Publicado
NEW ANDEAN BEE SPECIES OF CHILICOLA SPINOLA (HYMENOPTERA: COLLETIDAE, XEROMELISSINAE) WITH NOTES ON THEIR BIOLOGY
Especies nuevas de abejas andinas Chilicola Spinola (Hymenoptera: Colletidae,
Xeromelissinae) con notas sobre su biología
VICTOR H. GONZALEZ
CATALINA GIRALDO
Department of Ecology & Evolutionary Biology, 1200 Sunnyside Avenue,
Haworth Hall, University of Kansas, Lawrence, Kansas 66045, USA.
Current address: USDA-ARS Bee Biology and Systematics Laboratory, Utah State
University, Logan, UT, 84322, USA. victorgonzab@gmail.com
Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Apartado 7495, Bogotá D. C., Colombia. mcatagiraldo@yahoo.com.mx
ABSTRACT
We describe and illustrate three new bee species of the genus Chilicola Spinola that occur at high altitudes in the Eastern Andes of Colombia: C. (Anoediscelis) paramoides Gonzalez sp. nov., C. (Hylaeosoma) bochica Gonzalez sp. nov., and C. (Oroediscelis) deborahae Gonzalez sp. nov. We also provide biological notes and discuss the phylogenetic relationships of C. deborahae; nests of this species were found inside dry flower stems of Espeletia argentea Humb. & Bonpl. (Asteraceae) and a palynological analysis showed that 80 % of the pollen grains found in brood cells belonged to Arcytophyllum aff. nitidum and A. muticum (Rubiaceae). The ichneumonid wasp Grotea sp. is also recorded parasitizing brood cells of C. deborahae.
Key words. Apoidea, Colombia, Grotea, Páramo, Systematics.
RESUMEN
Describimos e ilustramos tres especies nuevas de abejas del género Chilicola Spinola que habitan en grandes alturas en la cordillera Oriental de Colombia: C. (Anoediscelis) paramoides Gonzalez sp. nov., C. (Hylaeosoma) bochica Gonzalez sp. nov., and C. (Oroediscelis) deborahae Gonzalez sp. nov. También presentamos notas biológicas y discutimos la relación filogenética de C. deborahae; los nidos de esta especie fueron encontrados dentro de inflorescencias secas de Espeletia argentea Humb. & Bonpl. (Asteraceae) y análisis palinológicos mostraron que el 80% de los granos de polen encontrados en las celdas de cría pertenecían a Arcytophyllum aff. nitidum y A. muticum (Rubiaceae). También se registra la avispa ichneumonida Grotea sp. parasitando celdas de cría de C. deborahae.
Palabras clave. Apoidea, Colombia, Grotea, Páramo, Sistemática.
INTRODUCTION
The diverse tropical Andean bee fauna is still poorly known taxonomically and ecologically (Gonzalez & Engel 2004, Gonzalez et al. 2005). Three subgenera (Anoediscelis Toro & Moldenke, Hylaeosoma Ashmead, and Oroediscelis Michener) and 28 species of Chilicola Spinola are known to occur in the Andes from Venezuela to Peru. Michener (2002) revised these species and explored the phylogenetic relationships of the 12 species of Oroediscelis, the only subgenus restricted to the Andes. Gonzalez & Michener (2004) described C. paramo, a species of Anoediscelis that did not show a close relationships to any of the remaining known species of the subgenus; they also provided an updated key to the Andean subgenera.
We describe and illustrate one species of each Andean subgenus of Chilicola. These new species occur in Páramos from the Eastern Andes of Colombia, and one of them is closely related to C. paramo. We also provide biological notes and discuss the phylogenetic relationships of C. deborahae sp. nov.
Materials and Methods
The morphological description and illustrations were made using an Olympus SZ60 stereomicroscope. Morphological terminology follows that of Michener (2007). Abbreviations used in the descriptions are: F, S, T, OD and PD for antennal flagellomere, metasomal sternum and tergum, ocellar diameter, and puncture diameter, respectively. Type specimens are deposited in the Instituto de Ciencias Naturales (ICN), Universidad Nacional de Colombia, Bogotá, Colombia, and the Snow Entomological Museum (SEMC), University of Kansas, Lawrence, Kansas, USA.
