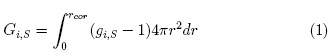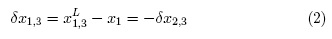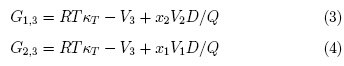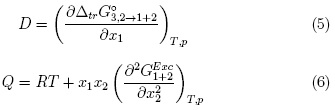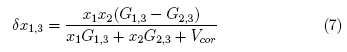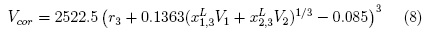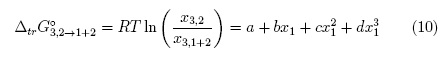PREFERENTIAL SOLVATION OF L-ARABINOSE AND DL-MALIC ACID IN ETHANOL + WATER MIXTURES
SOLVATACIÓN PREFERENCIAL DE L-ARABINOSA Y ÁCIDO DL-MÁLICO EN MEZCLAS ETANOL + AGUA
DOI:
https://doi.org/10.15446/mo.n54.62428Keywords:
L-arabinose, DL-malic acid, ethanol, solubility, IKBI, preferential solvation. (en)L-arabinosa, ácido DL-málico, etanol, solubilidad, IKBI, solvatación preferencial. (es)
By using the inverse Kirkwood-Buff integrals (IKBI) method, the differences between the local, around the solute and the bulk mole fractions of both solvents in saturated solutions of L-arabinose (compound 3) and DL-malic acid (compound 3) in ethanol (compound 1) + water (compound 2) binary mixtures were derived from their thermodynamic properties. Accordingly, it is found that these compounds are sensitive to preferential solvation effects; in this way, the preferential solvation parameter (dx1,3) for L-arabinose is slightly positive in water-rich mixtures but negative in those beyond 0.25 in ethanol mole fraction. In different way, the dx1,3 values of DL-malic acid are negative in almost all the compositions. The highest solvation by ethanol observed in water-rich mixtures for L-arabinose could be due mainly to polarity effects. Otherwise, the preference of these compounds for water in ethanol-rich mixtures could be explained in terms of the higher acidic behavior of water interacting with hydrogen-acceptor hydroxyl groups in L-arabinose and DL-malic acid.
Utilizando algunas propiedades termodinámicas clásicas de disolución se calcularon los parámetros de solvatación preferencial dx1,3) de L-arabinosa y ácido DL-málico en mezclas etanol + agua mediante el método de las integrales inversas de Kirkwood-Buff (IKBI, por sus siglas en inglés); estos parámetros dx1,3 corresponden a las diferencias entre las fracciones molares locales alrededor del soluto y en el grueso de la solución. Se observó que estos compuestos son sensibles a efectos específicos de solvatación según la composición de la mezcla cosolvente. Así, los valores de dx1,3 para la L-arabinosa son positivos en mezclas ricas en agua pero negativos en composiciones desde 0.25 en fracción molar de etanol hasta el etanol puro. Sin embargo, en el caso del ácido DL-málico los valores de dx1,3 son negativos en todas las composiciones cosolventes analizadas. En mezclas ricas en agua la mayor solvatación de la L-arabinosa por parte de las moléculas de etanol podría deberse principalmente a efectos de polaridad. De otro lado, la preferencia que manifiestan ambos compuestos por el agua en mezclas ricas en etanol, podría explicarse en términos de la mayor acidez del agua, la cual estaría interactuando con los grupos aceptores de hidrógeno presentes en los dos solutos.
PREFERENTIAL SOLVATION OF L-ARABINOSE AND DL-MALIC ACID IN ETHANOL + WATER MIXTURES
SOLVATACIÓN PREFERENCIAL DE L-ARABINOSA Y ÁCIDO DL-MÁLICO EN MEZCLAS ETANOL + AGUA
Zaira J. Cárdenas, Daniel M. Jiménez, Fleming Martínez
Grupo de Investigaciones Farmacéutico, Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia, Bogotá, Colombia.
