EXPERIMENTAL COMBUSTION ANALYSIS OF A HSDI DIESEL ENGINE FUELLED WITH PALM OIL BIODIESEL-DIESEL FUEL BLENDS
Keywords:
Heat release, palm oil biodiesel, diesel engines, exergy analysis. (es)Downloads
EXPERIMENTAL COMBUSTION ANALYSIS OF A HSDI DIESEL ENGINE FUELLED WITH PALM OIL BIODIESEL-DIESEL FUEL BLENDS
ANÁLISIS EXPERIMENTAL DE LA COMBUSTION DE UN MOTOR DIESEL DE AUTOMOCIÓN OPERANDO CON MEZCLAS DIESEL-BIODIESEL DE PALMA
JOHN AGUDELO
Grupo de Manejo Eficiente de la Energía GIMEL,
Universidad de Antioquia, jragude@udea.edu.co
ELKIN GUTIÉRREZ
Grupo de Manejo Eficiente de
la Energía GIMEL,
Universidad de Antioquia, elk.gtz@gmail.com
PEDRO BENJUMEA
Grupo de combustibles
alternativos, Facultad de Minas, Universidad Nacional de Colombia, pbenjume@unalmed.edu.co
Recibido para agosto 1 de 2008, aceptado enero 23 de 2009, versión final febrero 12 de 2009
ABSTRACT: Differences in the chemical nature between petroleum diesel fuels and vegetable oils-based fuels lead to differences in their physical properties affecting the combustion process inside the engine. In this work a detailed combustion diagnosis was applied to a turbocharged automotive diesel engine operating with neat palm oil biodiesel (POB), No. 2 diesel fuel and their blends at 20 and 50% POB by volume (B20 and B50 respectively). To isolate the fuel effect, tests were executed at constant power output without carrying out any modification of the engine or its fuel injection system. As the POB content in the blend increased, there was a slight reduction in the fuel/air equivalence ratio from 0.39 (B0) to 0.37 (B100), an advance of injection timing and of start of combustion. Additionally, brake thermal efficiency, combustion duration, maximum mean temperature, temperature at exhaust valve opening and exhaust gas efficiency decreased; while the peak pressure, exergy destruction rate and specific fuel consumption increased. With diesel fuel and the blends B20 and B50 the same combustion stages were noticed. However, as a consequence of the differences pointed out, the thermal history of the process was affected. The diffusion combustion stage became larger with POB content. For B100 no premixed stage was observed.
KEYWORDS: Heat release, palm oil biodiesel, diesel engines, exergy analysis.
RESUMEN: Debido a las diferencias entre los combustibles diesel y biodiesel en cuanto a estructura química, se derivan diferencias entre las propiedades físicas de éstos, afectando con ello el proceso de combustión en el motor. En este trabajo se aplica un modelo de diagnóstico del proceso de combustión a un motor diesel de automoción turboalimentado operando con biodiesel puro, diesel convencional (acpm) y mezclas al 20 y 50% en volumen (B20 y B50 respectivamente), operando a la misma potencia para estudiar solo el efecto del combustible, las pruebas se realizaron sin ninguna modificación en el motor ni en el sistema de inyección de combustible.
En el estudio experimental y de diagnóstico se observó que a medida que aumentaba la concentración de biodiesel en la mezcla se obtuvo una ligera reducción de la relación combustible/aire (0.39 para B0, y 0.37 para B100), en el avance de la inyección y en el inicio de la combustión. Además disminuyeron la eficiencia térmica efectiva, la duración de la combustión, la temperatura media máxima en el interior del cilindro, la temperatura de los gases a la salida de la válvula de escape y la eficiencia de los gases de escape; mientras que la presión máxima, la tasa de exergía destruida y el consumo específico de combustible aumentaron.
PALABRAS CLAVE: Calor liberado, biodiesel de palma, motores diesel, análisis energético.