To explore the phylogenetic relationships of C. deborahae sp. nov., we used the morphological characters and data set of Michener (2002) for the phylogeny of Oroediscelis (two outgroup species, 12 ingroup species, and 16 characters). We analyzed this data set using the wh* and max* commands in Nona (Goloboff 1993); all characters were considered non-additive. Trees were visualized and printed using Winclada (Nixon 1999, slow optimization). We only explored the phylogenetic relationships of this species because the phylogeny of the subgenera Anoediscelis and Hylaeosoma have not yet been studied.
Two nests of C. deborahae sp. nov. were found on August 23, 2003, at the Santuario de Fauna y Flora de Iguaque, 5º70'N, 73º46'W (Departamento of Boyacá). The nests were kept inside Ziplock plastic bags containing moistened cotton for about four weeks until adult emergence. Pollen samples from brood cells were treated and preserved following the acetolysis method of Erdtman (1986) and then mounted on glass slides. Pollen samples were examined at 40X and 100X magnification with a Leitz DIALUX 22 EB microscope, and are deposited in the palynological collection of the ICN.
Results
Chilicola (Anoediscelis) paramoides Gonzalez sp. nov.
Figs. 2, 4-9
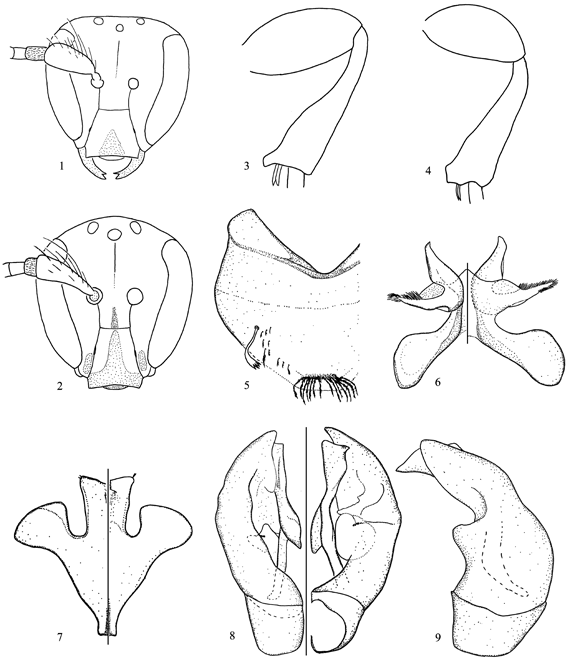
Diagnosis. This species is closely related to C. paramo. Both species differ from the species placed in the C. ashmeadi group by Michener (2002) in the distal stigmal perpendicular crossing submarginal cells near first submarginal crossvein, long scape (Figs. 1, 2), and the swollen hind femur of the male (Figs. 3, 4). C. paramoides sp. nov. can be separated from C. paramo by the shinier integument of frons, more extensive yellow markings on the face and legs, longer and narrower scape, shorter pedicel (Fig. 2), and the broader apical projection of the hind tibia (Fig. 4); the S6-S8 and genital capsule are also different (compare with Figs. 4-8 of Gonzalez & Michener 2004).
