Publicado
Diversidad taxonómica de Thomisidae (Araneae) en ambientes heterogéneos del sitio Ramsar Humedales Chaco, Argentina
Taxonomic diversity of Thomisidae (Araneae) in heterogeneous environments of the Ramsar site Chaco Wetlands, Argentina
DOI:
https://doi.org/10.15446/caldasia.v44n1.83581Palabras clave:
arañas cangrejo, complejidad vegetal, área prioritaria de conservación, disimilitud taxonómica (es)crab spiders, taxonomic dissimilarity, vegetation complexity, priority conservation area (en)
Descargas
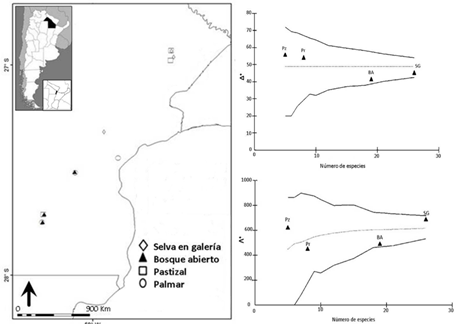
El sitio Ramsar Humedales Chaco se caracteriza por su heterogeneidad ambiental y elevada diversidad de flora y fauna. Sin embargo, la fragmentación y homogeneización en estos ambientes tiende a afectar negativamente a taxones con baja abundancia o riqueza como los Thomisidae. En este trabajo se analiza la diversidad taxonómica de los ensambles de Thomisidae en ambientes con distintos grados de complejidad vegetal. Los muestreos se efectuaron durante los años 2013 a 2016 en pastizales, palmares, bosques abiertos y selvas de galería de siete localidades, mediante las técnicas de golpeteo de follaje, aspirado y observación directa. Se obtuvieron en total 464 individuos y 34 especies/morfoespecies pertenecientes a cinco subfamilias, siete tribus y diez géneros. Los ambientes boscosos mostraron baja diversidad taxonómica (Δ+) y altos valores de variación taxonómica (Λ+), ello refleja un árbol filogenético de Thomisidae poco uniforme debido a una sobrerrepresentación de Tmarus. La elevada riqueza de este género en los bosques sugiere que el mecanismo de ensamblaje del género posiblemente esté mediado por filtros ambientales, donde las condiciones bióticas favorecen la coexistencia de especies, los cuales les permiten sobrevivir en determinados hábitats. En los pastizales y palmares las especies estuvieron uniformemente representadas en el árbol filogenético. Los bosques degradados presentaron una estructura específica y taxonómica más cercana a los ambientes de pastizal y palmar. La inclusión de las relaciones filogenéticas en los análisis de diversidad podría ser clave a la hora de implementar planes de manejo y conservación.
The Ramsar site Chaco Wetlands is characterized by its environmental heterogeneity and high diversity of flora and fauna. However, fragmentation and homogenization of environments tend to negatively affect taxa with low abundance or richness like the Thomisidae spiders. In this work, taxonomic diversity is analyzed for Thomisidae assemblages in those environments with varying degrees of plant complexity. The samples were carried out in grasslands, palm groves, open forests, and gallery forests of seven localities, during the years 2013 to 2016, by the techniques of foliage beating, vacuum sampling, and direct observation. A total of 464 individuals and 34 species/morphospecies were obtained. These samples belong to five subfamilies, seven tribes, and ten genera. Forested environments showed low taxonomic diversity (Δ +) and high values of taxonomic variation (Λ +), this reflects a phylogenetic tree of Thomisidae non-uniform due to an overrepresentation of species of the genus Tmarus. The high species richness of this genus in forests suggests that environmental filters possibly mediate the mechanism of assembly, where biotic conditions favor the coexistence of species, which allow them to survive in certain habitats. In the grasslands and palm groves, the species were uniformly represented in the phylogenetic tree. The degraded forests presented a specific and taxonomic structure closer to the grassland and palm grove environments. The inclusion of phylogenetic relationships in diversity analyzes could be key in implementing management and conservation plans.
