Jumping spider (Araneae: Salticidae) diversity in the understory of the Argentinian Atlantic Forest
Diversidad de arañas saltadoras (Araneae: Salticidae) del sotobosque del Bosque Atlántico argentino
DOI:
https://doi.org/10.15446/caldasia.v39n1.60527Palabras clave:
Salticid, community, Bamboo gaps, Chusquea ramosissima, rainforest, South America. (en)Saltícidos, comunidad, claros de Bambú, Chusquea ramosissima, bosque lluvioso, América del sur. (es)
Descargas
The Atlantic Forest is one of the key biodiversity hotspots in the world. Its understory can be dominated by species such as the native bamboo Chusquea ramosissima (tacuarembó), which can be an aggressive gap colonizer and change biotic and abiotic conditions compared to mature forest. A strong association has been observed between Salticidae spiders and microhabitat type, frequently linked to host plant structure. Since little is known about the diversity of spiders in tree-fall gaps of the Atlantic Forest, the differences in the Salticidae assemblages between three habitats –the understory of typical forest, Bamboo gaps (dominated by tacuarembó) and restoration areas (tacuarembó removal)– were explored in the southern tip of the Atlantic Forest, Upper Parana Atlantic Forest ecoregion in Misiones province, Argentina. A total of 515 jumping spiders (32 species; 106 adults) were collected. Differences in species composition were detected between habitats, where Forest understory and Bamboo gaps differ in almost 50% of the species. Bamboo gaps had the highest number of exclusive species, but the three habitats did not differ in their average taxonomic diversity indices. In addition, richness was higher in Bamboo gaps compared to Forest understory, which may reflect higher niche diversity in the former habitat.
El Bosque Atlántico es uno de los principales focos de biodiversidad en el mundo. Su sotobosque puede estar dominado por especies como el bambú nativo Chusquea ramosissima (tacuarembó), que puede ser un agresivo colonizador de claros o lagunas (gaps) y cambiar las condiciones bióticas y abióticas en comparación al bosque maduro. Se ha observado una fuerte asociación entre las arañas Salticidae y el tipo de microhábitats, con frecuencia vinculada a la estructura de la vegetación. Dado que se conoce poco acerca de la diversidad de arañas en los claros por caída de árboles del Bosque Atlántico, se exploraron las diferencias en los ensambles de Salticidae en tres hábitats: el Sotobosque de bosque típico, Claros de Bambú (tacuarembó) y en áreas de restauración (remoción del tacuarembó). La región de estudio se encuentra en el extremo sur del Bosque Atlántico, ecorregión Bosque Atlántico Alto Paraná en la provincia de Misiones, Argentina. En total se colectaron 515 arañas (32 especies; 106 adultos). Se detectaron diferencias en la composición de especies entre hábitats, donde el Sotobosque y los Claros de Bambú difirieron en cerca del 50% de las especies. Los Claros de Bambú tuvieron el mayor número de especies exclusivas, no obstante, los tres hábitats no difirieron en sus índices de diversidad taxonómica media. Además, la riqueza fue mayor en los Claros de Bambú comparados con el Sotobosque, lo que podría reflejar una mayor diversidad de nichos en el primer hábitat.
Recibido: 17 de octubre de 2016; Aceptado: 13 de marzo de 2017
ABSTRACT
The Atlantic Forest is one of the key biodiversity hotspots in the world. Its understory can be dominated by species such as the native bamboo Chusquea ramosissima (tacuarembó), which can be an aggressive gap colonizer and change biotic and abiotic conditions compared to mature forest. A strong association has been observed between Salticidae spiders and microhabitat type, frequently linked to host plant structure. Since little is known about the diversity of spiders in tree-fall gaps of the Atlantic Forest, the differences in the Salticidae assemblages between three habitats -the understory of typical forest, Bamboo gaps (dominated by tacuarembó) and restoration areas (tacuarembó removal)- were explored in the southern tip of the Atlantic Forest, Upper Parana Atlantic Forest ecoregion in Misiones province, Argentina. A total of 515 jumping spiders (32 species; 106 adults) were collected. Differences in species composition were detected between habitats, where Forest understory and Bamboo gaps differ in almost 50% of the species. Bamboo gaps had the highest number of exclusive species, but the three habitats did not differ in their average taxonomic diversity indices. In addition, richness was higher in Bamboo gaps compared to Forest understory, which may reflect higher niche diversity in the former habitat.