Description. Male. Body length 4.6 mm; forewing length 3.6 mm. Structure. Head 1.1x longer than broad; interalveolar distance 2x OD, 1.3x greater than alveolorbital distance; interocellar distance 2.4x OD, 1.1x ocellocular distance; ocelloccipital distance about 1.4x OD; scape 3x longer than broad (Fig. 2); pedicel subcylindrical, 1.1x longer than broad; F1 1.4x longer than broad, narrower and slightly longer than pedicel, shorter than F2 and F3 individually, F2 and F3 each 1.3x longer than broad, F4-F10 progressively broader and longer, F11 1.5x longer than broad. Hind femur swollen, 1.8x longer than broad, hind tibia with apical projection broader than in C. paramo (Fig. 4). S6-S8, and genital capsule as in Figs. 5-9. T7 with distal margin rounded. Coloration. Head and mesosoma mainly black except for the following yellow parts: maxillary palpus, hypostomal area, labrum, and mandible, except for red brown apex; clypeus, supraclypeal and lower paraocular areas as in Fig. 2.; antenna dark brown with apex of scape, pedicel, and ventral surface of flagellum yellowish; legs dark brown with apex of femora, outer surface of front tibia, and base and apex of remaining tibiae yellow; tarsi mostly yellow with outer surface of distal segments brownish; tegula dark brown; wings faintly smoky, veins and stigma dark brown; metasoma mainly dark brown, terga and sterna with translucent brownish distal margins. Pubescence. Whitish to light yellow; predominantly short and sparse, longer (2.0-2.5x OD) on frons, scape, vertex, anterior surface of mesepisternum, sides of propodeum and terga, and distal margin of sterna; pedicel densely covered by very short (0.5x OD), semierect hairs. Punctation. Head and mesosoma finely imbricate, punctures stronger and denser (= PD) on frons and vertex; basal area of propodeum weakly striate, striae ending before smooth and shiny distal margin, integument otherwise granular; metasoma finely lineolate with minute and sparse punctures.
Variations. In all paratypes the supraclypeal area is black; in one of them, the yellow markings of clypeus and lower paraocular area are also slightly more reduced than the holotype.
Holotype. ?, COLOMBIA. Boyacá: Arcabuco Prov., Santuario de Fauna y
Flora
Iguaque, Chaina, 2600 m, 16 March 2001, V. Gonzalez [ICN]. Paratypes. 4?, two
specimens with the same data as the holotype, one of them collected on June
20, 2001; remaining paratypes with the following data: idem, Carrizal, 3350
m, June 23 2000 [ICN, SEMC].
Etymology. This species is named using the Greek suffix "-oides", meaning "having the appearance of" or "like", referring to its resemblance to C. paramo.
Comments. This species and C. paramo represent a distinct group of Andean Anoediscelis
easily recognized by the characters mentioned in the diagnosis. The group has
been fully characterized by Gonzalez & Michener (2004, p. 28).
Chilicola (Hylaeosoma) bochica Gonzalez sp. nov.
Figs. 10-14
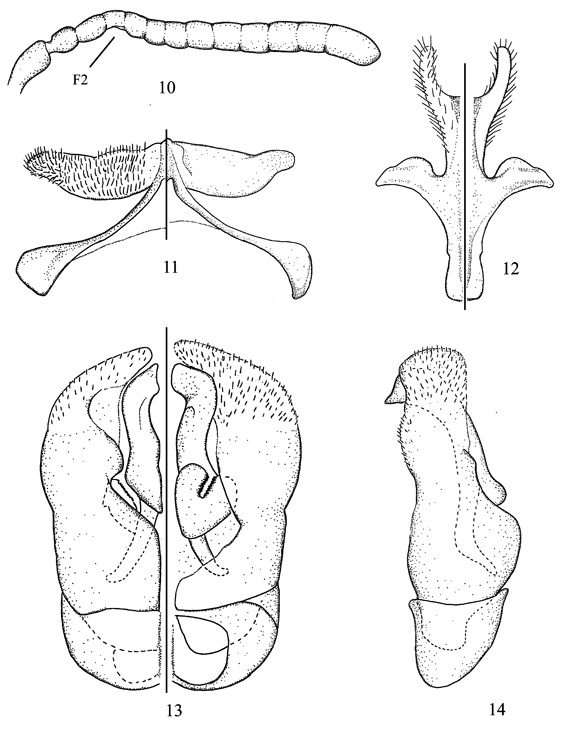
Diagnosis. In the key of Michener (2002) to the Andean species of Hylaeosoma, this species runs to C. canei Michener. However, the male of C. bochica sp. nov. can be separated from that species, and any other species of the subgenus, by the shape of S7 and S8 (Figs. 11, 12), and the modified antenna, with F2 ventrally concave and F3-F5 slightly crenulate (Fig. 10); the female can be separated from C. canei by the basal area of the propodeum with a strong median stria and the first recurrent vein basad to first submarginal crossvein.