1. INTRODUCTION
Biodiesel is attracting more attention every day as an alternative fuel for diesel engines, not only for its inherent characteristics: renewable, biodegradable, non toxic, oxygenated and free of sulphur and aromatics, but also for the main
current fossil fuel issues: reserves gradual depletion, environmental concerns and high volatility of oil prices.
The global market for biodiesel has undergone an accelerated growth in last years. The overall biodiesel production in the European Union (EU) increased from 1.9 million tonnes in 2004
to
3.2 in 2005 and to about 4.9 in 2006. In the USA ,
world’s second largest biodiesel player, production in 2006 amounted to
about 250 million gallons (Approx. 836000 tonnes)
Biodiesel is similar to conventional petroleum based diesel fuel in its
main characteristics and so it can be used neat or blended in existing diesel
applications without significant modifications to the engine. However,
differences in the chemical nature of both fuels lead to differences in their
physical properties, affecting engine performance, combustion process and
pollutant emissions. The extent of this effect mainly depending on biodiesel
production raw material, and engine type and operating parameters
Hamasaki
et al.
Abdul
et al.
Rakopoulos and Giakoumis
In this work a detailed combustion diagnosis model, including second law analysis, was applied to a turbocharged automotive diesel engine operating with neat POB, commercial grade No.2 diesel fuel and their blends at 20 and 50% biodiesel by volume (B20 and B50 respectively). The diagnosis model developed, making difference from other approaches followed in the reviewed works, takes into account the effect of the combustion products composition for each tested fuel. The study carried out allowed determining the effect of POB content on the main parameters characterizing the heat release process and also on the specific fuel consumption and the energy and exergy balances for the closed-valve period.
2. METHODOLOGY
2.1 Model description
Combustion diagnosis was carried out using a two species (air and
combustion products), single-zone model, based on the approach proposed by
Lapuerta et al.
In order to determine exergy, dead state was defined by a pressure of 101.325 kPa, a temperature of 298.15 K, and an ambient environment composition equal to that assumed for the air. The in-cylinder exergy balance, considering the blow-by as the only mass exchanged, is given by the following equation:
![]() (1)
(1)
The terms of this equation,
from left to right, account for the exergy related to the system (in-cylinder
gas mixture) (dEcyl), heat transfer (dEQ), work (dEW), blow-by (dEbb), fuel (dEf) and exergy destruction (dEd). The specific exergy of the
in-cylinder gas mixture (ecyl)
was obtained as
![]() (2)
(2)
where u, s and v are the internal energy, entropy and
specific volume, respectively. The subscript 0 refers to the dead state
condition. The thermodynamic properties of the in-cylinder gas mixture were
calculated assuming it as a mixture of ideal gases
The control volume exchanges
heat only with the combustion chamber walls at
the gas mean temperature. The exergy related to this process was calculated
considering that heat is leaving the system
![]() (3)
(3)
where ![]() is the heat transfer
to the walls. The exergy of work was obtained assuming that the
compression-expansion processes are internally reversible
is the heat transfer
to the walls. The exergy of work was obtained assuming that the
compression-expansion processes are internally reversible
![]() (4)
(4)
The blow-by mass was obtained
considering one-dimensional, compressible, isentropic flow
When a fraction of fuel is
burned, its chemical exergy is released according to the next expression
![]() (5)
(5)
where the fuel-burning rate ( ![]() ) was calculated from the fuel lower heating value (LHV) and the heat release rate (
) was calculated from the fuel lower heating value (LHV) and the heat release rate ( ![]() ) obtained from the diagnosis model:
) obtained from the diagnosis model:
![]() (6)
(6)
The chemical exergy of the
fuel, ![]() , was estimated from its composition and lower heating value
, was estimated from its composition and lower heating value
Carrying out a mass balance, replacing terms and solving (1) for the in-cylinder exergy destruction, the following equation is obtained:
![]() (7)
(7)
The chemical exergy of the
exhaust gases was neglected because it is too low and very difficult to recover
2.2
Test procedure and experimental equipment
Tests were carried out in an
instrumented automotive 2.5L, turbocharged high speed diesel engine located at the
Laboratorio de Máquinas Térmicas of the Universidad de Antioquia. Four fuel
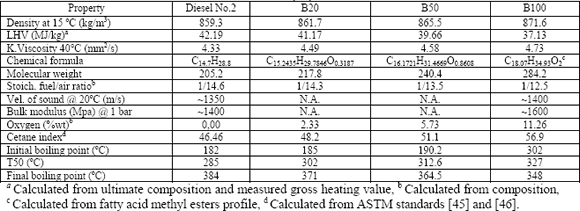
samples were tested (Table 1): commercial grade No.2 diesel fuel with an
elemental composition by weight of 87.2% carbon, 12.8% hydrogen and 0.0225%
sulphur, and an aromatic content of 29.3% (13% monoaromatics, 13.3% diaromatics
and 3% polyaromatics), neat palm oil biodiesel (B100), and their blends at 20
and 50% biodiesel by volume (B20 and B50 respectively). After each fuel was
tested, fuel pipes were drained prior to filling them with the next one. Then
the engine was warmed at least one hour to purge any of the remaining non test
fuel from the engine fuelling system.
