Published
Synthesis and characterization of polymers based on citric acid and glycerol: Its application in non-biodegradable polymers
DOI:
https://doi.org/10.15446/dyna.v82n190.42718Keywords:
polymers, biodegradability, citric acid, glycerol, hybrid. (es)Downloads
DOI: https://doi.org/10.15446/dyna.v82n190.42718
Synthesis and characterization of polymers based on citric acid and glycerol: Its application in non-biodegradable polymers
Síntesis y caracterización de polímeros a base de ácido cítrico y glicerol: Su aplicación en polímeros no biodegradables
Jaime Alfredo Mariano-Torres a, Arturo López-Marure b & Miguel Ángel Domiguez-Sánchez c
a Research Center for Applied Science and
Advanced Technology. IPN. Altamira. Mexico. jmarianot1400@alumno.ipn.mx
b Research Center for Applied Science and
Advanced Technology. IPN. Altamira. Mexico. arlopezm@ipn.mx
c Research Center for Applied Science and
Advanced Technology. IPN.
Altamira. Mexico. madominguezs@ipn.mx
Received: March 30th, 2014. Received in revised form: November 19th, 2014. Accepted: December 1st, 2014.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
The notable increase in global consumption of plastics
and their long residence time in the atmosphere show the great need for
biodegradable products. In this project, we developed biodegradable polymers
based on citric acid and glycerol. Their synthesis was carried out at different
conditions of constant temperature and concentration and they were synthesized
using an economically viable method. Characterization was undertaken using:
Acid number, FTIR spectroscopy, refractive index, viscosity, impact analysis,
tensile test, hardness, calorimetry, the % humidity (oven method with
recirculating air), and density determination, along with qualitative evidence
to corroborate their biodegradability. These elaborated polymers were mixed
with medical grade PVC formulation, obtaining a hybrid polymer, showing their
mechanical properties were changed.
Keywords: polymers; biodegradability; citric acid; glycerol; hybrid.
Resumen
El
notable incremento mundial en el consumo de plásticos y su largo tiempo de
residencia en el ambiente muestran la gran necesidad de productos con
características biodegradables. En este proyecto fueron desarrollados polímeros
biodegradables a base del ácido cítrico y del glicerol. La síntesis de estos se
lleva a cabo a diferentes condiciones de concentración y a temperatura
constante. Se desarrollaron mediante un proceso económicamente viable. Se caracterizaron por medio de las siguientes
técnicas: Numero ácido, espectroscopia infrarroja FTIR, índice de refracción,
viscosidad, análisis de impacto, ensayo de tensión, dureza, calorimetría, el %
de Humedad (método de la estufa con recirculación de aire), determinación de
densidad, además de pruebas cualitativas para corroborar su biodegradabilidad. Los
polímeros elaborados fueron mezclados con una formulación de PVC grado médico, obteniendo un polímero hibrido y se pudo observar que modifica sus
propiedades mecánicas.
Palabras clave: polímeros; biodegradabilidad; ácido cítrico; glicerol; hibrido.
1. Introduction
Degradation of synthetic plastics is very slow and can take up to 500 years. The "degradation" of these plastic particles generates smaller plastics, which despite no longer being evident, they accumulate in ecosystems in great quantities. Biodegradable polymers are plastics with similar properties but with a shorter degradation time, which could be used to offset this problem.
The biodegradable polymer developed in this work was produced using a poly condensation method and is part of polyester family. [1] It is important to know that polyesters are an important group of polymers with ester bonds (-co-o-) in the backbone. These polymers are interesting as biomaterials because the ester groups are hydrolytically degradable, so that, in contrast to polyamides, polyesters do not exhibit strong intermolecular forces. Therefore, their properties are more sensitive to their structure. [4]
Biodegradable materials combined with non-biodegradable materials are referred to as hybrid materials and have a significantly higher number of properties than each material separately.