Key words:
Salticid, community, Bamboo gaps, Chusquea ramosissima, rainforest, South America.RESUMEN
El Bosque Atlántico es uno de los principales focos de biodiversidad en el mundo. Su sotobosque puede estar dominado por especies como el bambú nativo Chusquea ramosissima (tacuarembó), que puede ser un agresivo colonizador de claros o lagunas (gaps) y cambiar las condiciones bióticas y abióticas en comparación al bosque maduro. Se ha observado una fuerte asociación entre las arañas Salticidae y el tipo de microhábitats, con frecuencia vinculada a la estructura de la vegetación. Dado que se conoce poco acerca de la diversidad de arañas en los claros por caída de árboles del Bosque Atlántico, se exploraron las diferencias en los ensambles de Salticidae en tres hábitats: el Sotobosque de bosque típico, Claros de Bambú (tacuarembó) y en áreas de restauración (remoción del tacuarembó). La región de estudio se encuentra en el extremo sur del Bosque Atlántico, ecorregión Bosque Atlántico Alto Paraná en la provincia de Misiones, Argentina. En total se colectaron 515 arañas (32 especies; 106 adultos). Se detectaron diferencias en la composición de especies entre hábitats, donde el Sotobosque y los Claros de Bambú difirieron en cerca del 50% de las especies. Los Claros de Bambú tuvieron el mayor número de especies exclusivas, no obstante, los tres hábitats no difirieron en sus índices de diversidad taxonómica media. Además, la riqueza fue mayor en los Claros de Bambú comparados con el Sotobosque, lo que podría reflejar una mayor diversidad de nichos en el primer hábitat.
Palabras claves:
Saltícidos, comunidad, claros de Bambú, Chusquea ramosissima, bosque lluvioso, América del sur.INTRODUCTION
The Atlantic Forest is a biome that hosts thousands of endemic species and has been internationally recognized as one of the key global biodiversity hotspots (Giraudo et al. 2008). It was considered one of the largest rainforests of the American continent, originally covering around 150 million ha, in highly heterogeneous environmental conditions (Morellato & Haddad 2000). Currently, due to an expansion of the agricultural frontier, deforestation, and urbanization among other factors, an estimated 11 to 16% remains, mostly as small fragments (smaller than 50 ha) (Ribeiro et al. 2009) in Brazil, Paraguay, and Argentina (Tabarelli et al. 2010). The understory of the Atlantic Forest has several Poaceae species such as the native bamboo Chusquea ramosissima Lindm. (tacuarembó), which can be an aggressive colonizer after human disturbance (Campanello et al. 2007, Gallardo et al. 2008) or in naturally opened habitat (Tabarelli & Mantovani 2000). Among the traits explaining its dominance is this production of large amounts of seeds with short dormancy periods, fast growth and efficient vegetative reproduction. It usually plays an important role in the structure and dynamics of forest ecosystems (Lima et al. 2012). Several studies about woody bamboos in the Atlantic Forest showed a variation in light quantity and quality, soil humidity, density, richness and tree regeneration, among others, compared with the mature forest (Campanello et al. 2007, Lima et al. 2012, Tabarelli & Mantovani 2000). Accordingly, both environments, forest and bamboo have particular microclimatic conditions and plant community structure at the understory level. Thus, one strategy for forest restoration is the removal of bamboo stumps, cutting the branches manually.
Spiders are a mega-diverse group comprising 46,438 described species as of January 2017 (World Spider Catalog 2017). They are generalist predators whose distribution and abundance respond to several environmental factors such as vegetation structure and microclimatic conditions (Baldissera & Rodrigues-Silva 2010, Huang et al. 2011). In tropical forests, habitat structure should be regarded as one of the most critical factors influencing the composition and richness of spider communities (Lima-Peres et al. 2007). There are few published studies on Atlantic Forest spider diversity; some usually focus on species richness, diversity and composition of assemblages (e. g. Castanheira et al. 2016, Rubio 2016), habitat fragmentation, and forestation (e. g. Baldissera et al. 2008, Baldissera & Rodrigues-Silva 2010, Lima-Peres et al. 2007, Lima-Peres et al. 2014). Salticidae, also known as jumping spiders, is a large family grouping 620 genera and 5,944 species (World Spider Catalog 2017). They are characterized by an acute visual system and being capable of very agile jumps. Generally, they are day hunters that actively seek their prey (Richmann & Jackson 1992). Cumming & Wesolowska (2004) observed a strong association between salticids and microhabitat type, frequently linked to host plant structure. The combination of narrow spatial niches occupied by most species and diverse microhabitats within a site would explain the high diversity of jumping spiders.
An additional feature that can be used for the analysis of species diversity is the degree of phylogenetic relatedness between species, which has great potential in setting conservation priorities and for environmental monitoring (Vane-Wright et al. 1991, Warwick & Clarke 1995, 1998, Clarke & Warwick 1999). Clarke & Warwick's taxonomic diversity indices are appropriate tools to assess this facet of biodiversity, because they do not depend on sampling effort and take into account the taxonomic arrangement of species as a crude approximation of their evolutionary diversity (Warwick & Clarke 1995, Clarke & Warwick 1998). Also, they are based on the idea that a community with closely related species is less biodiverse than a community with low relatedness among species (Rubio 2016).