Measurements were carried out in duplicate in order to guarantee their repeatability. Tests were executed without carrying out any modification on the engine or its fuel injection system (mass injected and injection timing). The engine was tested at 2000 rpm and 100 Nm. This mode was chosen because it was the point of minimum air-fuel ratio and maximum smoke opacity.
The air consumption was
measured with a hot-wire sensor (Magnetrol TA2, Accuracy ±0.5% full scale), and
fuel consumption with a Danfoss Masflo 6000 Coriolis-type mass flow sensor
(Accuracy ±0.1% of actual flow). For recording the instantaneous in-cylinder
pressure a Kistler 6056A piezoelectric pressure transducer installed in the
glow plug and a Kistler 5011B charge amplifier were used. Injection pressure
was recorded with an AVL 41DP 1200K piezoresistive pressure transducer,
installed at the exit of the injection pump. In order to guarantee confidence
in the combustion diagnosis results, 100 pressure curves were registered at
each operation mode
3. RESULTS AND DISCUSSION
3.1 Combustion diagnosis
As observed in Fig. 1, the
absolute fuel/air ratio (left axis) increased with POB content, this trend
being a consequence of a corresponding increase in fuel consumption. However,
the oxygen content of POB and its blends led to
a slight reduction of the fuel/air equivalence ratio from 0.39
(B0) to 0.37 (B100) (Fig. 1, right axis).
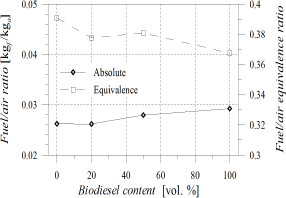
Figure 1. Fuel/air ratio (Left: absolute, Right:
equivalence)
The higher quantity of fuel
injected per stroke of the volumetric injection pump may be related to the
higher density of POB (Table 1). A similar behaviour has been reported for rape
seed oil biodiesel
As seen in Fig. 2, as POB
content increased the injection timing was advanced. This behaviour being related with biodiesel
higher density, speed of sound and bulk modulus leading to a faster increase in
injection pressure
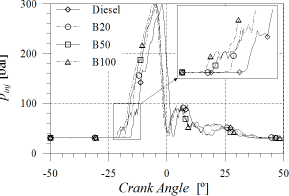
Figure 2. Injection-line pressure
It means that the injection pump (volumetric type) had to change its operating point although the engine speed and torque were maintained constant (maintaining the engine operation mode) since it had to inject more fuel at the same engine speed and so it had to start delivering fuel before.
As observed in Fig. 3, no significant differences in the in-cylinder pressure were registered during the compression and expansion strokes. The peak pressure increased about 12 bar when B100 was used instead of diesel fuel.