The main properties that allow these polymers to compete with other materials such as glass and metals are their chemical, physical and mechanical strengths. Based on these, the researches in this field have always been designed to increase the life of certain polymers. However, longevity can lead to problems. In recent years, synthetic polymer wastes have increased their percentage of the total solid waste. As a result, scientists have changed direction, and shifted towards the synthesis of degradable polymers, either by the effect of temperature (thermal degradation) due to contact with water (hydrolytic degradation), or by environmental effects such as sunlight (photo degradation) or organisms (biodegradation). [8].
In DIN FNK 103.2, it was found that plastic materials are called biodegradable if all their organic compounds undergo complete biodegradation processes. Environmental conditions and biodegradation rates are to be determined by standardized test methods.
Biodegradation is a process caused by biological activity leading to a change in the chemical structure of naturally occurring metabolic products.
In the ASTM Subcommittee D20.96 it arose that a biodegradable polymer is a degradable plastic in which the degradation results from the action of naturally occurring microorganisms such as bacteria, fungi and algae.
ISO 472 provides for biodegradable plastics: A plastic designed to undergo a significant change in its chemical structure under specific environmental conditions resulting in a loss of some properties that may vary as measured by standard test methods appropriate to the plastic and the application in a period of time that determines its classification. The change in the chemical structure results from the action of naturally occurring microorganisms. [9]
It is possible to consider biodegradation as a microorganism attack against a material; small fragments are obtained due to the rupture of bonds in its backbone. The biodegradation of plastics is generally a complex process. Due to the molecular size of polymers and their lack of solubility in water, the microorganisms are not capable of delivering the polymeric material to the cells where most biochemical processes take place, so they initially excrete enzymes that depolymerize extracellular material outside cells. The final products of this metabolic process are water, carbon dioxide, methane (anaerobic biodegradation) and organic matter [11].
2. Methodology
2.1. Materials
2.1.1. Citric acid
Anhydrous citric acid is produced in the form of translucent crystals and an odorless crystalline white powder with a strong acidic taste. It is very soluble in water and alcohol. It should be stored in airtight containers away from heat and moisture (at 24°C and 55% relative humidity).
Typical features of a commercial anhydrous citric acid are:
- Molecular Weight: 192.13 g/mol
- Solubility in water: 162 g/100 mL at 25 ° C.
- Purity: 99%
- Humidity: 0.3% max.
- Melting point: 153 ° C
- Color: White
- Appearance: Crystals.
2.1.2. Glycerol
Glycerin (also glycerol, 1, 2, 3-propanetriol, 1, 2, 3-Trihydroxypropane, molecular formula C3H8O3 and molecular mass 92.09 g/mol), is a colorless, viscous, hygroscopic liquid, which is very soluble in water but not in most other organic solvents.
The typical characteristics of glycerin are
- Physical state: Dense liquid
- Color: Colorless.
- Boiling point: 290 ° C
- Fusion point: 18 ° C
- pH: Neutral
- Relative density: 1.25 g/cm3
- Solubility: Soluble in water and alcohol. Insoluble in ether, benzene, chloroform, fixed and volatile oils. [3]
2.2. Synthesis
The biodegradable polymer was made essentially in three stages: Sample preparation, pre- polymerization and polymerization. Synthesis was carried in a simple and inexpensive way, in a short time and autocatalytically.
2.2.1. Sample preparation
Citric acid was dried at 105° C for one hour and glycerin was heated to 80° C with a vacuum of 5 psi to dry. In this project, three concentrations were handled, 1:1 (1 citric acid mole, 1 glycerol mole), 1:2 (1 citric acid mole, 2 glycerol mole), 1:3 (1 citric acid mole, 3 glycerol mole).
2.2.2. Prepolymerization
During this stage, the homogeneous mixture was slowly heated to a temperature of 150° C, and stirred continuously with a constant speed. The reactor was jacketed in order to conserve heat. During the increase of temperature, it was possible to observe how the mixture became more homogeneous and transparent.