To the best of our knowledge, there are few publications on the diversity of spiders in tree-fall gaps of the Atlantic Forest. For example, in the Northeast region of Brazil, Lima-Peres et al. (2014) reported tree-fall gap formation triggering significant changes in the spiders' assemblages in relation to the adjacent forest. Indeed, forest yielded the highest richness and number of exclusive species compared to edges and open gaps. The spider assemblages of the southern tip of the Atlantic Forest, Upper Parana Atlantic Forest ecoregion, are poorly explored compared to the spider fauna of Northern hemisphere or even lower latitudes of the Atlantic Forest.
We describe the Salticidae fauna of the understory of a typical forest, a forest dominated by bamboo, and a restoration habitat (explained below) and explore potential differences on spider fauna between them. Our working hypothesis is that the diversity of low-foliage salticids (understory up to 2 m) differs between open canopy forests dominated by bamboo, bamboo gaps (from here on), and closed canopy forest with no bamboo. Patches dominated by bamboo should have a higher Salticidae diversity due to higher foliage density, offering a higher number of potential niches.
METHODOLOGY
On the use of spiders
Within the context of a larger project on the biodiversity of the spiders from the understory of a wildlife reserve, we assessed a subset of data comprising adult Salticidae specimens, as potential indicators of global spider diversity. Salticidae represents a well-known taxon in this area (Rubio 2014, Galvis & Rubio 2016, Rubio 2016, Rubio & Baigorria 2016), thus, common errors derived from the use of morphospecies to characterize richness are strongly reduced. This allows a reliable interpretation of results.
Spiders are considered useful indicators of the conservation status of a habitat, because they respond to changes in environment conditions (Pearce & Venier2006). However, the use of such a large taxonomic group (all Araneae) generates identification of many of the entities that subsequently may result in misinterpretation of results (Bortolus 2008). The use of a limited taxon (e.g. a family sorted by a specialist) provides manageable and more accurate data knowledge. On the other hand, immature spider specimens are usually indistinguishable to the human eye, even that of a specialist, at the species, morphospecies, genus and, in some cases, family level. It is known that 60 - 70% of individuals in a spider community regularly found in the field are immature and this reduces the real size of useful study material. If immature specimens should be included, then there is a high risk of overestimation of richness, increse in rare species, and unreal species turnover.
Study area
The study area was in Urugua-í Wildlife Reserve ("Reserva de Vida Silvestre Urugua-í" -RVSU) in the Province of Misiones, northeast of Argentina (25° 59' South, 54°05'West) (Fig. 1a). The reserve contains 3200 ha of mixed rainforest. Local climate is classified as subtropical, and the study area is characterized by a semi-deciduous subtropical forest; mean annual precipitation ranges from 1700 to 2200 mm and with no marked dry season (Crespo 1982). The annual temperature ranges between 16-22 °C. The RVSU was exploited by the forest industry until the 1990's; however, it has a good conservation state (Paviolo et al. 2009).
Figure 1: Location of Urugua-í Wildlife Reserve and sampling area, Misiones Province, Argentina a. Studied habitats: Black dots = Forest understory; white dots = Bamboo gaps; yellow dots = Restoration sites. b. Forest understory. c. Bamboo gaps.
We selected three different understory habitats, i.e. representing lower strata: 1) Forest understory (Fig. 1b): shady and cool forest with closed canopy and low light intensity. The understory is relatively sparse, plant density is low, and dominated by ferns and seedlings of native trees such as Allophylus guaraniticus (A. St.-Hil.), Nectandra megapotamica (Spreng.), Euterpe edulis Mart. and some Araucaria angustifolia (Bertol.) specimens (Cabrera 1971). 2) Bamboo gaps (Fig. 1c): areas of open canopies in the forest resulting in warmer sites with high light intensity and dense vegetation that can exceed the height of a human adult, and thus are difficult to access. These gap areas have, according to Cabrera (1971), high dominance of woody bamboo such as Guadua trinii (Nees), Chusquea ramosissima Lindm., Chusquea tenella Nees, and Merostachys claussenii Munro. 3) Restoration sites: habitats under an experimental treatment, where the bamboo has been cut-down as a means to allow establishment of trees out-competed by bamboo. In these patches, bamboo stumps are manually cut down to <15 cm high so that the sapling of the original forest that remains underneath have access to light and can grow.