Description. Male. Body length 3.9-4.2 mm; forewing length 3.2 mm. Structure. Head 1.1x longer than broad; interalveolar distance 2.3x OD, 1.3x greater than alveolorbital distance; interocellar distance 2.1x OD, about as long as ocellocular distance; ocelloccipital distance about 1.2x OD; scape claviform, 1.7x longer than broad; pedicel subcylindrical, 1.3x longer than broad; F2 ventrally concave, F3-F5 slightly crenulate, remaining flagellomeres normal (Fig. 10); F1 1.1x longer than broad, slightly shorter than pedicel; F2 and F3 each about as long as wide, F4-F10 progressively broader and longer, F11 1.6x longer than broad, distinctly shiny apically. Legs unmodified. S2-S4 with distinct preapical sublateral tubercles, more conspicuous on S4; S5 with strong preapical transverse ridge (clearly visible in lateral view); S7, S8, and genital capsule as in Figs. 11-14. T2 and T3 with pregradular areas distinctly depressed; T7 with distal margin rounded. Coloration. Head and mesosoma mainly black except for the following yellow parts: maxillary palpus, mandible (except for red brown apex), and labrum; clypeus with an inverted T-shaped maculation; antennal flagellum dark brown with ventral surface yellow, except for apex of F11 black; legs dark brown with apex of femora, front tibia, and base and apex of remaining tibiae yellow; tarsi mostly light brown; tegula yellowish; wings faintly smoky, veins and stigma dark brown; metasoma mainly dark brown, terga and S1-S5 with translucent brownish distal margins; S6 yellowish. Pubescence. Whitish to light yellow, hairs denser on lower paraocular area, depression for antennal scape, vertex, and gena; scape uniformly covered by short hairs (= OD); mesepisternum and sterna with longer hairs than on head (1.5-2.0x OD). Punctation. Head and mesosoma dull, finely imbricate between punctures, punctures stronger and denser (= PD) on frons and vertex; lower gena lineolate, hypostomal area shiny; basal area of propodeum with several irregular longitudinal striae, integument otherwise imbricate; metasoma shiny, finely lineolate with minute and sparse (1-2x PD) punctures.
Female. As described for the male, except for: Structure. Head 1.2x longer than broad; interalveolar distance 3.0x OD, 1.6x greater than alveolorbital distance; interocellar distance 2.3x OD, 0.8x ocellocular distance; ocelloccipital distance about 1.6x OD; scape 2.9x longer than broad; pedicel slightly longer than broad; flagellomere normal, F1 longer than broad, about as long as combined length of F2 and F3. Coloration. Labrum and clypeus black; ventral surface of F1-F5 and fore tibia brownish. Pubescence. Similar to that of the male but with sparse hairs on lower paraocular area and depression for antennal scape, as in remaining areas of face. Punctation. Face with finer and sparser (1-2x PD) punctures than in the male.
Variations. In all paratypes the maxillary palpus and tegula are brownish.
Holotype. ?, COLOMBIA. Boyacá: Arcabuco Prov., Santuario de Fauna y
Flora
Iguaque, Chaina, 2600 m, 15 March 2001, V. Gonzalez [ICN]. Paratypes. 1? 3?,
one female with the same data as the holotype, remainder paratypes collected
on June 20, 2001 [ICN, SEMC].
Etymology. The specific name refers to the most influential deities of the Muiscas, a pre-Colombian culture that had a well developed political system in South America. The Muiscas lived in the highlands of modern-day departments of Boyacá and Cundinamarca (Colombia). Bochica brought law and morals, and taught the Muiscas agriculture and manual arts.
Chilicola (Oroediscelis) deborahae Gonzalez sp. nov.