Half an hour after starting the reaction, it began to generate water steam, since water is the byproduct of low molecular weight that is generated in this reaction of poly condensation. Within minutes water steam was observed leaving the reactor and moving to the condenser to achieve a steady drip of water. The water generated was stored and counted. This reaction was monitored by means of acidity, samples were taken every twenty minutes and the acid number was estimated, making it possible to know the efficiency of the reaction. The reaction had reached equilibrium and the reaction was stopped when very similar numbers were obtained.
2.2.3. Polymerization
During this stage the prepolymer was placed in a metal container and introduced in an oven at 170 °C for one hour to achieve a higher degree of polymerization. Fig. 1 shows the container with the prepolymer. (These parameters were used based on decomposition temperatures, bringing the sample to the limit, the time was established by the FTIR, samples were taken every 10 minutes and were characterized, and after 60 minutes, no changes in the intensities of peaks of spectra were seen).
the polymerization time ended, the polymer was left in the oven until reaching room temperature, in order to avoid a sudden change in temperature. After the curing time, the polymer was removed from the oven, was packaged and labeled and then taken to different characterizations.
2.3. Characterization
- Fourier transform infrared spectroscopy. Spectrum One computer was used. Attenuated total reflectance (ZnSe) was used. The elaborated polymers were analyzed by transmission, preparing the specimen with the Potassium Bromide (KBr) disc technique. Scan type: sample. Unit: %T. Scan number: 10. Scan time: 1 min Resolution 4cm-1 and interval of 650 to 400 cm-
- Refractometer (Refractive index). Atago Abbe refractometer was used at 25-26°C.
- Acidity. For this determination, the volumetric method was used. Employing KOH and phenolphthalein as reactive and indicator respectively for this analysis.
- Determination of humidity. A binder stove was used at 105°C for 1 a hour.
- Calorimetry. Thermal analyses were carried out on a differential scanning calorimeter DSC Perkin-Elmer Pyris-1. Nitrogen gas was used. Temperature program: Holded for 1.0 min at 30°C. Heated from 30°C to 400°C at 40°C/min. Sample weight 1 mg
- Determination of density. Density was determined through a buoyancy method using scales equipped with a Metter Toledo brand density determination kit.
2.4. Mechanical Testing
- Tensile tests. INSTRON Equipment was used for this test.
- Impact test. The impact test was performed on a computer CEAST ® Resil impactor by IZOD method.
- Hardness Test. Complex Shore D Durometer was used with ASTM D2240-00.
2.5. Biodegradation test
During these tests, the polymers were studied to different conditions, it is noteworthy that these tests gave us qualitative results and were considered a field test.
The first test consisted in keeping the polymer completely weatherproof, meaning a piece of our polymer was placed in a specific place in which to be exposed to different climatic factors, like the sun, rain, air, insects, microorganisms, etc., in order to observe their behavior in a time range.
The second test consisted in immerse our polymer in water (50 ml) to allow us to observe whether a hydrolytic degradation was occurring by the passing of time.
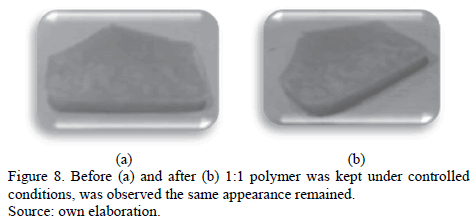
The third test was to keep the piece of polymer in controlled conditions at a temperature of 22°C, and in closed containers free of humidity and atmospheric pressure. This allowed us to compare the three exhibits.
It is noteworthy that the three samples correspond to the same polymer.