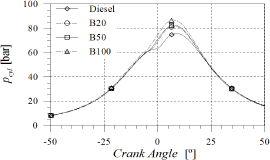
Figure 3. In-cylinder pressure
As seen in Fig. 4,
the start of combustion advanced as the blend became richer in POB, this trend, being
in agreement with the results obtained by several researches using biodiesel
produced from different raw materials
The fraction of fuel burned
during the premixed combustion stage decreased with POB content. A similar
behaviour with soybean oil biodiesel was reported by Zhang and Van Gerpen
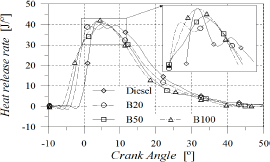
The heat release fraction shown in Fig. 5 can be used as an indicator of the overall rate of the combustion process and also as a way to verify the strength of the developed diagnosis model.
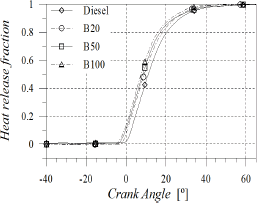
Figure 5. Heat release fraction
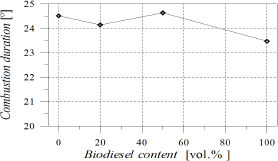
As POB content increased, combustion process became faster (Fig. 6), leading to a reduction in combustion duration which was estimated as the angular distance from 10 to 90% of the heat release fraction (Fig. 7). At 60 CA after TDC all fuels were completely burned. Crank angles for 25, 50 and 75% of heat release were reduced with POB content, causing that the combustion process remained centred in spite of injection timing and start of combustion advances. This behaviour may be favoured by the content of molecular oxygen in biodiesel.
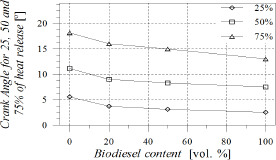
Figure 6. Angle for 25, 50 and 75% of heat release
The mechanisms by which biodiesel
undergoes combustion is not well understood yet, but it is likely that its long
carbon chains bonded to the ester functional group behave similarly to long
chain aliphatic hydrocarbons
As shown in Fig. 8, the
maximum mean in-cylinder temperature decreased with biodiesel content due to the
pressure gradient reduction, which affected the premixed combustion stage
causing that the angle corresponding to the maximum mean temperature moved
towards TDC. Since this behaviour remained along the expansion stroke, the
temperature at exhaust valve opening (EVO) decreased with POB content. These
results being in agreement with those reported by Canakci
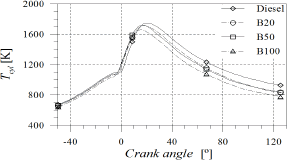
Figure 8. In-cylinder temperature
Although POB underwent the same combustion stages as No. 2 diesel fuel, the differences pointed out above, affected the thermal history of the process, causing a decrease in the brake thermal efficiency (ηb) and an increase in the brake specific fuel consumption (gf) as POB content was increased in the blend.
Comparing the performance of
B100 and the reference fuel, a reduction of 2% in ηb (Fig. 9 Left) and an increase of about 15% in gf (Fig. 9 Right) were
obtained. The gf increase
was also related with the lower LHV of
POB. Similar results have been reported by several researchers for soybean oil,
yellow grease and cooking oil biodiesel
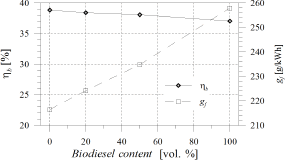
Figure 9. Left: Brake thermal efficiency,
Right: Brake specific fuel consumption
3.2 Second law analysis
Figure 10 shows the cumulative exergy destruction as a
function of crank angle and biodiesel content, expressed as a fraction of the
exergy supplied by the fuel. For all fuels, exergy destruction was low during
the compression stroke, rose sharply at the beginning of the combustion
process, and then became stable. This behaviour
indicates that

Figure 10. Dimensionless cumulative exergy destruction
4. CONCLUSIONS
The objective of this work was to study the combustion process in an automotive diesel engine operating with neat palm oil biodiesel, conventional diesel fuel and their B20 and B50 blends. Based on the experimental results, the following conclusions can be drawn as POB content was increased in the blend:
The injection timing and the start of combustion were advanced as reported for other types of biodiesel obtained from different raw materials. All fuels tested underwent the same combustion stages; however, small differences in start of injection and start of combustion affected the process thermal history.