Sampling method
Samples were obtained in September 2008, March 2009, June 2009, and September 2009 to account for potential seasonal variations in species presence or abundance. In September 2008, restoration habitats were reduced to bare ground due to very recent bamboo cuttings and thus no spider samples were collected. Within each habitat category, four patches were randomly selected, located at least 700 meters apart, except for the restoration sites in which three patches were sampled. Within each patch, samples were collected from three sites. Spiders were collected using the beating method (Coddington et al. 1991). Beating was done by firmly striking branches and foliage with a mallet, over a 69 x 95 cm white cloth quadrant, and striking was repeated five times. This is the most used methodology for salticid collecting (e.g. by Wayne Maddison Lab; for an excellent explanation, you can see https://waynemaddisonlab.wordpress.com/2014/04/22/video-on-how-to-collect-jumping-spiders/. All spiders dislodged were removed from the cloth with forceps and soft paintbrushes and placed into labelled plastic containers with 70% ethanol.
Adult spiders collected were identified using in the first instance the database websites by Metzner (2015) and Prószyński (2015), and by the use of original papers with descriptions and revisions for each corresponding salticid group. Within each gender, diagnostic characteristics were used to distinguish species. Due to the difficulty of assigning most immature spiders to species, juveniles (except subadults) were excluded from analysis. In a few cases the abbreviations "cf." (confer: compare with) and "aff." (affinis: related to; has an affinity to) were used to name individuals.
Statistical analyses
Habitats were compared using incidence data models. Incidences were the presence/absence records of each species detected for the sampling units. First, to estimate completeness of the sample, the sample coverage (C) representing the fraction of incidence probabilities that are associated with the detected species, were evaluated for each habitat. Next, for habitats with C >50%, the observed richness and the Chao2-bc value (i.e., an estimate of expected richness) were recorded. Chao2-bc, a corrected form of Chao2, uses the numbers of uniques, species detected in only one sampling unit, and duplicates, species detected in two sampling units, to estimate the number of undetected species (SpadeR software; Chao et al. 2015).
The Average Taxonomic Distinctness (Δ+) and Variation in Taxonomic Distinctness (Λ+) were the two taxonomic measures used following Clarke & Warwick (1998, 2001). These taxonomic indices are relevant measures of diversity due to their lack of dependence on sampling effort and having a statistical framework for the assessment of the significance of departure from expectation (Warwick & Clarke 1998). On one hand, Δ + is a direct measure of taxonomic diversity: a high value reflects high taxonomic diversity or low relatedness among species, while Λ + is a measure of unevenness in the taxonomic tree, reflecting the degree to which certain taxa are over- or under-represented in samples. For detailed assumptions and a complete interpretation, see Warwick & Clarke (1995) or Magurran (2004). For these analyses the species were classified into genera, tribes, subfamilies and family, reaching five levels; subfamily and tribes classification of salticids was based on Maddison (2015). Weights (ω) were determined by species richness in master data as 14.48 for species, 39.76 for genus, 76.83 for tribe, and 100 for subfamily. To detect differences in the taxonomic distinctness at each observed habitat category, a randomization test with 1,000 random samples, taking into account the number of species sampled, was also performed from the expected values derived from the species pool (Clarke & Warwick 1998). The null hypothesis assumes that each sample contains species randomly selected from the pool and that it should therefore fall within the 95% confidence intervals. All the analyses of taxonomic diversity measures and randomization tests were performed using PRIMER (Clarke & Gorley 2001).
To assess species/morphospecies similarity between pairs of habitats the popular and simple similarity measure βsim was used; this is a variant of S0rensen (1948) given by Lennon et al. (2001) that focuses on differences in species composition between two communities, and is calculated as  , where α = number of species shared between sites, b = total number of species that occur only in one site, and c = total number of species that occur in the other site. This index was chosen because it is independent of the relative abundances of the species/morphospecies and it is easy to calculate and interpret (Magurran 2004).
, where α = number of species shared between sites, b = total number of species that occur only in one site, and c = total number of species that occur in the other site. This index was chosen because it is independent of the relative abundances of the species/morphospecies and it is easy to calculate and interpret (Magurran 2004).
RESULTS
A total of 106 adults (46 males and 60 females), plus 409 juveniles, salticid spiders were collected among the three sampled habitats and 32 taxa were identified (Table 1), 25 to the species level and seven to the genus level. Seventeen species were singletons representing 53% of the total sample, and four doubletons representing 12.5% of the total. Only three species were relatively abundant with over ten specimens: Mopiopia comatula Simon, 1902 (22 specimens), Scopocira histrio Simon 1900 (19 specimens) and Saitis cyanipes Simon, 1901 (ten specimens). M. comatula and S. histrio were more abundant in Bamboo gaps than in Forest understory, and they were absent in Restoration sites. Bamboo gaps had the highest number of exclusive species (ten) followed by Restoration sites (eight) and Forest understory (four) (Fig. 2).