3. Results
3.1. Fourier transform infrared spectroscopy
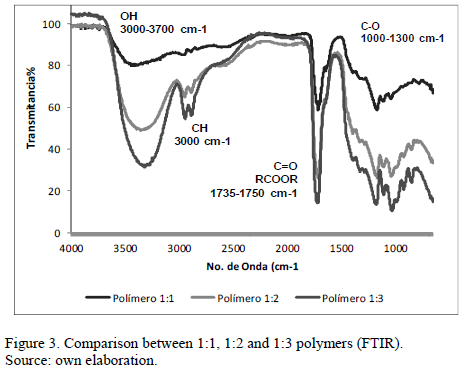
This technique allowed us to observe the functional groups present in our samples. The first sample analyzed was called 1:1. In spectrum 1, we observed the presence of certain characteristic functional groups of the polyesters of which our polymer is part, mainly OH groups, CH, C=O, RCOOR, CO.
In the case of 1:2 prepolymer, we basically observed the same groups with varying intensities. Mainly observed were OH groups, CH, C=O, RCOOR, CO.
In the case of the 1-3 polymers, the same groups were observed again, and again, with varying intensities. Those mainly observed were OH, CH, C = O, RCOOR, CO
Fig. 3 shows the spectrum of the three different polymers together. It is possible to observe that they have the same line, i.e., it shows the presence of the same functional groups, all three show the formation of ester groups. It is important to note that in the OH functional group, the intensity of this polymer is greater in 1:3. This is because this polymer has a higher concentration of glycerol, which has three hydroxides (OH) in its structure. The least intense is 1:1 as this has a lower concentration of glycerin.
The OH groups can also be indicative of the presence of water in the polymer since water is a by-product in our reaction and this could be reflected in the spectrum.
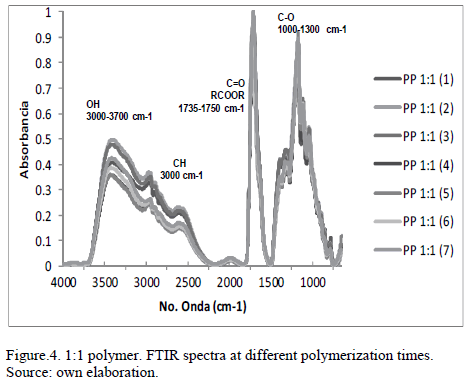
Fig. 4 shows a spectrum of seven lines representing 7 different polymerization times. The specters were taken every twenty minutes, and showed the following trend: The intensity of the line representing the OH and CH did decrease the polymerization step. This indicates that water is being generated. The line that represents the C=O and CO showed greater intensity with the passing of the polymerization, this indicates an increase of ester groups, which is typical in these types of polymers.
3.2. Refractometry (refractive index)
The refractive index is an optical property of materials. The values shown below are the average of several tests undertaken at the same temperature. It is noteworthy that these tests were performed following the ISO-17025-2006 standard.
Refractive index of the three types of polymer Citrate: 1:1, 1:2 and 1:3.
1:1 prepolymer 1.50 (25 ° C)
1:2 prepolymer 1.50 (26 ° C)
1:3 prepolymer 1.495 (26 ° C)
3.3. Acidity
Acidity was an important parameter when reactions were taking place, using this parameter controlled the equilibrium of the reaction. With the acid number, it was possible to calculate the amount of acid present in the sample and also the efficiency with which the reaction took place.
3.4. Humidity determination. (Method of recirculating air oven)
Mass loss as a percentage of the original mass of the sample is calculated using eq. 1:
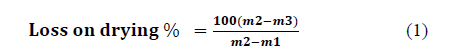
Where:
- m1 is the mass of the capsule = 22 316 g
- m2 is the mass of the capsule + sample before drying = 27.036 g
- - m3 is the mass of the capsule + sample after drying = 26.927 g
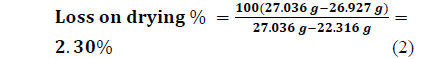
3.5. Calorimetry
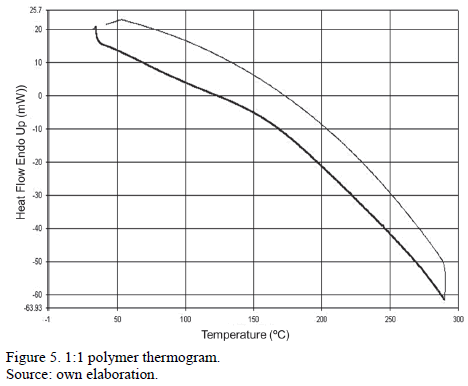
In the following DSC curve, a temperature change at 168° C was observed. This indicates a possible transition to a glassy amorphous state.