Fleming Martínez: fmartinezr@unal.edu.co
(Recibido: Octubre/2016. Aceptado: Diciembre/2016)
Abstract
By using the inverse Kirkwood-Buff integráis (IKBI) method, the differences between the local, around the solute and the bulk mole fractions of both solvents in saturated solutions of L-arabinose (compound 3) and DL-malic acid (compound 3) in ethanol (compound 1)+ water (compound 2) binary mixtures were derived from their thermodynamic properties. Accordingly, it is found that these compounds are sensitive to preferential solvation effects; in this way, the preferential solvation parameter (δx1,3) for L-arabinose is slightly positive in water-rich mixtures but negative in those beyond 0.25 in ethanol mole fraction. In different way, the δx1,3 values of DL-malic acid are negative in almost all the compositions. The highest solvation by ethanol observed in water-rich mixtures for L-arabinose could be due mainly to polarity effects. Otherwise, the preference of these compounds for water in ethanol-rich mixtures could be explained in terms of the higher acidic behavior of water interacting with hydrogen-acceptor hydroxyl groups in L-arabinose and DL-malic acid.
Keywords: L-arabinose, DL-malic acid, ethanol, solubility, IKBI, preferential solvation.
Resumen
Utilizando algunas propiedades termodinámicas clásicas de disolución se calcularon los parámetros de solvatación preferencial (δx1,3) de L-arabinosa y acido DL-málico en mezclas etanol + agua mediante el método de las integrales inversas de Kirkwood-Buff (IKBI, por sus siglas en ingles); estos parámetros δx1,3 corresponden a las diferencias entre las fracciones molares locales alrededor del soluto y en el grueso de la solución. Se observó que estos compuestos son sensibles a efectos específicos de solvatación según la composición de la mezcla cosolvente. Así, los valores de δx1,3 para la L-arabinosa son positivos en mezclas ricas en agua pero negativos en composiciones desde 0,25 en fracción molar de etanol hasta el etanol puro. Sin embargo, en el caso del ácido DL-málico los valores de δx1,3 son negativos en todas las composiciones cosolventes analizadas. En mezclas ricas en agua la mayor solvatación de la L-arabinosa por parte de las moléculas de etanol podrá deberse principalmente a efectos de polaridad. De otro lado, la preferencia que manifiestan ambos compuestos por el agua en mezclas ricas en etanol, podrá explicarse en términos de la mayor acidez del agua, la cual estará interactuando con los grupos aceptores de hidrogeno presentes en los dos solutos.
Palabras clave: L-arabinosa, acido DL-málico, etanol, solubilidad, IKBI, solvatación preferencial.
Introduction
L-Arabinose (also known as pectinose, C5H10O5, molar mass 150.13 g mol-1, CAS number 147-81-9, Fig. 1-B is a monosaccharide including an aldehyde functional group found in hemicelluloses and pectin [1]. DL-Malic acid (Hydroxybutanedioic acid, C4H6O5, molar mass 134.09 g mol-1, CAS number 617-48-1, Fig. 1-C) is a hydroxy-dicarboxylic organic acid present in several fruits and commonly used as food additive [1]. In the industrial manufacture of L-arabinose and DL-malic acid, these compounds are purified through crystallization from diluted or concentrated solutions as the final step. In this way, aqueous alcoholic mixtures are widely used with this purpose. Because the knowledge about the equilibrium solubility is a crucial factor for crystallization processes Jiang et al. [2] and Yuan et al. [3] studied, respectively, the solubility of L-arabinose and DL-malic acid in several ethanol + water mixtures at different temperatures.
Considering that the cosolvency or solvent blending has been employed for a long time to increase or decrease the solubility of organic compounds [4, 5], which is a desired effect to optimize the crystallization processes of solutes, a deep physical-chemical approach of the mechanisms involved in solubilization and/or desolubilization processes, including preferential solvation [6, 7], regains significance.