The combustion process became faster and was maintained centred. However the maximum pressure increased while the maximum mean temperature decreased as a consequence of a pressure gradient reduction close to TDC.
Brake thermal efficiency underwent a slight decrease, while specific fuel consumption increased about 16% comparing B100 with diesel fuel.
The differences in the exergy behaviour with biodiesel came from the combustion process. Expansion and compression strokes were similar and they were not affected by biodiesel.
The exergy destruction was higher for greater biodiesel content due to the faster combustion and to the chemical structure differences with diesel fuel, which may have led to greater entropy of mixing of the combustion products. As a consequence, the cumulative exergy destruction was increased.
5. ACKNOWLEDGEMENTS
The authors wish to acknowledge the financial support of COLCIENCIAS (Colombian Institute for Science and Technology Development Francisco José de Caldas) to the research project 1115-05-16882. They also acknowledge the collaboration of ICP (Colombian Institute of Petroleum).
REFERENCES
[1] CLARK, G. EBB calls for greater support for biodiesel in the EU. from http://www.biofuelreview.com/content/view/1105/. (Retrieved 04-12-2007).
[2] SZYBIST, J. P., SONG, J., ALAM, M. AND BOEHMAN, A. L. Biodiesel combustion, emissions and emission control. Fuel Processing Technology 88, 679-691, 2007.
[3] KNOTHE, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Processing Technology 86, 1059-1070, 2005.
[4] HAMASAKI, K., KINOSHITA, E., TAJIMA, H., TAKASAKI, K. AND MORITA, D. Combustion Characteristics of Diesel Engines with Waste Vegetable Oil Methyl Ester. The Fifth International Symposium on Diagnostics and Modeling of Combustion in Internal Combustion Engines (COMODIA 2001), Nagoya, 2001.
[5] SCHOLL, K. W. AND SORENSON, S. C. Combustion of Soybean Oil Methyl Ester in a Direct Injection Diesel Engine, SAE, 930934, 1993.
[6] ZHANG, Y. AND VAN GERPEN, J. H. Combustion analysis of esters of soybean oil in a diesel engine, SAE, 960765, 1996.
[7] NWAFOR, O. M. I. AND RICE, G. Performance of Rapeseed Oil Blends in a Diesel Engine. Applied Energy 54, 4, 345-354, 1996.
[8] CHOI, C. Y., BOWER, G. R. AND REITZ, R. D. Effects of biodiesel blended fuels and multiple injections on DI diesel engines, SAE, 970218, 1997.
[9] SENATORE, A., CARDONE, M., ROCCO, V. AND PRATI, M. V. A Comparative Analysis of Combustion Process in D. I. Diesel Engine Fueled with Biodiesel and Diesel Fuel, SAE, 2000-01-0691, 2000.
[10] SCHMIDT, K. AND VAN GERPEN, J. H. The effect of biodiesel fuel composition on diesel combustion and emissions, SAE, 961086, 1996.
[11] CANAKCI, M. AND VAN GERPEN, J. H. Comparison of Engine Performance and Emissions for Petroleum Diesel Fuel, Yellow Grease Biodiesel, and Soybean Oil Biodiesel. ASAE, Sacramento, 016050, 2001.
[12] LAPUERTA, M., ARMAS, O., AND RODRÍGUEZ-FERNÁNDEZ, J., Effect of biodiesel fuels on diesel engine emissions, Progress in Energy and Combustion Science, vol. 34, pp. 198-223, 2008.
[13] ALI, Y. AND HANNA, M. A. In-cylinder pressure characteristics of a DI heavy duty diesel engine on biodiesel fuel, SAE, 971683, 1997.
[14] SHAHEED, A. AND SWAIN, E. Combustion analysis of coconut oil and its methyl esters in a diesel engine. Proc. Instn. Mech. Engrs. Part A 213, 417-425, 1999.