3.6. Determination of density
This test was performed with an analytical balance with a kit for obtaining density data. This data was important for the realization of the mechanical tests, given that by using the volume and mass data to provide density, it was possible to calculate the grams of polymer needed to fill the frames and plates for performing this test. [5]
The density data were obtained only for the 1:1 polymer as the equipment used only handles solid samples.
d 1:1 polymer= 1.3 g/cm3
3.7. Tensile test
This test was performed to analyze the tensile strength and elongation behavior. Values shown in the table are very poor, given that, as mentioned above, it was not possible to undertake mechanical tests 1:2 and 1:3 [7].
The properties of reference polymer (Crystal polystyrene PS) are attractive for CD cases.
3.8. Impact test. IZOD
This test was performed to observe the impact strength of the IZOD method. The values shown in the table are very low, meaning that the polymer is very fragile. As mentioned above, it was not possible to perform mechanical tests 1:2 and 1:3 [6].
3.9. Hardness Test
This test could only be performed for polymer 1:1. The results tell us that it is a hard polymer when compared to PS, so it is possible to imagine the harshness of this material. The implementation of this test did not present complications.
1:1 polymer: 83.9 Shore D
Polystyrene: 85 to 90 Shore D
3.10. Biodegradation test
The first test was to put a piece of the polymer outdoors. This showed that the polymer is degradable, given that after a short period of time, it decreased in weight. This was clearly visible, and it was possible to observe how time affected surface degradation over the entire polymer. Affecting factors in this test were mainly sunlight, humidity, air, temperature and insects.
In the second test in which the polymer was left in water, the hydrolytic properties of the polymer were tested. We observed how the polymer swelled and the growth of fungi and microorganisms.
The third test which was to keep a piece of polymer citrate in controlled conditions allowed us to observe how the polymer manages to stay in good condition, maintaining the same visual characteristics. By touching the polymer, we were able to observe that it had lost strength but gained flexibility. By this, we deduced that there is a molecular rearrangement after molding.
4. Discussion
The Fourier transform infrared spectroscopy for sample 1:1 showed the presence of the characteristic functional groups of polyesters; the presence of OH, CH, C = O, RCOOR, CO groups was observed in all three samples (1:1, 1:2, 1:3). Importantly, the intensity of OH functional group was stronger in the 1:3 polymer. This is because this polymer has a higher concentration of glycerol, which has in its structure three OH. 1:1 showed the least intensity since it had a lower concentration of glycerol.
From the above, it follows that the difference in intensity of the line of the OH groups is that the polymers contain different amounts of glycerol, higher glycerol generated less viscous polymers and a higher efficiency reaction, and managed to saturate more citric acid protons. The presence of OH groups present may also indicate the presence of water in the polymer since water is a byproduct in this reaction and this could be reflected in the spectrum.
Moreover, the line representing the C = O and CO, showed greater intensity with the polymerization step, this indicates an increase of ester groups, which is typical in these types of polymers.
Acidity allowed us to calculate the amount of acid present in the sample and also the efficiency with which the reaction took place, indicating whether the reaction had reached equilibrium.
DSC results showed a thermal change at 168°C indicating a transition to its glassy state.
With respect to mechanical properties evaluated, tensile and impact assay showed a fragile behavior; with high levels of hardness (Shore D); however, there was a significant improvement in tensile strength at 100% out the polymerization at a temperature of 170°C.