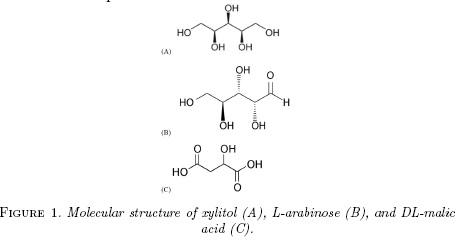
Regarding thermodynamic studies, some recent researches have been published based on the enthalpic and entropic contributions to the Gibbs energy of solution of these organic hydroxyl-compounds [3, 4]. Nevertheless, the preferential solvation, i.e. the cosolvent specific composition around L-arabinose and DL-malic acid molecules has not been studied. Therefore, the main goal of this research is to evaluate the preferential solvation of these compounds in ethanol + water cosolvent mixtures, based on some classical thermodynamic definitions [8, 9]. L-Arabinose and DL-malic acid were chosen as model solutes for this research owing their multiple pharmaceutical and chemical applications. Thus, this work is very similar to that presented previously in the literature for the preferential solvation of the sweetening agent xylitol (Fig. 1-A) in similar cosolvent mixtures [10].
Theoretical Background
The Kirkwood-Buff integrals (KBIs, Gi,S) are given by the following expression:
Here giS is the pair correlation function for the molecules of the solvent i in cosolvent mixtures around the solute (S), r the distance between the centers of the molecules of solute and solvent components, and rcor is a correlation distance for which gi,s (r > rCor) ≈ 1. The results are expressed in terms of the preferential solvation parameter δxi,S for the solute in the saturated solutions by the component solvents, i.e. ethanol and water [11]. For solvation of L-arabinose (component 3) or DL-malic acid (component 3), this parameter is defined for ethanol (component 1) as:
Where x1 is the mole fraction of ethanol in the bulk solvent mixture and xL1,3 is the local mole fraction of ethanol in the environment near to the solute. If δx1,3 > 0 the solute is preferentially solvated by ethanol; on the contrary, if it is < 0 the solute is preferentially solvated by water, within the correlation volume (Vcor = (4π/3)r3cor) and the bulk mole fraction of ethanol, x1 . Values of 8x1 3 are calculable from those of G1,3 and G2,3, which are obtained from thermodynamic data of the cosolvent mixtures with and without the solute dissolved on them [8].
Mathematical manipulation of the basic expressions reported by Newman [11] leads to practical expressions for the Kirkwood-Buff integrals (expressed in cm3 mol-1) for the individual solvent components as shown in equations 3 and 4 [6, 7]:
Here kT is the isothermal compressibility of the ethanol + water solvent mixtures
(expressed in GPa-1), V1 and V2 are
the partial molar volumes of the solvents in the mixtures (expressed in cm3 mol-1), and similarly, V3 is the partial molar volume of the
solute L-arabinose or DL-malic acid in these cosolvent mixtures (expressed in
cm3 mol-1). The function D is the derivative of the
standard molar Gibbs energies of transfer of the solute from neat water to
ethanol + water mixtures regarding to the ethanol proportion in the mixtures
(expressed in kJ mol-1, as also is RT). The function Q involves the
second derivative of the excess molar Gibbs energy of mixing of both solvents (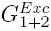 ) in function of
the water proportion in the mixtures (also expressed in kJ mol-1)
[6, 7]. Thus, functions D and Q are defined as:
) in function of
the water proportion in the mixtures (also expressed in kJ mol-1)
[6, 7]. Thus, functions D and Q are defined as:
Ben-Naim [12] demonstrated that the preferential solvation parameter can be calculated from the Kirkwood-Buff integrals as follows:
The correlation volume (Vcor) is commonly obtained by means of the following expression [6, 7]:
Here r3 is the molecular radius of the solute (expressed in nm) and may be calculated from the molar volume by using the Avogadro number (NAv) as:
However,
the definitive correlation volume requires iteration because it depends on the
local mole fractions. This iteration is performed by replacing δx1 t3 in the equation 2 to calculate 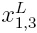 until a non-variant
value of Vcor is obtained.
until a non-variant
value of Vcor is obtained.