Referencias
Achitte-Schmutzler HC, Avalos G, Oscherov EB. 2016. Comunidades de arañas en dos localidades del sitio Ramsar Humedales Chaco, Argentina. Cuad. Inv. UNED 8(2):115-121. doi: https://doi.org/10.22458/urj.v8i2.1548
Argañaraz CI, Rubio GD, Gleiser RM. 2017. Jumping spider (Araneae: Salticidae) diversity in the understory of the Argentinian Atlantic Forest. Caldasia. 39(1):157-168. doi: https://doi.org/10.15446/caldasia.v39n1.60527
Avalos G, Rubio GD, Bar ME, González A. 2007. Arañas (Arachnida, Araneae) asociadas a dos bosques degradados del Chaco húmedo en Corrientes, Argentina. Rev. Biol. Trop. 55(3-4):899-909. doi: https://doi.org/10.15517/rbt.v55i3-4.5965
Avalos G, Damborsky MP, Bar ME, Oscherov EB, Porcel E. 2009. Composición de la fauna de Araneae (Arachnida) de la Reserva Provincial Iberá, Corrientes, Argentina. Rev. Biol. Trop. 57(1-2):339-351. doi: https://doi.org/10.15517/rbt.v57i1-2.11325
Avalos G, Bar ME, Oscherov EB, González A. 2013. Diversidad de Araneae en cultivos de Citrus sinensis (Rutaceae) de la Provincia de Corrientes, Argentina. Rev. Biol. Trop. 61(3):1243-1260. doi: https://doi.org/10.15517/rbt.v61i3.11938
Bacaro G, Ricotta C, Mazzoleni S. 2007. Measuring beta-diversity from taxonomic similarity. J. Veg. Sci. 18(6):793-798. doi: https://doi.org/10.1111/j.1654-1103.2007.tb02595.x
Bacaro G, Rocchini D, Duprè C, Diekmann M, Carnesecchi F, Gori V, Chiarucci A. 2012. Absence of distance decay in the similarity of plots at small extent in an urban brownfield. Community Ecol. 13(1):36-44. doi: https://doi.org/10.1556/comec.13.2012.1.5
Benjamin SP. 2011. Phylogenetics and comparative morphology of crab spiders (Araneae: Dionycha, Thomisidae). Zootaxa. 3080(1): 1-108. doi: https://doi.org/10.11646/zootaxa.3080.1.1
Benjamin SP. 2013. On the crab spider genus Angaeus Thorell, 1881 and its junior synonym Paraborboropactus Tang and Li, 2009 (Araneae: Thomisidae). Zootaxa. 3636(1): 71-80. doi: https://doi.org/10.11646/zootaxa.3635.1.7
Benjamin SP. 2015. On the African crab spider genus Geraesta Simon, 1889 (Araneae: Thomisidae). Afr Invertebr. 56(2): 309-318. doi: https://doi.org/10.5733/afin.056.0205
Benjamin SP. 2017. A new species of Angaeus from Malaysia with possible affinity to related genera within Stephanopinae (Araneae: Thomisidae). Zootaxa. 4337(2): 297-300. doi: https://doi.org/10.11646/zootaxa.4337.2.10
Benjamin SP, Dimitrov D, Gillespie RG, Hormiga G. 2008. Family ties: molecular phylogeny of crab spiders (Araneae: Thomisidae). Cladistics. 24(5): 708-722. doi: https://doi.org/10.1111/j.1096-0031.2008.00202.x
Bonaldo AB, Marques MAL, Pinto-da-Rocha R, Gardner T. 2007. Species richness and community structure of arboreal spider assemblages in fragments of three vegetacional types at Banhado Grande wet plain, Gravataí river, Rio Grande do Sul, Brazil. Iheringia, Ser. Zool. 97(2):143-151. doi: https://doi.org/10.1590/S0073-47212007000200003
[CAA] Catálogo de Arañas de Argentina. c2018. Catálogo de Arañas de Argentina. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, en línea. [Revisada en: 7 Dic 2020]. http://sites.google.com/site/catalogodearanasdeargentina/
Capetillo-Piñar N, Villalejo Fuerte MT, Tripp-Quezada A. 2015. Distinción taxonómica de los moluscos de fondos blandos del Golfo de Batabanó, Cuba. Lat. Am. J. Aquat. Res. 43(5):845-855.
Cardoso P, Pekár S, Jocqué R, Coddington JA. 2011. Global Patterns of Guild Composition and Functional Diversity of Spiders. PLoS ONE 6(6): e21710. doi: https://doi.org/10.1371/journal.pone.0021710
Cavender‐Bares J, Kozak KH, Fine PVA, Kembel SW. 2009. The merging of community ecology and phylogenetic biology. Ecol. Let. 12(7):693-715. doi: https://doi.org/10.1111/j.1461-0248.2009.01314.x
Clarke KR, Gorley RN. 2001. PRIMER v5: User manual/tutorial. Plymouth: Plymouth Marine Laboratory.
Clarke KR, Warwick RM. 1998. A taxonomic distinctness and its statistical properties. J. Appl. Ecol. 35(4):523-531. doi: https://doi.org/10.1046/j.1365-2664.1998.3540523.x
Clarke KR, Warwick RM. 2001. Change in marine communities: an approach to statistical analysis and interpretation. Second edition, PRIMER-E. United Kingdom: Plymouth Marine Laboratory Ltd.