Degradation tests showed a gradual surface degradation, but over time, the entire polymer was affected; in aqueous medium, the hydrolytic properties of the polymer were observed, and the formation of fungi and microorganisms with the passage of time was observed.
Based on the functional properties obtained, the fact that they are hydrolytically degradable is of great interest in the field of biomaterials.
A hybrid polymer was achieved for sample 1:1, whereby hardness increased when the concentration of citric acid was increased. In the case of the other two samples, low viscosity impeded further mechanical tests.
Immediate applications are compostable bags and packaging, and due to its high rate of degradation in water, it could be a good candidate for drug delivery systems.
By undertaking tests at a temperature of 170°C, at which polymerization takes place, we also found that it can serve as an adhesive for glass and ceramics.
5. Conclusions
A novel method was developed to obtain polyesters based on citric acid and glycerol.
This work demonstrated that longer synthesis times generate a longer and higher crosslinking polymerization, which provides more water resistance to this polymer. It was also possible to confirm Halpern's conclusion about crosslinking behavior [10].
This polymer (1:1) after molding at room temperature is hard, very inflexible and fragile; however, after a week, the polymer becomes flexible and loses its hardness. This is due to the increase in humidity present in the polymer days after molding.
The polymer is obtained by autocatalizable synthesis, which is one factor that makes our reaction inexpensive as well as the fact that the materials for producing this polymer are cheap. In other works, a catalyst was used.
The three types of prepolymer and polymer obtained have different physical, chemical and mechanical properties, which allow a greater spectrum of applications for this family of polymers.
Biodegradation tests indicate that the 1:1 polymer is a biodegradable polymer, which exhibits hydrolytic properties, which means that it degrades rapidly in the presence of water.
It was also observed that polymer 1:1 first presented a partial biodegradation in which a surface degradation was observed, which then led to total degradation. This behavior was explained in 2003 by Gonzalez. [4]
These polymers (1:1, 1:2 and 1:3) have ester groups, which tend to be biodegradable and it is known that their bonds are hydrolyzed by the action of microorganisms. It is known that carbon-carbon bonds presented in our polymer are biodegraded by oxidation; the first degradation occurs with the hydroxyl groups and the second in the carbon chain.
This family of polymers has a heteroatom (oxygen) in its backbone carbon atoms, which acts as a potential attack point for enzymatic hydrolysis and oxidative breakdown.
5. References
[1] Callister, W.D., Introducción a la ciencia e ingeniería de los materiales, Vol. 2, Nueva York, USA, Reverte. , 1996, pp. 601-604
[2] Rivada-Núñez, F.J., Planta industrial de producción de ácido cítrico a partir de mezclas de remolacha. Cádiz, Universidad de Cádiz, España, 2008.
[3] Ferrero, A. J., Rosa, I. M., Veneciano, E. Proceso de purificación de la glicerina obtenida del biodiesel a pequeña escala. Centro de Investigación en Tecnología Lactocárnica. Universidad Tecnológica Nacional/Facultad Regional Villa Maria, Argentina, 2010.
[4] González, C., Anexo C: Polímeros biodegradables con aplicaciones en suturas quirúrgicas. 2004, 32 P.
[5] Toledo, M., Balanzas analíticas. 2013.
[6] Colombiana, E.D., Máquina de Impacto (Manual). Laboratorio de Producción. Facultad de Ingeniería Industrial. Escuela Colombiana de Ingeniería Julio Gravito. Bogotá, Colombia, 2008.
[7] Propiedades mecánicas de los materiales: Ensayos estáticos y dinámico. 2010.
[8] Escudero-Castejón, L., Moreno Grau J.M., Biodegradabilidad y toxicidad de materiales plásticos, aplicación de las normas UNE-EN ISO 14852 y 11348-3, Bs. Tesis, Departamento de Ingeniería Industrial, Universidad Politécnica de Cartagena, Cartagena, Colombia, 2011.
