. Food Chem. 54(1), 41-47.
Olckers, T., J. Medal, and D. Gandolfo. 2002. Insect herbivores associated with species of Solanum (Solanaceae) in Northeastern Argentina and Southeastern Paraguay, with reference to biological control of weeds in South Africa and the United States of America. Fla. Entomol. 85(1), 254-260.
Pariona, N., P. Castellanos, and E. León. 2007. Capacidad entomocida de cepas nativas de Beauveria sp. sobre Schistocerca piceifrons peruviana (Lynch Arribalzaga, 1903). Rev. Peru. Biol. 14(2), 253-257.
Pedrosa-Macedo, J., T. Olckers, M. Vitorino, and M. Caxambu. 2003. Phytophagous arthropods associated with Solanum mauritianum Scopoli (Solanaceae) in the first plateau of Paraná, Brazil: a cooperative project on biological control of weeds between Brazil and South Africa. Neotrop. Entomol. 32(3), 519-522.
Raigon, M., J. Prohens, J. Munoz-Falcón, and F. Nuez. 2008. Comparison of eggplant landraces and commercial varieties for fruit content of phenolics, minerals, dry matter and protein. J. Food Compos. Anal. 21(5), 370-376.
Raymons, M. 1985. Présentation d'un programme basic d'analyse log-probit pour microordinateur. Cah. ORST OM, Sér. Ent. Méd. et Parasitol. 23(2), 117-121.
Roberts, D.W. and R.A. Humbre. 1984. Entomopathogenic fungi. pp. 1-12. In: Roberts, D.W. and R. James (eds.). Infection processes of fungi. The Rockefeller Foundation, New York, NY .
Salgado, K. and S. Regino. 2001. Identificación y ciclo de vida de la chinche de encaje en berenjena (Solanum melongena L.) en el Sinú medio. Undergraduate thesis. Faculty of Agricultural Sciencies, Universidad de Córdoba, Monteria, Colombia.
San José, R., A. González, M. Sánchez, M. Cámara, J. Prohens, and F. Nuez. 2007. Variación de parámetros físico-químicos en las berenjenas escarlata (Solanum aethiopicum) y gboma (S. macrocarpon). pp. 352-355. In: XI Congreso SECH. Sociedad Española de Ciencias Hortícolas, Albacete, Spain.
Sadilova, E., F. Stintzing, and R. Carle. 2006. Anthocyanins, colour and antioxidant properties of eggplant (Solanum melongena L.) and violet pepper (Capsicum annuum L.) peel extracts. Z. Naturforsch. C. 61(7-8), 527-535.
Torres, G. 2003. Fluctuación poblacional de Corythaica cyathicollis (Costa) (Hemiptera: Tingidae) en el cultivo de berenjena en el sinu medio. Undergraduate thesis. Faculty of Agricultural Sciencies, Universidad de Córdoba, Monteria, Colombia.
Torres, J.L.R. 1995. Influência da adubação nitrogenada e fatores climáticos sobre a flutuação populacional de Corythaica cyathicollis (Costa, 1864) (Hemiptera: Tingidae) em jiló (Solanum gilo Raddi) em Seropédica - RJ. M.Sc. thesis. Institute of Agronomy, Universidade Federal Rural do Rio de Janeiro, Seropédica, Brazil.
Torres, P. and A. López. 1997. Estudios básicos sobre el control microbiológico del gusano blanco de la papa (Premnotrypes vorax) con Beauveria spp. y Metarhizium sp. Rev. Colomb. Entomol. 23(1-2), 83-88.
Vélez, P., F. Posada, P. Marín, M. González, E. Osorio, and A. Bustillo. 1997. Técnicas para el control de calidad de formulaciones de hongos entomopatógenos. Bul. Tech. Cenicafé 17, 37.
Whitaker, B. and J. Stommel. 2003. Distribution of hydroxycinnamic acid conjugates in fruit of commercial eggplant (Solanum melongena L.) cultivars. J. Agric. Food Chem. 51(11), 3448- 3454.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Article abstract page views
Downloads
License
Copyright (c) 2012 Agronomía Colombiana

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
© Centro Editorial de la Facultad de Ciencias Agrarias, Universidad Nacional de Colombia
Reproduction and quotation of material appearing in the journal is authorized provided the following are explicitly indicated: journal name, author(s) name, year, volume, issue and pages of the source. The ideas and observations recorded by the authors are their own and do not necessarily represent the views and policies of the Universidad Nacional de Colombia. Mention of products or commercial firms in the journal does not constitute a recommendation or endorsement on the part of the Universidad Nacional de Colombia; furthermore, the use of such products should comply with the product label recommendations.
The Creative Commons license used by Agronomia Colombiana journal is: Attribution - NonCommercial - ShareAlike (by-nc-sa)

Agronomia Colombiana by Centro Editorial of Facultad de Ciencias Agrarias, Universidad Nacional de Colombia is licensed under a Creative Commons Reconocimiento-NoComercial-CompartirIgual 4.0 Internacional License.
Creado a partir de la obra en http://revistas.unal.edu.co/index.php/agrocol/.