A new mathematical model for coal flotation kinetics
Un nuevo modelo matemático para la cinética de flotación de carbones
DOI:
https://doi.org/10.15446/dyna.v84n203.62593Keywords:
coal flotation, flotation rate, kinetic model (en)Recibido: 11 de febrero de 2017; Revisión recibida: 12 de septiembre de 2017; Aceptado: 4 de octubre de 2017
Abstract
This study describes the development and formulation of a novel mathematical model for coal flotation kinetic. The flotation rate was considered as a function of chemical, operating and petrographic parameters for a global flotation order n. The equation for flotation rate was obtained by dimensional analysis using the Rayleigh method. It shows the dependency of flotation kinetic on operating parameters, such as air velocity and particle size; chemical parameters, such as reagents dosage and solids content; and mineral and maceral composition of coal. The flotation rate equation integrates the kinetic coefficient and the intrinsic characteristics of coal with dimensional consistency, and it is expressed by three dimensionless numbers which have physical chemical meaning. The model also exhibits similarities with traditional transport phenomena models represented by dimensionless numbers and predicts the flotation kinetic constant of a Colombian coal sample showing a good correlation between experimental and calculated values.
Keywords:
coal flotation, flotation rate, kinetic model..Resumen
Este estudio describe el desarrollo y formulación de un nuevo modelo matemático para la cinética de flotación de carbón. La velocidad de flotación se considera una función de parámetros químicos, operacionales y petrográficos para la flotación global de orden n. La ecuación de velocidad de flotación se obtuvo por análisis dimensional usando el método de Rayleigh. Este método muestra la dependencia de la cinética de flotación sobre los parámetros de operación tales como velocidad del aire y tamaño de partícula; parámetros químicos tales como dosis de reactivos y contenidos de sólidos; y composición mineral y maceral del carbón. La ecuación de velocidad de flotación integra el coeficiente cinético y las características intrínsecas del carbón con consistencia dimensional, y se expresa por tres números adimensionales que tienen significado químico físico. El modelo también muestra similitudes con los modelos tradicionales de fenómenos de transporte representados por números adimensionales y predice la constante cinética de flotación de un carbón Colombiano mostrando buena correlación entre los valores experimentales y calculados.
Palabras clave:
flotación de carbón, tasa de flotación, modelo cinético..1. Introduction
The flotation rate constant is necessary to scale-up flotation units on an industrial scale from results obtained in laboratory. This constant also offers a way to evaluate and predict the performance of flotation equipment and determine the effect of operating parameters on flotation efficiency. As a result of this, many years of research have tried describing the flotation process with mathematical models involving the flotation rate constant and coefficients with physical, chemical and/or statistical meaning. These efforts have shown that the adjustment grade of each model to experimental results is sensitive to many factors and depend on the mineral type, the equipment and the operating parameters. For that reason, there is no predominantly acceptable model in the research area [1]. Each model may be applied to the flotation of a material on specific conditions and this leads to many kinetic expressions with restricted applications on the flotation of different minerals [2].
Some sources [3-5] state that the classic first-order flotation model is the most widely accepted approach and the model most widely used to optimize the design of a flotation circuit. Although there are numerous mathematical models, most of them are derived from the statement that the flotation follows a first-order kinetic, and some of these models are modified versions of the classic model including other parameters or functions (e.g. the flotation rate distribution). Kelsall’s kinetic model [3], for example, incorporates two rate terms instead of one rate constant, but still considers the flotation as a first-order process. Even studies of different approaches as multi-phase second-order kinetic models [2], concluded that the best fit of the experimental data was observed in first-order kinetic models.
The classic flotation model, which relates the kinetics of a chemical reaction to the flotation kinetic, is one of the most applied models [3-5] and still constitutes the research base of recent mineral flotation studies published in 2015 - 2017 [6-12]. This model assumes that flotation occurs as a chemical reaction (A + B → C) where the bubble establishes a bond with a solid particle to produce the bubble-particle attachment, as shown in Fig. 1.
Figure 1: Approach of the classic flotation model
As a chemical reaction in a constant-volume batch reactor, the kinetic of the flotation process is described by eq. (1), where Cp and Cb are the concentrations of particles and bubbles respectively, t is the time, n and m are the orders of reaction and k is the flotation rate constant.