Results and Discussion
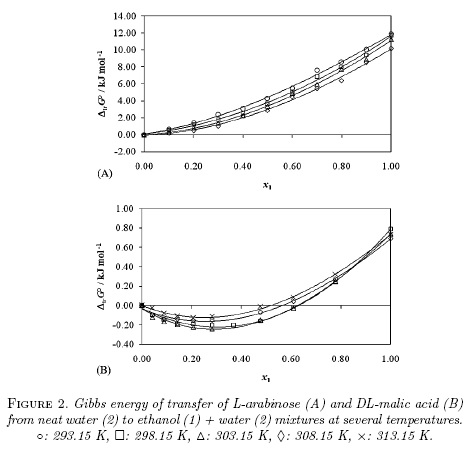
Standard molar Gibbs energy of transfer of these organic compounds from neat water to ethanol + water mixtures was calculated and correlated to regular third degree polynomials from the drug solubility data taken from [2, 3] by using equation 10. Table 1 and Fig. 2 show the Gibbs energy of transfer behavior at all the temperatures studied. Temperatures from 293.15 to 313.15 K were considered owing the thermodynamic quantities required for IKBI calculations have been reported in this range [10, 13]. The respective polynomial coefficients are shown in Table 2.
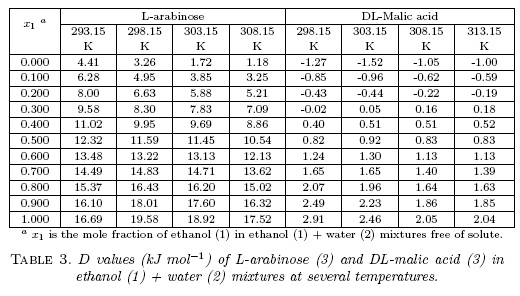
D values were calculated from the first derivative of polynomial models solved according to the cosolvent mixtures composition. This procedure was performed varying by 0.05 in mole fraction of ethanol but in the following tables the respective values are reported varying only by 0.10 to save space in the article. D values are reported in Table 3.
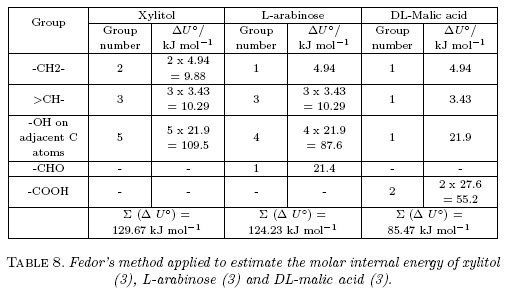
Q and RTKT values of the binary aqueous-ethanol mixtures at all temperatures, as well as the partial molar volumes of ethanol and water were taken from those reported in previous studies with other solutes [10, 13, 14]. Otherwise, in a first approach the molar volume of these compounds were considered here as independent of the cosolvent composition and temperature, and also as equivalent to those presented in solid state. Thus, these values were calculated by considering the density values reported in the literature (1.585 g cm-3 for L-arabinose and 1.601 g cm-3 for DL-malic acid) [15]. In this way, the molar volume values of 83.75 and 94.72 cm3 mol-1 were obtained, respectively. Furthermore, from these values the molecular radiuses (r3) of both compounds were calculated by using the equation 9 as 0.335 nm for L-arabinose and 0.321 nm for DL-malic acid.