Figs. 15-22
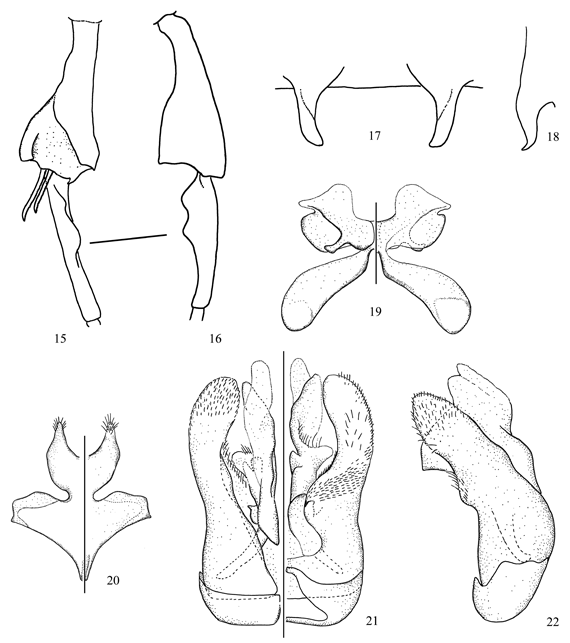
Diagnosis. In the key to the species of Oroediscelis (Michener 2002), the male of this species runs to C. bigibbosa Michener whereas the female runs to C. benoistiana Michener and C. quitensis Benoist. The male of C. deborahae sp. nov. differs from C. bigibbosa in the unmodified front and middle femora, longer preapical processes of S4 (Figs. 17, 18), and smaller ventral lobes of the hind basitarsus (Fig. 16). The preapical processes of S4 are flattened apically and pointed in lateral view as in C. espeleticola Michener; however, it can be separated by the punctuation of the face, vertex and gena, and the shape of the hind tibia, hind basitarsus, S7 and S8 (compare with Fig. 22 of Michener 2002). The strongly striate vertex and gena of the female of C. deborahae sp. nov. distinguishes this species from C. benoistiana and C. quitensis.
Description. Male. Body length 7.2 mm; forewing length 4.8 mm. Structure. Head about as long as broad; malar space about two-thirds as long as broad; interalveolar distance 1.5x OD, about as long as alveolorbital distance; interocellar distance 2.1x OD, 0.8x ocellocular distance; ocelloccipital distance about 1.4x OD; scape 3.3x longer than broad; pedicel about as long as broad; F1 2.0x longer than pedicel, 2.1x longer than broad, about as long as F2 and F3 individually; F2 and F3 about as long as wide, F4-F10 progressively shorter. Hind tibia and basitarsus as in Figs. 15, 16. S2 and S3 with low preapical sublateral tubercles; S4 with preapical processes flattened at tips, pointed in lateral view (Figs. 17, 18); S7, S8, and genital capsule as in Figs. 19-22. T7 with distal margin weakly emarginated medially. Coloration. Mainly black except for: mandible yellow basally, reddish brown apically; inferior paraocular area yellow; antennal flagellum with dorsal surface light brown; tarsi dark brown; tegula, wing veins and stigma dark brown; wing membrane faintly smoky. Pubescence. Whitish to light yellow; head (including scape) and mesosoma sparsely covered with long, branched hairs (= 3.0x OD); hind tibia with preapical brush. Distal margin of T1-T6 with weak lateral fasciae; sides of S6 with long tuft (= 2.5x OD) of yellow hairs, preapical fringe of yellowish hairs surpassed by yellow, transparent lamella. Punctation. Clypeus and lower supraclypeal area with sparse punctures (1-2x PD), integument shiner on distal half of clypeus, finely imbricate to lineolate between punctures; supraclypeal area duller than clypeus, with few punctures laterally; frons with coarse punctures, as close as they can be; upper paraocular area, vertex, and gena strongly striate; hypostomal area shiny, finely imbricate. Mesosoma finely imbricate with finer and closer punctures on scutum and scutellum; basal half of metepisternum and sides of propodeum weakly striate; basal area of propodeum with several irregular longitudinal striae. Terga finely lineolate with minute and sparse (1-2x PD) punctures on discs, distal margins smooth and shiny.