The classic model considers that flotation follows a first-order kinetic, with n = 1 [13]. Since the airflow is kept constant during the process, the flotation rate depends on the solid particles and is linearly dependent on their concentration. Therefore, the flotation process is represented by eq. (2) as a differential equation.
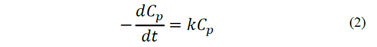
After integration, the kinetic expression in terms of the concentration of particles that remain in the system at t = t, Ct, and the initial concentration of particles, C0, is given by eq. (3).

In the case of coal flotation, the combustible recovery, R, is expressed by eq. (4) as a function of the initial concentration and the concentration of remaining particles. Then, the coal flotation kinetic in terms of combustible recovery is given by eq. (5). According to this model, the flotation rate constant can be obtained from a data set of combustible recovery as a function of time, which can be measured in conventional flotation equipment [14].
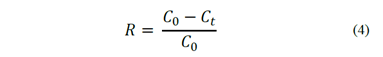

Since it is not possible to obtain 100% combustible recovery in real flotation systems, the classic model is modified, including a maximum recovery parameter, R∞, which represents the maximum recovery after a long flotation time. Thus, the flotation kinetic can be expressed by eq. (6). Despite of the acceptance of this first-order kinetic model, there is an unresolved controversy about the order of the flotation process [5]. Klassen and Mokrousov [15] suggested that the flotation order n most frequently equals 1, less frequently equals 2 and seldom 3 or more. De Bruyn and Modi [16] observed first order rates for the flotation of fine particles if the solids content in the pulp is less than 5.2%. Tomlinson and Fleming [17] also observed that the flotation is a first-order process when the solids concentration in the pulp is low. When the concentration is high, the flotation behaves as a zero-order process.
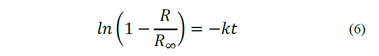
Following the approach of the classic first-order model, Yoon and Mao [18] proposed that the flotation rate constant is a function of both hydrodynamic parameters and surface phenomena, given by eq. (7), where Sb is the superficial surface area rate of bubbles, and P is the probability of the particles being collected by air bubbles.
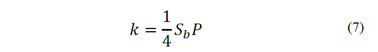
The superficial surface area rate of bubbles, Sb, is defined as the bubble surface area moving out of the cell per unit time per unit cross-sectional area, and it is a function of the superficial gas velocity and the bubble size. The probability of collection, P, is a function of the hydrodynamics of the system and all dominant surface forces found in flotation [19]. The complexity of calculating k, from surface and hydrodynamic parameters, justified the appearance of kinetic expressions as a function of operating parameters affecting the flotation and can be measured more easily [20]. Zhang [21] presented a review of kinetic expressions based on the classic flotation model. Szatkowski and Freyberger [22] expressed the flotation rate constant as a function of operating parameters as particle size, dp, bubble size, db, air concentration in pulp, Ca, air flow, J, and pulp volume, Vs. The flotation rate is represented by eq. (8), where A is a coefficient.
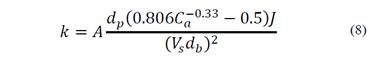
Inoue and Imaizumi [23] suggested that the flotation rate constant is defined by the agitation velocity of the conventional flotation equipment. k is given by eq. (9) where Naf is the airflow number calculated from the features of the agitation system, represented by eq. (10), i is the specific agitation energy, obtained from the agitation power and the characteristics of the pulp, given by eq.(11), and A and B are coefficients.

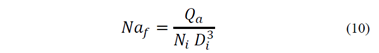
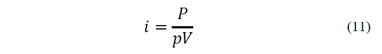
Where Qa is the airflow rate, Ni is the rotating velocity of the impeller, Di is the impeller diameter, P is the agitation power, p is the apparent density of the stirred pulp and V is the pulp volume. Other authors related the flotation rate with particle size [24,25]. These studies agree that the dependence of the flotation rate constant with the particle size, dp, is represented by eq. (12), where A is the coefficient of proportionality and n is a parameter between 1 and 2.