Table 1: Abundance data of adult specimens for species and morphospecies collected per habitat category in Urugua-í Wildlife Reserve, Misiones Province, Argentina.

Figure 2: Number of exclusive or shared Salticidae species between habitat categories in Urugua-í Wildlife Reserve, Misiones Province, Argentina.
The Average Taxonomic Distinctness (Δ+) index had a slightly higher value of taxonomic salticid diversity in Restoration sites than in the other two places, although not significantly different among the three habitat categories from the expected by random (Table 2, Fig. 3). A comparable pattern was found with the index of Variation in Taxonomic Distinctness (Λ+), but in this case Restoration sites and Forest understory had similar higher values than Bamboo gaps, the A+ value of the latter was statistically lower from the value calculated with the randomization test (Table 2).
*: statistically significant different from the predicted value by a randomization test (P < 0.05).Table 2: Taxonomic diversity values of jumping spiders (Salticidae) per habitat category in Urugua-í Wildlife Reserve, Misiones Province, Argentina according to the indices proposed by Clarke & Warwick (1998)
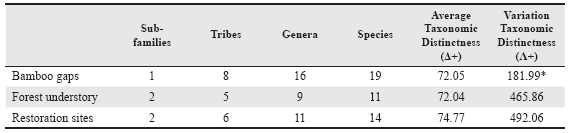
Figure 3: Funnel graph representing the 95 % confidence limits for Average Taxonomic Distinctness (Δ+) and Variation in Taxonomic Distinctness (Λ+) of jumping spiders (Salticidae) per habitat category in Urugua-í Wildlife Reserve, Misiones Province, Argentina.
Sample coverage was considered adequate for Forest understory (90%) and Bamboo gaps (75%). As Restoration sites had very low number of specimens, data were not enough to estimate coverage based on presence-absence counts. Besides, when considering abundance data, coverage was only 14%, thus this habitat was used only to describe the taxonomic diversity and species found.
Taking all the above results into account, the samples obtained from the Bamboo gaps and Forest understory were considered to have provided acceptable representations of the species present at both habitats. The expected richness (Chao2-bc for incidences) was 39.12 ± 16.10 for Bamboo gaps and 11.39 ± 0.85 for Forest understory, compared to an observed richness of 19 and eleven species, respectively. Similarity between Bamboo gaps and Forest understory was βsmi = 0.45.
DISCUSSION
For this study 515 individuals of Salticidae were collected from a total 86.53 m2 beating area. We identified 106 adult salticid specimens, a relatively high number when compared to other studies such as Marfil et al. (2016) who focused only on salticids and collected 975 (214 adults) on a natural reserve, combining beating net and manual sampling in five different areas, collecting monthly over three years, or Castanheira et al. (2016) who gathered 640 salticids (adult + juvenile) at Pedra Branca State Park, Rio de Janeiro, Brazil, using a combination of six active (hand collections, sweeping net, beating tray, sieving, cryptic) and one passive (pitfall) collecting techniques. On the other hand, several studies on the diversity of Atlantic Forest spiders, such as those of Lima-Peres et al. (2014) and Baldissera & Rodriguez-Silva (2010) collected fewer specimens (49 and 32 salticids, respectively) with comparatively higher sampling effort: Lima-Peres et al. (2014) sorted 480 m2 leaf-litter samples complemented with 120 h of active search, while Baldissera & Silva (2010) sorted 270 m2 beating tray samples. Both Lima-Peres et al. (2014) and Baldissera & Rodriguez-Silva (2010) highlight that Salticidae is the third most abundant family after Theridiidae and Linyphiidae.
In the present study, from a total of 32 species identified, 21 (65%) were singletons or doubletons. Frequent rare species, although indicative of insufficient sampling effort, are relatively common in spider community studies, due to a combination of their high diversity and practical sampling issues (Magurran 2004). For example, Rubio (2014) examined 463 salticid records from Misiones from several sources such as museum arachnid collections and literature and found that 78.3% were rare species in the Atlantic Forest. In a small tropical suburban study site in Zimbabwe that was surveyed over four years, 67% of a 40-species assemblage of jumping spiders were uncommon or rare species (Cumming & Wesolowska 2004). Another study on Tanzania mountain forest canopies described Salticidae as the third richest family in terms of number of species (15 species) but with a more equitable abundance (4 singletons and 1 doubleton) (SØrensen 2004).
The richness of this family observed in the present study was also in line with other recent similar surveys, such as Castanheira et al. (2016) who reported 45 species of jumping spiders in an Atlantic Forest; and Marfil et al. (2015) whom collected 29 species in a southern mostly subtropical forest. In Urugua-í Wildlife Reserve, in all 32 species were collected (Table 1). Considering that species coverage was insufficient in the Restoration sites and that estimated number of species was higher than observed in Bamboo gaps, it is expected that a higher number of species should be detected at RVSU with higher sampling effort or a combination of sampling techniques.