Cruz-Elizalde R, Ramirez-Bautista A, Johnson JD, Moreno CE. 2014. Community structure of reptiles from the southern portion of the Chihuahuan Desert Region, Mexico. North-West. J. Zool. 10(1):173-182.
Ellingsen KE, Clarke KR, Somerfield PJ, Warwick RM. 2005. Taxonomic distinctness as a measure of diversity applied over a large scale: the benthos of the Norwegiancontinental shelf. J. Anim. Ecol. 74(6): 1069-1079. doi: https://doi.org/10.1111/j.1365-2656.2005.01004.x
García-de Jesús S, Moreno-Ortega CE, Morón MA, Castellanos I, Pavón NP. 2016. Integrando la estructura taxonómica en el análisis de la diversidad alfa y beta de los escarabajos Melolonthidae en la Faja Volcánica Transmexicana. Rev. Mex. Biodiver. 87(3):1033-1044. doi: http://dx.doi.org/10.1016/j.rmb.2016.06.003
Grismado CJ, Achitte-Schmutzler HC. 2020. The crab spider genus Uraarachne Keyserling (Araneae: Thomisidae) in Argentina, Uruguay and Paraguay: a proposal of its senior synonymy over Plancinus Simon, and description of four new species. Rev. Mus. Argent. Cienc. Nat. 22(1): 91-130. doi: http://dx.doi.org/10.22179/REVMACN.22.665
Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 4(1):1-9.
Hidalgo G, Toledo W, Granados-Barba A. 2015. Diversidad y distinción taxonómica de la macrofauna en fondos blandos de la plataforma norte y suroccidental cubana. Lat. Am. J. Aquat. Res. 43(5):845-855. DOI: https://doi.org/10.3856/vol43-issue5-fulltext-5
Hodge S, Vink CJ, Banks CJ, Bowie MH. 2007. The use of tree-mounted artificial shelters to investigate arboreal spider communities in New Zealand nature reserves. J. Arachnol. 35(1):129-136. doi: https://doi.org/10.1636/ST-06-19.1
Hore U, Uniyal VP. 2008. Effect of prescribed fire on spider assemblages in Terai grasslands, India. Turk. J. Arachnol. 1(1):15-36. DOI: https://doi.org/10.1636/CT07-53.1
Humedales Chaco. c2019. Secretaría General de la Nación. Ambiente y Desarrollo Sustentable. [Revisada en: 27 May 2019]. https://www.argentina.gob.ar/ambiente/agua/humedales/sitiosramsar/chaco
Izsák C, Price ARG. 2001. Measuring b-diversity using a taxonomic similarity index, and its relation to spatial scale. Mar. Ecol. Prog. Ser. 215:69-77. doi: https://doi.org/10.3354/meps215069
Jiménez-Valverde A, Lobo JM. 2006. Establishing reliable spider (Araneae. Araneidae and Thomisidae) assemblage sampling protocols: estimatio of species richness, seasonal coverage and contribution of juvenile data to species richness and composition. Acta Oecol, 30(1): 21-32. doi: https://doi.org/10.1016/j.actao.2006.01.001
Kong A, Thorleifsson G, Frigge ML, Vilhjalmsson BJ, Young AI, Thorgeirsson TE, Benonisdottir S, Oddsson A, Halldórsson BV, Masson G, Gudbjartsson DF, Helgason A, Bjornsdottir G, Thorsteinsdottir U, Stefansson K. 2018. The nature of nurture: Effects of parental genotypes. Sci. 359(6374):424-428. doi: https://doi.org/10.1126/science.aan6877
Lehtinen PT. 2003. Taxonomic notes on the Misumenini (Araneae: Thomisidae: Thomisinae), primarily from the Palaearctic and Oriental regions. En; Logunov DV y Penney D, editores. 2003 European Arachnology 2003 (Proceedings of the 21st European Colloquium of Arachnology, St.-Petersburg, 4-9 August 2003). Moscow: Arthropoda Selecta. p. 147-184.