[9] Müller, R.J., Biodegradability of polymers: Regulations and methods for testing, in Steinbuchel A, Biopolymers Vol. 10, Wiley VCH, pp. 365-392. https://doi.org/10.1002/3527600035.bpola012
[10] Halpern, J., Urbanski, R., Weinstock, A., Iwing, D., Mathers, R. and Von Recum, H., A biodegradable thermoset polymer made by esterification of citric acid and glycerol. Society for Biomaterials. 5 (102), pp. 1467-1477, 2013 https://doi.org/10.1002/jbm.a.34821
[11] Valero-Valdivieso, M.F. and Ortegón, Y., Biopolymers: Progress and prospects, DYNA, 80 (181), pp. 171-180, 2013.
J.A. Mariano-Torres, received his Bs. degree in Chemical Engineering in 2007 from Instituto Tecnológico de Ciudad Madero, Mexico and a MSc. degree in Advanced Technology in 2013 from Instituto Politécnico Nacional in Mexico. Currently, he is a PhD student from Instituto Politécnico Nacional and works as professor in Universidad del Noreste. His research focuses on biodegradable polymers and their applications.
A. López-Marure, received his Bs. degree in Metallurgy Engineering in 1994 from the Instituto Politécnico Nacional in Madero Mexico, a MSc. degree in Metallurgy extractive in 1996 from Instituto Politécnico Nacional in Mexico, a MSc. degree in Material Science in 1997 from INPG in France, and a PhD degree in Inorganic Chemistry in 1999 from UCB Lyon, France. He has worked on projects in the aerospatial, petrol and mining fields, emphasizing on new materials. He is a full professor in the Materials Department, CICATA Altamira, Instituto Politecnico Nacional, Mexico.
M.A. Dominguez-Sánchez, received his Bs. degree in Chemical Engineering, and a MSc. degree in Polymers from Instituto Politécnico Nacional in Mexico. He has worked on the analysis of materials in the petrochemical industry, and is a Professor in the Polymers Department, CICATA Altamira, Instituto Politecnico Nacional, Mexico.
How to Cite
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Jiqian Li, Hezhi He, He Zhang, Mohong Xu, Qun Gu, Zhiwen Zhu. (2022). Preparation of thermoplastic starch with comprehensive performance plasticized by citric acid. Journal of Applied Polymer Science, 139(25) https://doi.org/10.1002/app.52401.
2. Lucas R.F. Figueiredo, Neymara C. Nepomuceno, José D.D. Melo, Eliton S. Medeiros. (2021). Glycerol-based polymer adhesives reinforced with cellulose nanocrystals. International Journal of Adhesion and Adhesives, 110, p.102935. https://doi.org/10.1016/j.ijadhadh.2021.102935.
3. T. Sopcak, L. Medvecky, T. Csanádi, M. Giretova, R. Stulajterova, R. Sedlák, F. Kromka, M. Streckova, M. Vojtko, K. Balázsi. (2024). Reinforcement of hydroxyapatite bone cement via thin glycerol-citrate polyester infiltration: Microstructural, mechanical and in-vitro evaluation. Surfaces and Interfaces, 52, p.104955. https://doi.org/10.1016/j.surfin.2024.104955.
4. Roger Borges, Camila Cristina Vieira Velloso, Camila Reis de Godoy, Cristiane Sanchez Farinas, Caue Ribeiro. (2024). Modulation of starch-based film properties for potential application as coating systems. Polymer Bulletin, 81(15), p.13765. https://doi.org/10.1007/s00289-024-05345-3.
5. Marina S. Azerêdo, Mário A. B. S. Nunes, Lucas R. F. Figueiredo, Juliano E. Oliveira, Gustavo D. Tonoli, Silvio de Barros, Eliton S. Medeiros. (2022). Environmentally friendly adhesives derived from glycerol-based polymers. Journal of Adhesion Science and Technology, 36(1), p.98. https://doi.org/10.1080/01694243.2021.1915619.