The assessment of taxonomic diversity offers a different perspective on the jumping spider communities. Bamboo gaps and Forest understory are similar in average taxonomic distinctness even if the former has higher number of species, mainly because most genera have one species. However, structure across the taxonomic units differed. Bamboo gaps had lower than expected value of variation in taxonomic distinctness, indicating evenness in the distribution of species in the higher-level taxa. This is clear-cut because almost every genus has one single species. In Forest understory, out of the five tribes collected, most species belonged to the Euophryini tribe; although this was also a frequent tribe in terms of numbers of species in Bamboo gaps, species were more evenly distributed among eight tribes in the latter habitat. This equitable representation of taxa in the community structure is an issue that should be studied in depth to understand processes and mechanisms leading to this pattern as well as for the interpretation of its implications for ecological functioning. Jumping spiders represent a well-known taxon in this area, according to Rubio (2014) and numerous Galiano papers between 1962 and 1999 (Rubio 2014). Rubio (2016) showed taxonomic dissimilarity in Salticidae among ecoregions and highlighted the significant contribution of P-taxonomic diversity to the regional jumping spider richness in Misiones province located in northeast Argentina. The present results agree in showing changes in spider assemblages according to different environments.
The results suggest that the Bamboo gaps are richer in Salticidae species than the understory of closed canopy forests, which is consistent with the intermediate disturbance hypothesis predicting the highest diversity under intermediate level of disturbances (Connell 1978), such as forests gaps. Also, the differences in species richness may be related to the more complex vertical structure of the Bamboo gaps. Moreover, the similarity measure shows that the Forest undestor and Bamboo gaps differ in almost 50% of the species found. Even though this is a preliminary result, and some of the differences in species composition may be artefacts due to the presence of singletons and uniques in the dataset, some differences may be explained by differing habitat preferences or requirements of some species. In fact, in an Atlantic Forest remnant in Recife, northeast Brazil, differences in species assemblages were detected between tree-fall gaps and forest using hand-searching (Lima-Peres et al. 2007). Although Lima-Perez et al. (2014) found the highest number of exclusive species occurring in the forest (in Bahia, Brazil), our results contrast with their because fewer exclusive species were detected in the Forest understory compared to Bamboo gaps, highlighting the higher richness of the latter habitat.
AUTHOR CONTRIBUTIONS
GDR conceived and designed the sampling; CIA and GDR collected data and processed samples; GDR, CIA, and RMG analyzed and interpreted the data and wrote the manuscript.
ACKNOWLEDGEMENTS
We wish to thank Fundación Vida Silvestre Argentina (FVSA) for their hospitality, lodging and funding; Ministerio de Ecología y Recursos Naturales Renovables (MEyRNR) of Misiones Province for collecting permits; the co-collectors for their assistance in the field, sampling and design: Emiliano H. Ocampo, Lisandro H. Negrete, Julián N. Lescano, Laura Aréjolas and Claudia E. Moreno. C.I. Argañaraz holds a doctoral scholarship from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and is a doctorate of Facultad de Ciencias Exactas Físicas y Naturales, Universidad Nacional de Córdoba (UNC); G.D. Rubio and R.M. Gleiser are Career Researchers of CONICET. Funding: this work was partly supported by the "Fondo para la Investigación Científica y Tecnológica" (FONCyT): grant PICT-2013-1664 given to GDR.
LITERATURE CITED
Referencias
Baldissera, R., G. Ganade, A.D. Brescovit & S.M. Hartz. 2008. Landscape mosaic of Araucaria forest and forest monocultures influencing understory spider assemblages in southern Brazil. Austral Ecology 33: 45−54.
Baldissera, R. & V. Rodrigues-Silva. 2010. Diversity and composition of arbustive spiders in an Atlantic Forest fragment and two adjacent areas. Neotropical Biology and Conservation 5: 77−85.
Bortolus, A. 2008. Error cascades in the biological sciences: the unwanted consequences of using bad taxonomy in ecology. Ambio 37:114−118.
Cabrera, A.L. 1971. Fitogeografía de la República Argentina. Boletín de la Sociedad Argentina de Botánica 14: 1−42.
Campanello P.I., M.G. Gatti, A. Ares, L. Montti & G. Goldstein. 2007. Tree regeneration and microclimate in a liana and bamboo-dominated semideciduous Atlantic forest. Forest Ecology and Management 252: 108−117.
Castanheira, P., A. Perez-G & R.L.C. Baptista. 2016. Spider diversity (Arachnida: Araneae) in Atlantic Forest areas at Pedra Branca State Park, Rio de Janeiro, Brazil. Biodiversity Data Journal 4:e7055.