Based on this relationship, Mohns [26] obtained a second order polynomial model for k as a function of the particle size, given by eq. (13), where A, B and C are coefficients.
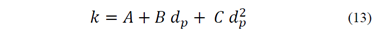
Cilek [27] found a statistical model represented by eq. (14) for the flotation rate constant as a function of the airflow rate (x1), the froth thickness (x2) and the feed grade (x3), with a-g coefficients. This model integrates the grade, a characteristic of the mineral, as a factor affecting the kinetics of flotation.
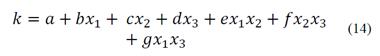
2. Formulation of the new mathematical model for coal flotation kinetics
The proposed model allows for the calculation of an “average” flotation rate constant for all particles in the system and under all flotation conditions. This is the simplest discrete model type with a single rate constant assuming a feed of particles with the same floatability. To be able to compare with the classic flotation model, the population-balance models also are discrete kinetic models where the particles in the system are divided into groups, and the particles in any one group are similar in dimension and composition [3,28]. Those particles in the system have distinct characteristics affecting their floatability (particle size and hydrophobicity) and the proposed model will apply only when these features are taken into account. This includes, for example: a good liberation degree (i.e. particle size reduction accounting the mineral liberation) and narrow particle size distributions. These applicability conditions of this type of model were included in the present work.
According to the classic flotation model, the change in the concentration of coal particles, Cp, over time, in conventional flotation equipment, can be expressed by eq. (15), where t represents the flotation time, n is the order of the process and k is the flotation rate constant. If the volume of the system is kept constant throughout the process, the flotation kinetic may be represented by eq. (16), where M represents the mass of coal that remains in the equipment and k is a complex constant. The flotation rate constant may be expressed by several empirical correlations where k is related with operating parameters such as particle size, airflow, and pulp concentration [20-26]. In spite of the effort to obtain a general equation for k, these correlations are limited to a first-order kinetic and do not show dimensional consistency regarding the parameters and exponents involved.
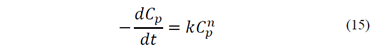
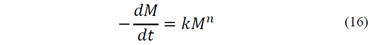
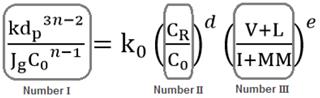
One of the reasons for this is that the flotation kinetic constant is highly affected by other parameters such as the reagents dosage and the characteristics of the mineral. In coal flotation, k is influenced by factors related to the chemistry of the process, the petrographic characteristics of the coal and operating conditions. Chemical parameters include solids concentration in the pulp and frother and/or collector dosage. Operating parameters include airflow velocity and particle size. In terms of petrographic characteristics, this includes the floatable macerals and non-floatable particles content in the coal. By considering all these independent variables, an equation to determine k can be derived by dimensional analysis using the Rayleigh method [29]. According to this method, the flotation rate constant, k (g1-n/s) can be expressed theoretically by eq. (17).

where Jg is the airflow velocity (cm/s), C0 is the solid concentration in the pulp (g/cm3), dp is the particle size (cm), CR is the frother concentration (g/cm3), and Mf and Mi are the floatable macerals and the non-floatable particles volume in the coal feed (cm3), respectively. In general terms, eq. (17) can be expressed in an exponential form by eq. (18), where the exponents a, b, c, d, e and f make the equation dimensionally consistent.
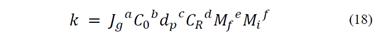
By substituting the corresponding dimensions of each parameter, the expression given by eq. (19) is obtained, where M is the unit of mass (g), L is the unit of length (cm), t is the unit of time (s) and Lm is the unit of length for macerals in coal (cm, for volumetric content of macerals).

Solving for the exponents: a = 1, b = 1 - n - d, c = 2 - 3n and f = -e. After substitution of these exponents, eq. (18) becomes eq. (20).

By grouping the parameters with the same exponent, eq. (20) can be expressed by eq. (21).