Female. As described for the male, except for: Body length 6.2-7.1 mm; forewing length 5.1 mm. Structure. Head 1.1x longer than broad; interalveolar distance about 2.0x OD, subequal to alveolorbital distance; interocellar distance 2.5x OD, slightly shorter than ocellocular distance; ocelloccipital distance about 1.8x OD; scape about 4.0x longer than broad; pedicel slightly longer than broad; F1 longer than pedicel, 1.8x longer than broad; F2 and F3 each about as long as broad, shorter than F1. Coloration. Mandible and lower paraocular area black. Pubescence. Metasoma with longer and denser hairs than in male. Punctation. Clypeus dull, with fine and sparse punctures; striae on upper paraocular area, vertex and gena weaker than in the male.
Holotype. ?, COLOMBIA. Cundinamarca: PNN Chingaza, La Siberia 4º31'N, 73º45'W, 3170 m, Malaise, 14-31-V-2001. E. Raigoso Leg [ICN]. Paratypes. 1?, 5?; one female with the same data as the holotype except for: Valle del Fraylejon, Malaise, 02-17-VIII-2000. A. Pérez; remaining paratypes with the following data: Boyacá: Arcabuco Prov., Santuario de Fauna y Flora de Iguaque, camino a la laguna, 5º70'N, 73º46'W, 3400-3600 m, Aug 23 2004, in stems of Espeletia argentata. V. H. Gonzalez [ICN, SEMC].
Etymology. This species honors Dr. Deborah Smith (University of Kansas), friend and mentor who has provided much inspiration and support to the first author.
Phylogenetic relationships. A total of 10 most parsimonious trees (L = 44, CI = 65, RI = 74) were obtained when including C. deborahae sp. nov. in the analysis of the data set of Michener (2002); five nodes collapsed in the consensus tree. The resulting topology was similar to that of Fig. 35 in Michener (2002), except for: C. maculipes Michener and C. bigibbosa were sister to the remaining species of Oroediscelis; C. deborahae sp. nov., C. cuzcoensis Michener, and C. espeleticola were in a polytomy with the clade that includes C. quitensis and remaining species. However, we obtained three most parsimonious trees (L = 45, CI = 66, RI = 75) when we included an additional character to Michener's data set (Apex of preapical process of S4: 0 = not apically flattened and pointed in lateral view; 1 = apically flattened and pointed in lateral view, Figs. 17, 18). Only two nodes collapsed in the consensus tree and C. deborahae sp. nov. and C. espeleticola were sister species. According to this analysis, the preapical processes of S4 with flattened and pointed apex is a synapomorphy that supports such relationship.
Biological notes. As described for other Andean species of Chilicola, such
as C. espeleticola and C. paramo (Michener 2002, Gonzalez & Michener 2004),
nests of C. deborahae sp. nov. were also found inside dead, dry, broken, pithy
flowering stems of living plants of Espeletia argentea Humb. & Bonpl. (Asteraceae).
The two stems containing nests had diameters that ranged from 8.0 to 18 mm.
Nests consisted of unbranched tunnels through the axes of the stems. The tunnel
diameter was 5 mm and length (measured from nest entrance to the upper end of
the cell) was 13 mm. Each nest had five cell partitions; one nest had three
cells with bee pupae and two with larva and pollen; the other nest had two cells
with larva and pollen, two cells with bee pupae, and one long cell (about twice
the size of a bee cell) with a pupa of the ichneumonid wasp genus Grotea Cresson
(Hymenoptera: Ichneumonidae, Labeninae). Cells with bee contents were cylindrical
and 8 mm in length.
We found pollen grains from 17 plant species and types (nine genera in 12 families)
within cells of C. deborahae sp. nov. (Table 1). In both
nests, about 80 % of the pollen belonged to Arcytophyllum aff. nitidum and A.
muticum (Rubiaceae).