Chao, A., K.H. Ma, T. C. Hsieh & C.H. Chiu. 2015. Online Program SpadeR (Speciesrichness Prediction and Diversity Estimation in R). Program and User’s Guide published at http://chao.stat.nthu.edu.tw/wordpress/software_download/.
Clarke, K.R. & R.N. Gorley. 2001. PRIMER v5: User manual/tutorial. Plymouth: Plymouth
Marine Laboratory.
Clarke, K.R. & R.M. Warwick. 1998. A taxonomic distinctness index and its statistical properties. Journal of Applied Ecology 35: 523−531.
Clarke, K.R. & R.M. Warwick. 1999. The taxonomic distinctness measure of biodiversity: weighing of step lengths between hierarchical levels. Marine Ecology Progress Series 184:21−29.
Clarke, K.R. & R.M. Warwick. 2001. Change in marine communities: An approach to statistical analysis and interpretation 2nd ed. PRIMER-E. Ltd, Plymouth Marine Laboratory, United Kingdom. 176 pp.
Coddington, J.A., C.E. Griswold, D.S. Dávila, E. Peñaranda & S.F. Larcher. 1991. Designing and testing sampling protocols to estimate biodiversity in tropical ecosystems. In: Dudley, E.C. (ed). Unity of Evolutionary Biology: Proceedings of The Fourth International Congress of Systematic and Evolutionary Biology. Dioscorides Press: 44−60.
Connell, J.H. 1978. Diversity in tropical rain forest and coral reefs. Science 199: 1302−1310.
Crespo, J.A. 1982. Ecología de la comunidad de mamíferos del Parque Nacional Iguazú, Misiones. Revista del Museo Argentino de Ciencias Naturales, Ecología 3: 45−162.
Cumming, M.S. & W. Wesołows ka. 2004. Habitat separation in a species-rich assemblage of jumping spiders (Araneae: Salticidae) in a suburban study site in Zimbabwe. Journal of Zoology 262: 1−10.
Gallardo, A., L. Monttini & S.P. Bravo. 2008. Effects of tacuarembó (Chusquea ramosissima, Poaceae) on seed dispersal process in Misiones forest. Austral Ecology 18: 347−356.
Galvis, W. & G.D. Rubio. 2016. On new records and distribution of ten species of the genus Lyssomanes Hentz from southern South America (Araneae: Salticidae: Lyssomaninae). Acta Arachnologica 65: 19−25.
Giraudo, A.R., S.D. Matteuchi, J. Alonso, J. Herrera & R.R. Abramson. 2008. Comparing bird assemblages in large and small fragments of the Atlantic Forest hotspot. Biodiversity and Conservation 17: 1251−1265.
Huang, P.S., I.M. Tso, H.C. Lin, L.K. Lin & C.P. Lin. 2011. Effects of thinning on spider diversity of an East Asian subtropical plantation forest. Zoological Studies 50: 705−717.
Lennon, J.J., P. Koleff, J.J.D. Greenwood & K.J. Gaston. 2001. The geographical structure of British bird distributions: diversity, spatial turnover and scale. Journal of Animal Ecology 70: 966−979.
Lima, R.A.F., D.C. Rother, A.E. Muler, I.F. Lepsch & R.R. Rodrigues. 2012. Bamboo overabundance alters forest structure and dynamics in the Atlantic forest hotspot. Biological Conservation 147: 32−39.
Lima-Peres, M.C., J.M. Cardoso Da Silva & A.D. Brescovit. 2007. The influence of treefall gaps on the distribution of web-building and ground hunter spiders in an Atlantic Forest remnant, northeastern Brazil. Studies on Neotropical Fauna and Environment 42: 49−60.
Lima-Peres, M.C., K.R. Benati, A. Rodrigues S. De Andrade, M.V.A. Guimarães, T. Da Silva Melo, A.D. Brescovit & J.H.C. Delabie. 2014. Tree-fall gaps effects on spider (Araneae) assemblages in an Atlantic Forest landscape in Northeastern Brazil. Open Journal of Animals Science 4: 118−133.
Maddison, W.P. 2015. A phylogenetic classification of jumping spiders (Araneae: Salticidae). Journal of Arachnology 43(3): 231−292.
Magurran, A.E. 2004. Measuring biological diversity. Blackwell Science Ltd. Malden, MA. Marfil, M.F., C.L. Scioscia, A. Armendano & A. Gonzalez. 2015. Diversity of Salticidae (Arachnida: Araneae), in the historical and natural reserve “Martín Garcia Island”, Argentina. Journal of Natural History 50: 689−700.
Metzner, H. 2015. Worldwide database of jumping spiders (Arachnida, Araneae, Salticidae). http://www.jumping-spiders.com/index.php. Accessed 02 May 2016.