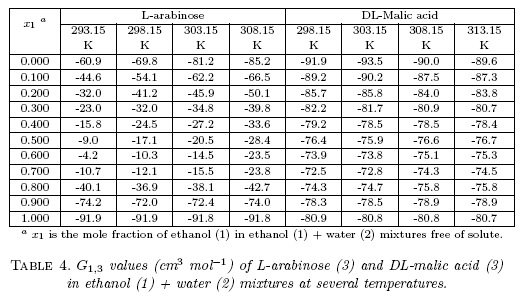
Table 4 shows that all the G1,3 values of both compounds are negative with the maximum values in neat ethanol for L-arabinose and neat water for DL-malic acid. In different way, Table 5 shows that G2,3 values of L-arabinose are negative in water-rich mixtures but positive beyond in the mixture with X1 ≥0.30 reaching maximum value in the mixture with x1 _0.80. Otherwise, G2,3 values of DL-malic acid are negative in almost all mixtures with the exception of X1 _0.80 at all temperatures and X1 _0.90 at 298.15 K and 303.15 K. These results could be interpreted as the preference of both solutes by water molecules in ethanol-rich mixtures.
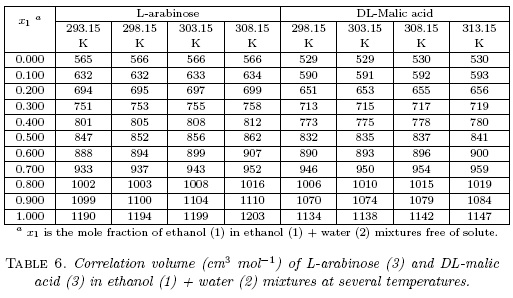
As was already mentioned, to use the IKBI method, the correlation volume of both compounds was iterated three times by using the equations 2, 7 and 8) to obtain the values reported in Table 6. It is noteworthy that these values are almost independent on temperature in water-rich mixtures but they increases slightly in ethanol-rich mixtures. This could be a consequence of the higher thermal expansibility of ethanol compared with water [16].
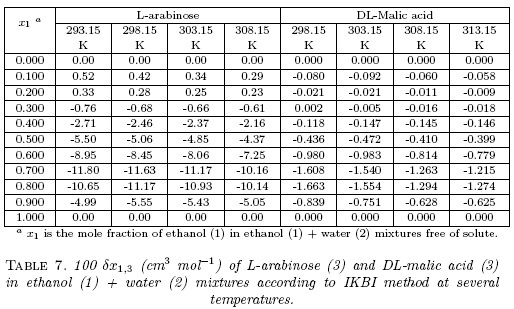
The values of δx1,3 vary non-linearly with the ethanol proportion in these aqueous mixtures at all the temperatures studied (Table 7 and Fig. 3). In water-rich mixtures, the addition of ethanol to water makes positive the δx1,3 values of L-arabinose from pure water to the mixture with x1 =0.20 reaching a maximum of 4.2 x 10-3 in the mixtures with x1 =0.10 at 298.15 K. This maximum diminishes with the temperature arising. On the contrary, the δx1,3 values of DL-malic acid are slightly negative in water-rich mixtures, except in x1 =0.30 at 298.15 K, where a really low positive value is observed. Nevertheless, it is not easy to assign these δx1,3 values to preferential solvation effects because they are lower than 0.01 and could be owing to uncertainties propagation in the IKBI calculations [12,17].
Otherwise, from these ethanol proportions up to neat ethanol, the δx1,3 values are significantly negative, and hence, L-arabinose and DL-malic acid are preferentially solvated by water in ethanol-rich mixtures. Both compounds act as Lewis acids in solution owing the hydrogen atoms in their -OH groups (Fig. 1) to establish hydrogen bonds with proton-acceptor functional groups in the solvents (oxygen atoms in -OH groups). Additionally, these compounds could act as Lewis bases due to the free electron pairs in the oxygen atoms of their hydroxyl and carbonyl groups (Fig. 1) to interact with the acidic hydrogen atoms in both solvents.