DISCUSSION
Given the high abundance of pollen of Arcytophyllum aff. nitidum and A. muticum in the sample, it seems that C. deborahae sp. nov. rely heavily on these plants. However, our sample size was very small and restricted to a single locality. It would be interesting to know the role of C. deborahae sp. nov. in the pollination biology of Arcytophyllum; as far it is known, the tubular white or red flowers of these montane plants are primarily visited by small (1-5 mm in body length) syrphid flies (García-Robledo & Mora 2007).
The Grotea specimen, collected from nests of C. deborahae sp. nov., is presumably
one of the six undescribed species of the genus that occur in Colombia (Herrera
2006). It represents the first record of Grotea parasitizing nests of Chilicola
subgenus Oroediscelis, although it has also been recorded from nests of other
stem nesting bees, including Chilicola (Chilicola) venticola Packer, Ceratina,
Manuelia (Apidae), and Megachile (Megachilidae) (Gauld 2000, Packer 2004).
Acknowledgments
We thank J. Rodríguez, P. Sepúlveda, J. Koch, K. Huntzinger,
and two anonymous reviewers for their comments and suggestions that improved
the manuscript; C. D. Michener, M. S. Engel, Z. Falin, and J. Thomas for access
to the bee collection at SEMC; Prof. O. Rangel for laboratory facilities at
ICN, A. Herrera for identifying the ichneumonid wasp, and The Instituto Alexander
von Humboldt (Villa de Leyva) and el Sistema de Parques Nacionales Naturales
de Colombia for their outstanding logistical support and for generously providing
both permission to work in the park and lodging to V.G. during this study.
LITERATURE CITED
1. ERDTMAN, G. 1986. The acetolysis method in a revised description. Svensk
Botanisk Tidskift Lund 54: 561-564.
2. GARCÍA-ROBLEDO, C. & F. MORA. 2007. Pollination biology and the
impact of floral display, pollen donors, and distyly on seed production in Arcytophyllum
lavarum (Rubiaceae). Plant Biology 9(4): 453-461.
3. GAULD, I. D. 2000. The Ichneumonidae of Costa Rica, 3. Memoirs of the American
Entomological Institute 63:1-453.
4. GOLOBOFF, P. A. 1993. NoName (NONA), version 1.5.1. Program and documentation,
Fundación and Instituto Miguel Lillo, Tucumán.
5. GONZALEZ, V. H. & M. S. ENGEL. 2004. The Tropical Andean bee fauna (Insecta:
Hymenoptera: Apoidea), with examples from Colombia. Entomologische Abhandlungen
62 (1): 65-75.
6. GONZALEZ, V. H. & C. D. MICHENER. 2004. A new Chilicola from Colombian
Páramo (Hymenoptera, Colletidae, Xeromelissinae). Journal of Hymenoptera
Research 13(1): 24-30.
7. GONZALEZ, V. H., OSPINA, M., & D. BENNETT. 2005. Abejas altoandinas de
Colombia: Guía de campo. Instituto de Investigación de Recursos
Biológicos Alexander von Humboldt, Bogotá, D.C.
8. HERRERA, A. 2006. Labeninae (Hymenoptera: Ichneumonidae) de Colombia. Estudio
taxonómico preliminar. Undergraduate thesis. Universidad de Antioquia,
Medellín.
9. MICHENER, C. D. 2002. The bee genus Chilicola in the tropical Andes, with
observations on nesting biology and a phylogenetic analysis of the subgenera
(Hymenoptera: Colletidae, Xeromelissinae). Scientific Papers, Natural History
Museum, The University of Kansas 26: 1-47.
10. MICHENER, C. D. 2007. The Bees of the World. Johns Hopkins University Press,
Baltimore, MD, 2nd Edition.
11. NIXON, K. C. 1999. WINCLADA, version 0.9.99tuc.13, beta. Cornell University,
Ithaca, New York.
12. PACKER, L. 2004. Taxonomic and behavioural notes on the Patagonian Xeromelissinae
with the description of a new species (Hymenoptera: Colletidae). Journal of
the Kansas Entomological Society 77(4): 805-820.
Recibido: 05/12/2008
Aceptado: 04/03/2009
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2009 Caldasia

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).