A proportionality constant, k0, can be introduced in the equation and constants d and e can be renamed as a and b, respectively. Therefore, eq. (21) can be rewritten as eq. (22).
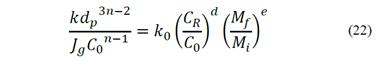
Due to the fact that coal macerals have differences in both hydrophobicity and electro kinetic surface properties, they have different behaviors in the flotation process. The decreasing hydrophobicity order for coal macerals is liptinite > vitrinite > inertinite [30]. On this basis, the percentage by volume of floatable macerals, Mf, can be considered as the percentage by volume of liptinite, L, and vitrinite, V; whereas the percentage by volume of non-floatable particles, Mi, can be considered as the percentage by volume of inertinite, I, and mineral matter content, MM (%, v/v). Thus, eq. (22) can be expressed as eq. (23).
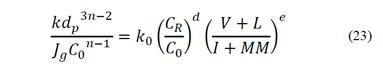
The left side term of eq. (23) relates to the flotation rate constant, k, with operating parameters such as airflow velocity, particle size and solids concentration in the pulp. The first factor on the right side relates to the solids concentration, which also affects the chemistry of the process and the reagent concentration; and the factor on the far right represents the relationship between the percentage of floatable and non-floatable coal material. From eq. (23), the flotation rate constant can be calculated by eq. (24).
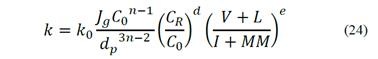
Eq. (24) allows for the calculation of an average flotation rate constant for all particles in the pulp, assuming a feed of
particles with the same floatability. Since the particles in the flotation system have distinct characteristics affecting their floatability (e.g. particle size and hydrophobicity), the proposed model has application only when these features are controlled (i.e. high mineral matter liberation and narrow particle size distributions). By substituting eq. (24) into eq. (16), the coal flotation kinetic is given by eq. (25).

A well as exhibiting dimensional consistency, the dimensionless numbers in the model represented by eq. (23), as shown in Fig. 2, have a physical-chemical meaning.
Figure 2: Dimensionless numbers in the coal flotation kinetic model
The dimensionless number I represents the relationship between the kinetics of the process and the factors affecting the hydrodynamic process. Dimensionless number II is associated with the chemical characteristics of the flotation process and dimensionless number III considers the coal’s characteristics by the relationship between floatable and non-floatable fractions. Those dimensionless numbers were based according to the effect of chemical reagents dosage and solids concentration on the chemistry of froth flotation [31], the effect of the hydrophobicity of coal macerals [30], and the operating variables affecting the hydrodynamics of the process [32].
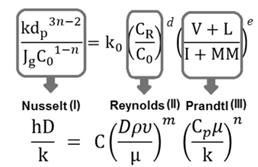
The coal flotation kinetics model resembles transport phenomena models represented by dimensionless numbers, for instance, the equation used to calculate heat transfer coefficients which is a function of Nusselt, Reynolds and Prandtl dimensionless numbers. This analogy is shown in Fig. 3.
Figure 3: Analogy of the coal flotation kinetic model and the heat transfer coefficient model
The heat transfer driving force given by the Reynolds number, can be related to the dimensionless number II in the model, which represents the chemistry of the process. Since chemical reagents dosage and the solids concentration affect the collision, attachment and stability processes between particles and bubbles which govern particle recovery [31], the relation of parameters in the dimensionless number II can be considered as the driving force of the overall flotation process. The Prandtl number provides a combination of properties of the fluid while the dimensionless number III is a combination of the properties of the coal defining its natural hydrophobicity or hydrophilicity [30]. The Nusselt number represents the ratio of the convective and conductive heat transfer of a fluid, two mechanisms of heat transfer, whereas the dimensionless number I relates to the main recovery mechanisms in the flotation, comparing the flotation kinetics, which considers recovery by true flotation, with the hydrodynamic features of the process defining recovery by entrainment [32]. The Rayleigh method has also been useful to develop kinetic models of other processes as char combustion for pulverized coal, where the physical characteristics of the char, the chemical characteristics of the coal/char combustion process and the composition of the coal are related to the chemical reaction rate coefficient through dimensionless numbers [33].
3. Validation of the flotation rate new mathematical model for coal flotation kinetics (special case of n = 1)
Following the classic first-order model approach, the flotation rate constant can be expressed as eq. (26), by substituting n = 1 in eq. (24).