Based on these preferential solvation results, it is probable that in water-rich mixtures, where the DL-malic acid is apparently preferentially solvated by ethanol molecules, this compound is slightly acting as Lewis acid with ethanol molecules because this cosolvent is more basic than water as described by their Kamlet-Taft hydrogen bond acceptor parameters, i.e. β = 0.75 and 0.47 for ethanol and water, respectively [18, 19]. On the other hand, in ethanol-rich mixtures, where both L-arabinose and DL-malic acid are preferentially solvated by water, these compounds could be acting mainly as Lewis bases in front of water because water is more acidic than ethanol as also described by their Kamlet-Taft hydrogen bond donor parameters, i.e. α =1.17 and 0.86 for water and ethanol, respectively [18, 20].
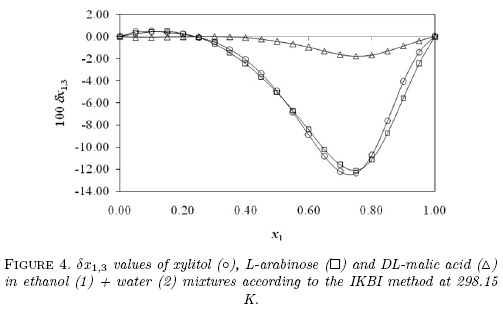
Furthermore, Fig. 4 compares the preferential solvation behavior of these compounds including xylitol at 298.15 K [10]. It is noteworthy that the behaviors exhibited by L-arabinose and xylitol are very similar, being both of them highly preferentially solvated by water in ethanol-rich mixtures, with maximum δx1,3 values higher than -0.12 in the mixture with x1 =0.75; whereas, the preferential solvation of DL-malic acid by water is not so high as the other two compounds with a maximum δx1,3 value of -1.76 x 10-2 in the mixture of x1 =0.75. These three hydroxyl-compounds present different functional groups as follows: xylitol is a polyhydroxy-alcohol, L-arabinose is a polyhydroxy-aldehyde, and DL-malic acid is a hydroxy-dicarboxylic acid. For this reason, as a polarity criterion [21], the Hildebrand solubility parameters (δ3 expressed in MPa1/2) were calculated for these compounds based on the Fedors method [22]. δ3 values were calculated as (ΔU°/V°)1/2, with ΔU° (expressed in J mol-1) as the molar internal energy and V° (expressed in cm3 mol-1) the molar volume [21]. Table 8 shows the respective ΔU° values for these compounds. In this way, í3 values are as follows: 36.0 MPa1/2 for xylitol, 36.2 MPa1/2 for L-arabinose, and 31.9 MPa1/2 for DL-malic acid. It is noteworthy that solubility parameters of xylitol and L-arabinose are very similar as also are their preferential solvation behaviors. Thus, the í3 value is the lowest being the less polar compound and therefore, the DL-malic acid preferential solvation by water in ethanol-rich mixtures is significantly lower compared with xylitol and L-arabinose.
Finally, it is important to note that all these results about preferential solvation of these compounds are in good agreement with those described previously in the literature, which were based in more basic dissolution thermodynamic approaches [2,3,23].
Conclusions
Quantitative values for the local mole fractions of ethanol and water around these compounds were derived based on the IKBI method applied to some literature equilibrium solubility values in ethanol + water mixtures at several temperatures. Thus, these compounds are preferentially solvated by water in mixtures beyond 0.20 or 0.25 in mole fraction of ethanol at all temperatures considered. It is noteworthy that these negative δx1,3 values diminish as temperature arises for both compounds. It can also be concluded for these compounds that the less polar a compound is its δx1,3 magnitude also is.
References
[1] S. Budavari et al., The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed. (Merck & Co, 2001).
[2] L. Jiang et al., J. Mol. Li, 211, 406 (2015).
[3] Y. Yuan et al., Fluid Phase Equilib. 377, 27 (2014).
[4] J. Swarbrick and J. Boylan, Encyclopedia of Pharmaceutical Technology, Vol. 3 (Marcel Dekker, Inc. New York, 1988) pp. 375-398.