[15] YAMANE, K., UETA, A. AND SHIMAMOTO, Y. Influence of Physical and Chemical Properties of Biodiesel Fuel on Injection, Combustion and Exhaust Emission Characteristics in a DI-CI Engine. The Fifth International Symposium on Diagnostics and Modeling of Combustion in Internal Combustion Engines (COMODIA 2001), Nagoya, COMODIA, 3-08, 2001.
[16] KINOSHITA, E., HAMASAKI, K. AND JAQIN, C. Diesel combustion of palm oil methyl ester, SAE, 2003-01-1929, 2003.
[17] KINOSHITA, E., HAMASAKI, K. AND MYO, T. Combustion characteristics of a diesel engine with palm oil methyl ester and its blended fuel with gas oil. FISITA 2004. World Automotive Congress, Barcelona, FISITA, 2004.
[18] ABDUL, A. A., FARID, S. M. AND AFIQ, A. M. Performance of palm oil-based biodiesel fuels in a single cylinder direct injection engine. Palm Oil Developments 42, 15-27, 2005.
[19] RAKOPOULOS, C. D., SCOTT, M. A., KYRITSIS, D. C. AND GIAKOUMIS, E. G. Availability analysis of hydrogen/natural gas blends combustion in internal combustion engines. Energy, 33, No. 2, 248-255, 2008.
[20] RAKOPOULOS, C. D. AND GIAKOUMIS, E. G. Second-law analyses applied to internal combustion engines operation. Progress in Energy and Combustion Science 32, 2-47, 2006.
[21] LAPUERTA, M., ARMAS, O. AND HERNÁNDEZ, J. J. Diagnosis of DI Diesel combustion from in-cylinder pressure signal by estimation of mean thermodynamic properties of the gas. Applied Thermal Engineering 19, 513-529, 1999.
[22] GORDON, S. AND MCBRIDE, B. (1994). Computer program for calculation of complex chemical equilibrium compositions and applications. I Analysis, NASA
[23] GORDON, S. AND MCBRIDE, B. (1996). Computer program for calculation of complex chemical equilibrium compositions and applications. II Users manual and program description, NASA
[24] HERNÁNDEZ, J. J., LAPUERTA, M. AND PÉREZ-COLLADO, J. A combustion kinetic model for estimating diesel engine NOx emissions. Combustion Theory and Modelling 10, 4, 639-657, 2006.
[25] WOSCHNI, G. A universally applicable equation for the instantaneous heat transfer coefficient in the internal combustion engine, SAE, 670931, 1967.
[26] HSU, B. D. Practical diesel-engine combustion analysis., Society of Automotive Engineers - SAE, Warrendale, 2002.
[27] AGUDELO, A. F., AGUDELO, J. R. AND BENJUMEA, P. N. Combustion diagnosis of biofuels in internal combustion engines, Imprenta Universidad de Antioquia, Medellín, [In Spanish] 2007.
[28] FOSTER, D. E. An overview of zero-dimensional thermodynamic models for IC engine data analysis, SAE, 852070, 1985.
[29] CATON, J. A. Results from the second-law of thermodynamics for a spark-ignition engine using an engine cycle simulation. Fall Technical Conference, ASME-ICED, ASME, 35-49, 1999.
[30] SHAPIRO, H. N. AND VAN GERPEN, J. H. Two zone combustion models for second law analysis of internal combustion engines, SAE, 890823, 1989.
[31] LAPUERTA, M., BALLESTEROS, R. AND AGUDELO, J. R. Effect of the gas state equation on the thermodynamic diagnostic of diesel combustion. Applied Thermal Engineering 26, 1492-1499, 2006.
[32] VALERO, A. AND LOZANO, M. Los balances de energía, entropía, exergía y energía libre. Métodos para el diagnóstio de instalaciones industriales. Ingeniería Química Mayo, 143-153, 1987.
[33] MORAN, M. J. AND SHAPIRO, H. N. Fundamentals of Engineering Thermodynamics, John Wiley & Sons, The Atrium,, 2006.