6. Tim Huber, Sean Feast, Simone Dimartino, Wanwen Cen, Conan Fee. (2019). Analysis of the Effect of Processing Conditions on Physical Properties of Thermally Set Cellulose Hydrogels. Materials, 12(7), p.1066. https://doi.org/10.3390/ma12071066.
7. Amanda Maria Leal Pimenta, Silvânio Rodrigues dos Santos, Nelson de Abreu Delvaux Júnior, Marcos Koiti Kondo, Ignacio Aspiazú. (2021). Tree resin as a cultivation strategy under water deficit. Pesquisa Agropecuária Tropical, 51 https://doi.org/10.1590/1983-40632021v5167901.
8. John Garner, Kinam Park. (2025). Nuplon: New synthetic polymers fully degradable in water. Journal of Controlled Release, 377, p.744. https://doi.org/10.1016/j.jconrel.2024.11.067.
9. Ireen Parvin Nitu, Summia Rahman, Md. Nazrul Islam, Md. Ashaduzzaman, Md. Iftekhar Shams. (2022). Preparation and properties of jute stick particleboard using citric acid–glycerol mixture as a natural binder. Journal of Wood Science, 68(1) https://doi.org/10.1186/s10086-022-02039-0.
10. Yadong Zhao, Carl Moser, Gunnar Henriksson. (2018). Transparent Composites Made from Tunicate Cellulose Membranes and Environmentally Friendly Polyester. ChemSusChem, 11(10), p.1728. https://doi.org/10.1002/cssc.201800627.
11. T. Sopcak, L. Medvecky, M. Giretova, R. Stulajterova, J. Brus, M. Urbanova, F. Kromka, M. Podobova, M. Faberova. (2021). Fabrication of a glycerol-citrate polymer coated tricalcium phosphate bone cements: Structural investigation and material properties. Journal of Polymer Research, 28(6) https://doi.org/10.1007/s10965-021-02596-w.
12. Jennifer M. Castro, Mercedes G. Montalbán, Noelia Martínez-Pérez, Daniel Domene-López, Juana M. Pérez, Francisco M. Arrabal-Campos, Ignacio Fernández, Ignacio Martín-Gullón, Juan C. García-Quesada. (2023). Thermoplastic starch/polyvinyl alcohol blends modification by citric acid–glycerol polyesters. International Journal of Biological Macromolecules, 244, p.125478. https://doi.org/10.1016/j.ijbiomac.2023.125478.
13. Igor F. Pimenta, Lucas R. F. Figueiredo, Adillys M. C. Santos, Juliano E. Oliveira, Eliton S. Medeiros. (2022). Development of controlled release fertilizer systems for KCl using glycerol‐based polymers. Journal of Applied Polymer Science, 139(10) https://doi.org/10.1002/app.51747.
14. Jaime Alfredo Mariano-Torres, Arturo López-Marure, Margarita García-Hernández, Gustavo Basurto-Islas, Miguel Ángel Domínguez-Sánchez. (2018). Synthesis and Characterization of Glycerol Citrate Polymer and Yttrium Oxide Nanoparticles as a Potential Antibacterial Material. MATERIALS TRANSACTIONS, 59(12), p.1915. https://doi.org/10.2320/matertrans.M2018248.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2015 DYNA

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The author of a paper accepted for publication in any of the journals published by the School of Mines will yield all the property to the National University of Colombia rights free of charge, within which include article: the right to edit, publish, reproduce and distribute both print and digital media, as well as including in an article in international indexes and / or databases, likewise, it enables the publisher to use images, tables and/or graphic material presented in Article for designing covers or posters of the magazine. By assuming the economic rights of the article, it may be reproduced partially or totally in any printed or digital media without express permission of the same.