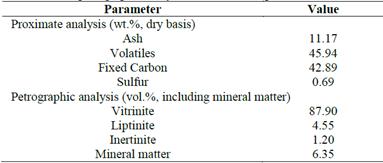
The required coefficients to validate the kinetic model shown in eq. (26) were derived from experimental data of kinetic flotation runs using a coal sample from the state of Antioquia-, Colombia, under different operating conditions. Table 1 shows the proximate analysis and the maceral composition of the coal sample.
Source: The authors.Table 1: Proximate and petrographic analysis of the coal sample.
The kinetic tests were carried out in a 135 cm high 3.9 cm diameter batch glass flotation column. Kerosene and Aerofroth-65 were used as the collector and frother respectively and the frother/collector ratio and the pulp concentration were kept constant at 1.6 and 7.6% w/w, respectively. Three particle sizes (less than 150, 250 and 500 µm), three airflows (0.82, 0.89 and 1.03 cm/s) and three frother concentrations (2400, 2600 and 2800 g/ton) were considered to complete a 33 factorial experimental design with the flotation rate constant as response variable. In all experimental runs, froth samples were collected after 5, 15, 35 and 60 seconds of flotation. From the results of combustible recovery as a function of time, the kinetic constant was determined by linear regression from the solution of eq. (16) for n = 1, given by (27).

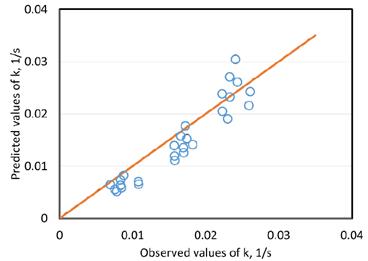
For the validation of the proposed kinetic expressed in eq. (26), a non-lineal least-square method was used to find the values of the constants k0, d and e, that minimized the sum of the squares of the residuals between the experimental kinetic constant and the model calculations. The minimum and maximum values of k from experimental data, the parameters, k0, d and e, and the standard error of the regression, S, are presented in Table 2. The standard error of the regression represents the average distance that the observed values fall from the predicted values of k using the eq. (26) and the parameters of Table 2. The observed and calculated values of the flotation kinetic constant for the coal sample are shown in Fig. 4.
Source: The authors.Table 2: Validation of the flotation kinetic model given by eq. (26)