[34] DINCER, I. AND CENGEL, Y. A. Energy, entropy and exergy concepts and their roles in thermal engineering. Entropy 3, 116-149, 2001.
[35] RAKOPOULOS, C. D. Evaluation of a spark ignition engine cycle using first and second law analysis techniques. Energy Conversion and Management 34, 12, 1299-1314, 1993.
[36] BOZZA, F., NOCERA, R., SENATORE, A. AND TUCCILLO, R. Second law analysis of turbocharged engine operation, SAE, 910418, 1991.
[37] LI, J., ZHOU, L., PAN, K., JIANG, D. AND CHAE, J. Evaluation of the thermodynamic process of indirect injection diesel engines by the first and second law, SAE, 952055, 1995.
[38] SZARGUT, J., MORRIS, D. R. AND STEWARD, F. R. Exergy analysis of thermal, chemical, and metallurgical processes, Hemisphere Publishing Corporation, New York, 1988.
[39] KOTAS, T. J. The exergy method of thermal plant analysis, Krieger Publishing Company, Malabar, Florida, 1995.
[40] KYRITSIS, D. C. AND RAKOPOULOS, C. D. Parametric study of the availability balance in an internal combustion engine cylinder, SAE, 2001-01-1263, 2001.
[41] RAKOPOULOS, C. D. AND KYRITSIS, D. C. Comparative second-law analysis of internal combustion engine operation for methane, methanol, and dodecane fuels. Energy 26, 705-722, 2001.
[42] RAKOPOULOS, C. D. AND GIAKOUMIS, E. G. Development of cumulative and availability rate balances in a multi-cylinder turbocharged indirect injection diesel engine. Energy Conversion and Management 38, 4, 347-369, 1997.
[43] RAKOPOULOS, C. D. AND GIAKOUMIS, E. G. Availability analysis of a turbocharged diesel engine operating under transient load conditions. Energy 29, 1085-1104, 2004.
[44] BRUNT, M. AND EMTAGE, A. Evaluation of IMEP routines and analysis errors, SAE, 960609, 1996.
[45] ASTM (2005). Standard Test Method for Calculated Cetane Index by Four Variable Equation. Philadelphia, ASTM. D4737-04.
[46] ASTM (2005). Standard test method for Calculated Cetane Index of distillate fuels. Philadelphia, ASTM. D976.
[47] LAPUERTA, M., RODRÍGUEZ, J. AND AGUDELO, J. R. Diesel particulate emissions from used cooking oil biodiesel. Bioresource Technology 99, 731-740, 2008.
[48] APARICIO, C., GUIGNON, B., RODRIGUEZ-ANTON, L. M. AND SANZ, P. D. Volumetric properties of sunflower methyl ester oil at high pressure. Journal of agricultural and food chemistry 55, 7394-7398, 2007.
[49] APARICIO, C., GUIGNON, B., RODRÍGUEZ-ANTÓN, L. M. AND SANZ, P. D. Determination of rapeseed methyl ester oil volumetric properties in high pressure (0.1 to 350 MPa). Journal of agricultural and food chemistry Vol. 89, 1, 1319, 2007.
[50] CANAKCI, M. Combustion characteristics of a turbocharged DI compression ignition engine fueled with petroleum diesel fuels and biodiesel. Bioresource Technology 98, 1167-1175, 2007.
[51] GRABOSKI, M. S. AND MCCORMICK, R. L. Combustion of fat and vegetable oil derived fuels in diesel engines. Progress in Energy and Combustion Science 24, 125-164, 1998.
How to Cite
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Download Citation
Article abstract page views
Downloads
License
Copyright (c) 2009 DYNA

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The author of a paper accepted for publication in any of the journals published by the School of Mines will yield all the property to the National University of Colombia rights free of charge, within which include article: the right to edit, publish, reproduce and distribute both print and digital media, as well as including in an article in international indexes and / or databases, likewise, it enables the publisher to use images, tables and/or graphic material presented in Article for designing covers or posters of the magazine. By assuming the economic rights of the article, it may be reproduced partially or totally in any printed or digital media without express permission of the same.