[5] A. Jouyban, Handbook of Solubility Data for Pharmaceuticals (CRC Press, 2010).
[6] Y. Marcus, J. Mol. Liq. 140, 61 (2008).
[7] Y. Marcus, Acta Chim. Slov 56, 40 (2009).
[8] Y. Marcus, Solvent Mixtures: Properties and Selective Solvation(CRC Press, 2002).
[9] Y. Marcus, Preferential solvation in mixed solvents. In: Fluctuation Theory of Solutions: Applications in Chemistry, Chemical Engineering, and Biophysics, edited by P. Smith, E. Matteoli, and J. O'Connell (CRC Press, Taylor & Francis Group, 2013).
[10] D. Delgado, E. Vargas, and F. Martínez, Rev. Colomb. Quím. 4 2, 59 (2013).
[11] K. E. Newman, Chem. Soc. Rev. 23, 31 (1994).
[12] A. Ben-Naim, Pure Appl. Chem. 62, 25 (1990).
[13] D. R. Delgado, M. A. Peña, and F. Martínez, Rev. Colomb. Cienc. Quím. Farm. 42, 298 (2013).
[14] D. R. Delgado and F. Martínez, J. Mol. Liq. 193, 152 (2014).
[15] D. R. Lide, Handbook of Chemistry and Physics, 84th ed. (CRC Press, 2003).
[16] J. Jimíenez, J. Manrique, and F. Martínez, Rev. Colomb. Cienc. Quím. Farm. 33, 145 (2004).
[17] Y. Marcus, Pure Appl. Chem. 62, 2069 (1990).
[18] Y. Marcus, The Properties of Solvents (John Wiley & Sons, 1998).
[19] M. J. Kamlet and R. W. Taft, J. Am. Chem. Soc. 98, 377 (1976).
[20] R. W. Taft and M. J. Kamlet, J. Am. Chem. Soc. 98, 2886 (1976).
[21] A. F. M. Barton, Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed. (CRC Press, 1991).
[22] R. F. Fedors, Polymer Eng. Sci. 14, 147 (1974).
[23] Z. Wang, Q. Wang, X. Liu, W. Fang, Y. Li, and H. Xiao, Korean J. Chem. Eng. 30, 931 (2013).
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Abolghasem Jouyban, Fleming Martinez, William E. Acree. (2017). Comment on “Measurement and Correlation of the Solubility of Maltitol in Different Pure Solvents, Methanol–Water Mixtures, and Ethanol–Water Mixtures”. Journal of Chemical & Engineering Data, 62(6), p.1919. https://doi.org/10.1021/acs.jced.7b00204.
2. Darío A. Tinjacá, María M. Muñoz, Abolghasem Jouyban, Fleming Martínez, William E Acree. (2019). Equilibrium solubility, preferential solvation and apparent specific volume of sucrose in some {cosolvent (1) + water (2)} mixtures at 298.2 K. Physics and Chemistry of Liquids, 57(2), p.259. https://doi.org/10.1080/00319104.2018.1439028.
3. Victoria Inyang, David Lokhat. (2020). Kinetic studies on propionic and malic acid reactive extraction using trioctylamine in 1-decanol. Chemical Papers, 74(10), p.3597. https://doi.org/10.1007/s11696-020-01194-2.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2017 MOMENTO - Physics Journal

This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Those authors who have publications with this journal, accept the following terms:
a. The authors will retain their copyright and will guarantee the publication of the first publication of their work, which will be subject to the Attribution-SinDerivar 4.0 International Creative Commons Attribution License that permits redistribution, commercial or non-commercial, As long as the Work circulates intact and unchanged, where it indicates its author and its first publication in this magazine.
b. Authors are encouraged to disseminate their work through the Internet (eg in institutional telematic files or on their website) before and during the sending process, which can produce interesting exchanges and increase appointments of the published work.