Figure 4: Predicted and observed values of flotation kinetic constant k
According to the Fig. 4 and the results presented in Table 2, the proposed model shows notable agreement with experimental kinetic constants at various operating conditions using the Antioquia coal sample. The resulting model can be considered a technical tool to predict the flotation process behavior from traditional coal analysis data (proximate and petrographic analysis) and known and/or easily calculated typical operating conditions (particle size, airflow velocity, pulp concentration and reagent dosage), to help in the design, evaluation or optimization of flotation units.
4. Conclusions
Most of the flotation kinetic models found in the literature are empirical and do not have dimensional consistency. A theoretical analysis can be a more descriptive model for the microprocesses of the flotation with practical applications where all of the complex parameters involved are known or measured with specialized equipment. The coal flotation kinetics model developed in this work is new and was obtained by dimensional analysis where petrographic, chemical and operating parameters involved in flotation were correlated with the flotation rate constant using the Rayleigh method. The equation obtained is dimensionally consistent and is given by a combination of three dimensionless numbers which have physical-chemical meaning. These dimensionless numbers represent the hydrodynamical components of the process; the chemical factors of flotation; and the characteristics of the coal in terms of the floatable and non-floatable material content. Moreover, the new model exhibits similarities with models of dimensionless numbers used in transport phenomena. The model was validated to predict the flotation kinetic constant (for the special case of first-order) of a sample of Colombian coal and it showed that there is a good correlation between experimental and predicted values under various operating conditions involving particle size, airflow velocity and frother concentration. The proposed model offers an approximation of the expected behavior of the flotation process when the coal characteristics and operating conditions are known.
Acknowledgments
The authors gratefully acknowledge the grant given by the Colombian Institute of Science and Technology (Colciencias) to Juan Guerrero in order to carry out his doctoral studies.
References
References
Ahmed, N. and Jameson, G.J., Flotation kinetics. Mineral Processing and Extractive Metallurgy, Review 5, pp. 77-99, 1989. DOI: 10.1080/08827508908952645
Saleh, A.M., A study on the performance of second order models and two phase models in iron ore flotation. Physicochemical Problems of Mineral Processing, 44, pp. 215-230, 2010.
Bu, X., Xie, G., Peng, Y., Ge, L. and Ni, C., Kinetics of flotation. Order of process, rate constant distribution and ultimate recovery. Physicochemical Problems of Mineral Processing, 53, pp. 342-365, 2017.
Yianatos, J., Bergh, L., Vinnett, L., Contreras, F. and Diaz, F., Flotation rate distribution in the collection zone of industrial cells. Minerals Engineering, 23, pp. 1030-1035, 2010. DOI: 10.1016/j.mineng.2010.05.008.
Polat, M. and Chander, S., First-order flotation kinetics models and methods for estimation of the true distribution of flotation rate constants. International Journal of Mineral Processing, 58, pp. 145-166, 2000. DOI: 10.1016/S0301-7516(99)00069-1
Luo, C., He, Y., Bu, X. and Wang, S., And improved classic flotation kinetic model of narrow size slime. Journal of China University of Mining and Technology, 44, pp. 477-482, 2015.
Vinnett, L., Alvarez-Silva, M., Jaques, A., Hinojosa, F. and Yianatos, J., Batch flotation kinetics: Fractional calculus approach. Minerals Engineering, 77, pp. 167-171, 2015. DOI: 10.1016/j.mineng.2015.03.020
Ni, C., Kie, G., Jin, M., Peng, Y. and Xia, W., The difference in
flotation kinetics of various size fraction of bituminous coal between rougher and cleaner flotation processes. Powder Technology, 292, pp. 210-216, 2016. DOI: 10.1016/j.powtec.2016.02.004
Alvarez-Silva, M., Vinnett, L., Langlois, R. and Waters, K.E., A comparison of the predictability of batch flotation kinetic models. Minerals Engineering, 99, pp. 142-150, 2016. DOI: 10.1016/j.mineng.2016.08.019
Ai, G., Yang, X. and Li, X., Flotation characteristics and flotation kinetics of fine wolframite. Powder Technology, 305, pp. 377-381, 2017. DOI: 10.1016/j.powtec.2016.09.068
Xing, Y., Gui, X., Cao, Y., Wang, Y., Xu, M., Wang, D. and Li, C., Effect of compound collector and blending frother on froth stability and flotation performance of oxidized coal. Powder Technology, 305, pp. 166-173, 2017. DOI: 10.1016/j.powtec.2016.10.003
Zhang, N.N., Zhuo, C.C., Pan, J.H., Xia, W., Liu, C., Tang, M.C. and Cao, S.S., The response of diasporic-bauxite flotation to particle size based on flotation kinetic study and neural network simulation. Powder Technology, 318, pp. 272-281, 2017. DOI: 10.1016/j.powtec.2017.06.010