Morellato, L.P. & C.F.B. Haddad. 2000. Introduction: The Brazilian Atlantic Forest. Biotropica 32: 786−792.
Paviolo, A., C. Deangelo, Y. Di Blanco, I. Agostini, E. Pizzio, R. Melzew, C. Ferrari, L. Palacio & M.S. Dibitetti. 2009. Efecto de la caza y el nivel de protección en la abundancia de los grandes mamíferos del Bosque Atlántico de Misiones. Biodiversidad y manejo de recursos naturales. Contribuciones para la conservación y manejo del Parque Nacional Iguazú. Buenos Aires. pp. 237−274.
Pearce, J.L. & L.A. Venier. 2006. The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: A review. Ecological Indicators 6: 780−793.
Prószyński, J. 2015. Monograph of Salticidae (Araneae) of the world. http://www.peckhamia.com/salticidae/salticidae.php. Accessed 15 April 2016.
Ribeiro, M.C., J.P. Metzger, A.C. Martensen, F.J. Ponzoni & M.M. Hirota. 2009. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biology Conservation 142: 1141−1153.
Richman, D.B. & R.R. Jackson. 1992. A review of the ethology of jumping spider (Araneae: Salticidae). Bulletin of British Arachnology Society 9: 33−37.
Rubio, G.D. 2014. Baseline richness of Salticidae (Araneae) from Misiones, Argentina. Peckhamia 118.1: 1−21.
Rubio, G.D. 2016. Using a jumping spider fauna inventory (Araneae: Salticidae) as an indicator of their taxonomic diversity in Misiones, Argentina. Revista de Biología Tropical 64: 875−883.
Rubio, G.D. & J. Baigorria. 2016. New species and the first known male of the jumping spider Ceriomura Simon (Araneae: Salticidae: Gophoini) with the faunistic revision of gophoines from Misiones, Argentina. Zoology and Ecology 26(4): 265−274.
Sørensen, L.L. 2004. Composition and diversity of the spider fauna in the canopy of a montane forest in Tanzania. Biodiversity Conservation 13: 437−452.
Tabarelli, M. & W. Mantovani. 2000. Gapphase regeneration in a tropical montane forest: the effects of gap structure and bamboo species. Plant Ecology 148: 149−155.
Tabarelli, M., A.V. Aguiar, M.C. Ribeiro, J.P. Metzger & C.A. Peres. 2010 Prospects for biodiversity conservation in the Atlantic Forest: Lessons from aging human-modified landscapes. Biology Conservation 143: 2328−2340.
Vane-Wright, R.I., C.J. Humphries & P.H. Williams. 1991. What to protect? Systematics and the agony of choice. Biological Conservation 55: 235−254.
Warwick, R.M. & K.R. Clarke. 1995. New “biodiversity” measures reveal a decrease in taxonomic distinctness with increasing stress. Marine Ecology Progress Series 129: 301−305.
Warwick, R.M. & K.R. Clarke. 1998. Taxonomic distinctness and environmental assessment. Journal of Applied Ecology 35: 532−543.
World Spider Catalog 18.0. 2017. Natural History Museum Bern. http://wsc.nmbe.ch. Accessed 06 February 2017.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Gonzalo D. Rubio, Cristian E. Stolar, Diana V. Ohashi, Julián E. Baigorria. (2019). Jumping spiders (Araneae: Salticidae) in agroecosystems: a case study to know how friendly some crops can be for native fauna. Studies on Neotropical Fauna and Environment, 54(2), p.133. https://doi.org/10.1080/01650521.2019.1629174.
2. Bin-lu Liu, Yan-bin Yao, Zi-han Cai, Zhong-jing Wang, Ke-ke Liu. (2025). Diversity of jumping spiders (Araneae, Salticidae) in the Jinggangshan National Nature Reserve, Jiangxi, China. ZooKeys, 1239, p.183. https://doi.org/10.3897/zookeys.1239.140810.
3. Helga Cecilia Achitte-Schmutzler, Gilberto Avalos, Elena Beatríz Oscherov. (2022). Diversidad taxonómica de Thomisidae (Araneae) en ambientes heterogéneos del sitio Ramsar Humedales Chaco, Argentina. Caldasia, 44(1), p.119. https://doi.org/10.15446/caldasia.v44n1.83581.
4. Régis Céréghino, Bruno Corbara, Yann Hénaut, Camille Bonhomme, Arthur Compin, Alain Dejean. (2019). Ant and spider species as surrogates for functional community composition of epiphyte-associated invertebrates in a tropical moist forest. Ecological Indicators, 96, p.694. https://doi.org/10.1016/j.ecolind.2018.05.037.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Caldasia

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).