Machado M, Guzati C, Viecelli R, Molina-Gómez D, Teixeira RA. 2019a. A taxonomic review of the crab spider genus Sidymella (Araneae, Thomisidae) in the Neotropics. Zoosyst Evol. 95(2): 319-344. doi: https//doi.org/10.3897/zse.95.34958 29
Machado M, Teixeira RA, Milledge GA. 2019b. On the Australian bark crab spiders genus Stephanopis: taxonomic review and description of seven new species (Araneae: Thomisidae: Stephanopinae). Rec Aust Mus. 71: 217-276. doi:https://doi.org/10.3853/j.2201-4349.71.2019.1698
Machado M, Guzati1 C, Viecelli R, Molina-Gómez D, Teixeira RA. 2019. A taxonomic review of the crab spider genus Sidymella (Araneae, Thomisidae) in the Neotropics. Zoosyst. Evol. 95(2): 319-344. doi: https://doi.org/10.3897/zse.95.34958
Machado M, Texeira RA. 2021. Phylogenetic relationships in Stephanopinae: systematics of Stephanopis and Sidymella based on morphological characters (Araneae: Thomisidae). Org. Divers. Evol. doi: https://doi.org/10.1007/s13127-020-00472-x
Moreno CE. 2001. Métodos para medir la biodiversidad. Zaragoza: Manuales y Tesis, Sociedad Entomológica Aragonesa.
Moreno CE, Castillo‐Campos G, Verdú JR. 2009. Taxonomic diversity as complementary information to assess plant species diversity in secondary vegetation and primary tropical deciduous forest. J. Veg. Sci. 20(5):935-943. doi: https://doi.org/10.1111/j.1654-1103.2009.01094.x.
Morrone JJ. 2001. Biogeografía de América Latina y el Caribe. Zaragoza: Manuales y Tesis, Sociedad Entomológica Aragonesa.
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature. 403(6772):853-858. doi: https://doi.org/10.1038/35002501
Nadal MF, Achitte-Schmuztler HC, Zanone I, González PY, Avalos G. 2018. Diversidad estacional de arañas en una reserva natural del Espinal en Corrientes, Argentina. Caldasia 40(1):129-143. doi: http://dx.doi.org/10.15446/caldasia.v40n1.67362
Ono H. 1988. A revisional study of the spider family Thomisidae (Arachnida, Araneae) of Japan. [Tesis]. [Tokyo]: Kyoto University.
Pérez-Hernández CX. 2019. Distintividad taxonómica: Evaluación de la diversidad en la estructura taxonómica en los ensambles. En: Moreno CE, editora. La biodiversidad en un mundo cambiante: Fundamentos teóricos y metodológicos para su estudio. México: Universidad Autónoma del Estado de Hidalgo/Libermex. p. 285-306.
Peterson AT, Soberón J, Sánchez-Cordero V. 1999. Conservatism of ecological niches in evolutionary time. Science. 285(5431):1265-1267. doi: https://doi.org/10.1126/science.285.5431.1265
Podgaiski LR, Ott R, Rodrigues ENL, Buckup EH, Marques MAL. 2007. Araneofauna (Arachnida, Araneae) do Parque Estadual do Turvo, Rio Grande do Sul, Brasil. Biota Neotrop. 7(2):197-212. doi: https://doi.org/10.1590/S1676-06032007000200023
Ramírez MJ. 2014. The morphology and Phylogeny of Dionychan Spiders (Araneae: Araneomorphae). Bull. Am. Mus. Nat. Hist. 390: 1-374. doi: https://doi.org/10.5531/sd.sp.5
Ricetti J, Bonaldo AB. 2008. Spiders diversity and richness estimates in four vegetations types of Serra do Cachimbo, Para, Brazil. Iheringia Ser. Zool. 98(1): 88-99. doi: https://doi.org/10.1590/S0073-47212008000100013
Rodríguez-Artigas SM, Ballester R, Corronca JA. 2016. Factors that influence the beta-diversity of spider communities in northwestern Argentinean Grasslands. PeerJ 4:e1946. doi: https://doi.org/10.7717/peerj.1946
Rodrigues ENL, Mendonça Jr MS. 2012. Spider guilds in the tree-shrub strata of riparian forests in southern Brazil. J. Arachnol. 40(1):39-47. doi: https://doi.org/10.1636/P10-105.1
Rubio GD, Corronca JA, Damborsky MP. 2008. Do spider diversity and assemblages change in different contiguous habitats? A case study in the protected habitats of the Humid Chaco ecoregion, north-east Argentina. Environ. Entomol. 37(2):419-430. doi: https://doi.org/10.1093/ee/37.2.419
Rubio GD, Moreno CE. 2010. Orb-weaving spider diversity in the Iberá marshlands (Argentina). Neotrop. Entomol. 39(4):496-505. doi: https://doi.org/10.1590/S1519-566X2010000400006
Rubio GD. 2016. Using a jumping spider fauna inventory (Araneae: Salticidae) as an indicator of their taxonomic diversity in Misiones, Argentina. Rev. Biol. Trop. 64(2):875-883. doi: https://doi.org/10.15517/rbt.v64i2.19722
Sathianandan TV, Mohamed KS, Vivekanandan E. 2011. Species diversity in fished taxa along the southeast coast of India and the effect of the Asian Tsunami of 2004. Mar. Biodiver. 42(2):179-187. doi: https://doi.org/10.1007/s12526-011-0103-2
Schirmel J, Thiele J, Entling MH, Buchholz S. 2016. Trait composition and functional diversity of spiders and carabids in linear landscape elements Agriculture. Ecosys. Environ. 235:318-328. doi: https://doi.org/10.1016/j.agee.2016.10.028
Silva-Matos DM, Fonseca GD, Silva-Lima L. 2005. Differences on post-fire regeneration of the pioneer trees Cecropia glazioui and Trema micrantha in a lowland Brazilian Atlantic Forest. Rev. Biol. Trop. 53(1-2):1-4.