Brozek, M. and Mlynarczykowska, A., Analysis of kinetics models of batch flotation. Physicochemical Problems of Mineral Processing, 41, pp. 51-65, 2007.
Wierink, G., A computational framework for coupled modelling of three-phase systems with soluble surfactants. PhD dissertation, Aalto University, Helsinki, Finland, 2012.
Klassen, V.I. and Mokrousov, V.A., An introduction to the theory of flotation. Butterworths, London, 1963.
De Bruyn, P.L. and Modi, H.J., Particle size and flotation rate of quartz. Trans. AIME, 205, pp. 415-419, 1956.
Tomlinson, H.S. and Fleming, M.G., Flotation rate studies. VI International Mineral Processing Congress Proceedings, 1965, pp. 563-579.
Yoon, R. and Mao, L., Application of extended DLVO theory, IV derivation of flotation rate equation from first principles. Journal of Colloid and Interface Science, 181, pp. 613-626, 1996. DOI: 10.1006/jcis.1996.0419
Sherrel, I.M., Development of flotation rate equation from first principles under turbulent flow conditions. PhD dissertation. Virginia Polytechnic Institute and State University, Blacksburg, VA, 2004.
Piñeres, J.L., Fenómenos superficiales y cinéticos de la separación del grupo maceral vitrinita en fracciones beneficiadas de carbones colombianos obtenidas por flotación burbujeante. PhD dissertation, Universidad del Valle, Cali, Colombia, 2008.
Zhang, J.G., Factor affecting the kinetics of froth flotation. PhD Dissertation, Department of Mining and Mineral Engineering, University of Leeds, Great Britain, 1989.
Stokowski, H. and Freyberger, W.L., Model describing mechanism of the flotation process. Transaction of the Institution of Mining and Metallurgy, 94, C 61-69, 1985.
Inoue, T. and Imauzumi, T., A series of work related to flotation kinetics. 4th Joint Meeting MMIJ-AIME Proceedings, 1980. pp. 84-100.
Trahar, W.J., A rational interpretation of the role of particle size in flotation. International Journal of Mineral Processing, 8, pp. 289-327, 1981. DOI: 10.1016/0301-7516(81)90019-3
Hernáinz, F. and Calero, M., Froth flotation: kinetic models based on chemical analogy. Chemical Engineering and Processing, 40, pp. 269-275, 2001. DOI: 10.1016/S0255-2701(00)00125-2
Mohns, C.A., Effect of particle size on coal flotation kinetics. MSc. Thesis. Department of Mining Engineering, Queen’s University, Kingston, Canada, 1997.
Cilek, E.C., Estimation of flotation kinetic parameters by considering interactions of the operating variables. Minerals Engineering, 17, pp. 81-85, 2004. DOI: 10.1016/j.mineng.2003.10.008
Jovanovic, I. and Miljanovic, I., Modelling of flotation processes by classic mathematical methods - A review. Archives of Mining Sciences, 60(4), pp. 905-919, 2015. DOI: 10.1515/amsc-2015-0059
Perry, R., Green, D.W. andMaloney, J.O., Perry’s chemical engineers’ handbook, vol. 2. 6th ed. McGraw Hill, New York, 1992, pp. 2-120.
Arnold, B. and Aplan, F., The hydrophobicity of coal macerals. Fuel, 68(5), pp. 651-658, 1989. DOI: 10.1016/0016-2361(89)90168-3
Rao, S.R., Surface chemistry of froth flotation. Kluwer Academic / Plenum Publisher. New York, 2004, pp. 660-695.
Wang, L., Peng, Y., Runge, K. and Bradshaw, D., A review of entrainment: Mechanisms, contributing factors and modelling in flotation. Minerals Engineering, 70, pp. 77-91, 2015. DOI: 10.1016/j.mineng.2014.09.003
Barranco, R., Rojas, A., Barraza, J. and Lester, E., A new char combustion kinetic model 1. Formulation. Fuel, 88, pp. 2335-2339, 2009. DOI: 10.1016/j.fuel.2009.02.005
How to Cite
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Piyush Malhotra, Bivas Kumar Pradhan, Nikkam Suresh. (2024). Flotation and oil agglomeration of high ash LVMC coal after deshaling by jigging: a comparative study using different collectors. International Journal of Coal Preparation and Utilization, , p.1. https://doi.org/10.1080/19392699.2024.2305931.
2. Agnieszka Saramak, Daniel Saramak. (2022). Coal Modeling Investigations in International Collaboration in the Light of Bibliometric Analysis of the Problem. Energies, 15(16), p.6040. https://doi.org/10.3390/en15166040.
3. K. L. Bharath, Suresh Nikkam, G. Udayabhanu. (2022). Beneficiation of high-ash Indian coal fines by froth flotation using bio-degradable-oil as a collector. International Journal of Coal Preparation and Utilization, 42(9), p.2685. https://doi.org/10.1080/19392699.2021.1876681.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2017 DYNA

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The author of a paper accepted for publication in any of the journals published by the School of Mines will yield all the property to the National University of Colombia rights free of charge, within which include article: the right to edit, publish, reproduce and distribute both print and digital media, as well as including in an article in international indexes and / or databases, likewise, it enables the publisher to use images, tables and/or graphic material presented in Article for designing covers or posters of the magazine. By assuming the economic rights of the article, it may be reproduced partially or totally in any printed or digital media without express permission of the same.