Silva-Moreira T, Machado M. 2016. Taxonomic revision of the crab spider genus Epicadus Simon, 1895 (Arachnida: Araneae: Thomisidae) with notes on related genera of Stephanopinae Simon, 1895. Zootaxa, 4147(3): 281–310. doi: https://doi.org/10.11646/zootaxa.4147.3.4
Sirvid PJ, Moore NE, Chambers GK, Prendergast K. 2013. A preliminary molecular analysis of phylogenetic and biogeographic relationships of New Zealand Thomisidae (Araneae) using a multi-locus approach. Inverterb. Syst. 27(6): 655-672. doi: https://doi.org/10.1071/IS13025
Sokal RR, Rohlf FJ. 1995. Biometry: The principles and practice of statistics in biological research. J. Royal Stat. Soc. 133(1):102. doi: https://doi.org/10.2307/2343822
Tews J, Brose U, Grimm V, Tielborger K, Wichmann MC, Schwager M, Jeltsch F. 2004. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J. Biogeogr. 31(1):79-92. doi: https://doi.org/10.1046/j.0305-0270.2003.00994.x
Uetz GW. 1991. Habitat structure and spider foraging. En: Bell SS, McCoy ED, Mushinsky HR, editores. Habitat Structure: The Physical Arrangement of Objects in Space. London: Chapman &Hall. p. 325-348. DOI: https://doi.org/10.1007/978-94-011-3076-9_16
Velásquez-Puentes FJ, Bacon CD. 2016. Una introducción a la estructura filogenética de comunidades: Un caso de estudio en palmas de Bolivia. Ecol. Bol. 51(2):126-140.
Warwick RM, Clarke KR. 1995. New ' biodiversity ' measures reveal a decrease in taxonomic distinctness with increasing stress. Mar. Ecol. 129:301-305. doi: https://doi.org/10.3354/meps129301
Warwick RM, Light J. 2002. Death assemblages of mollusks on St. Martin´s Flats, Isles of Scilly: a surrogate for regional biodiversity? Biodiver. Conser. 11(1):99-112. doi: https://doi.org/10.1023/A:1014094829984
Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33: 475-505. doi: https://doi.org/10.1146/annurev.ecolsys.33.010802.150448
Wheeler WC, Coddingtonb JA, Crowleya LM, Dimitrov D, Goloboffe PA, Griswoldf CE, Hormiga G, Prendinia L, Ramírez MJ, Sierwald P, Almeida-Silva, Alvarez-Padilla F, Arnedok MA, Benavides Silva LR, Benjamin SP, Bond JE, Grismado CJ, Hasand E, Hedin M, Izquierdo MA, Labarque FM, Ledford J, Lopardo L, Maddison WP, Miller JA, Piacentini LN, Platnick NI, Polotow D, Silva-Davila D, Scharff N, Szuts T, Ubick D, Vink CJ, Wood HM, Zhang J. 2016. The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics. 33(6):1-43. doi: https://doi.org/10.1111/cla.12182
[WSC] Word Spider Catalog. c2018. The world spider catalog. Natural History Museum Bern, on line. [Revisada en: 7 Dic 2020]. http://www.wsc.nmbe.ch/
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Norberto Capetillo-Piñar, Manuel Zetina Rejón, Susana Perera-Valderrama, Arturo Tripp Quezada, Alejandro Bosch Callar, José Espinosa Sáez, Yuliesky Garcés. (2022). Do Changes in the Length of the List of Reference Species Influence the Results of the Average Taxonomic Distinctness?. Thalassas: An International Journal of Marine Sciences, 38(2), p.1013. https://doi.org/10.1007/s41208-022-00445-1.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2021 Caldasia

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).























