Publicado
Estudio calorimétrico comparativo de la adsorción sobre carbón activado de fenol y acetaminofén desde solución acuosa
Comparative calorimetry study of the phenol and acetaminophen adsorption on activated carbon in aqueous solution
DOI:
https://doi.org/10.15446/rcciquifa.v44n1.54284Palabras clave:
Solución acuosa de fenol, Solución acuosa de acetaminofén, Carbón activado, Termogramas, Entalpía de inmersión (es)Aqueous solution of phenol, Aqueous solution of acetaminophen, Activated carbon, Thermogram, Enthalpy of immersion (en)
Comparative calorimetry study of the phenol and acetaminophen adsorption on activated carbon in aqueous solution
Estudio calorimétrico comparativo de la adsorción sobre carbón activado de fenol y acetaminofén desde solución acuosa
Valentina Bernal a
Juan Carlos Moreno-Piraján b
a Departamento de Química, Facultad de Ciencias, Universidad Nacional de Colombia, Cra 30 No. 45-03, Bogotá, D. C., Colombia. * Correo electrónico: lgiraldogu@unal.edu.co.
b Departamento de Química, Facultad de Ciencias, Universidad de los Andes, Cra. 1a No. 18A-10, Bogotá, D. C., Colombia.
Recibido para evaluación: 9 de noviembre de 2014.
Aceptado para publicación: 16 de marzo de 2015.
Summary
Immersion enthalpies were determined for aqueous solutions of phenol, acetaminophen and water on three different carbons that differed in terms of oxygen groups on the surface. The intensity of the energy interaction between these compounds and the activated carbons was determined. The activated carbons had similar values for surface area and micropore volume, i.e. approximately 850 m2.g-1 and 0.35 cm3.g-1, respectively, but differed in the content of surface chemical groups that interacted with the solutes in aqueous solution. The values obtained for the enthalpies of immersion of activated carbon in water were between -32 and -66 J.g-1. The enthalpies of immersion of activated carbon for acetaminophen and phenol solutions with concentrations between 100 mg.L-1 and 10 mg.L-1 were between -18 and -157 J.g-1, indicating greater interaction between the two solutes and activated carbons with a high surface content of reduced chemical groups.
Key words: Aqueous solution of phenol, aqueous solution of acetaminophen, activated carbon, thermogram, enthalpy of immersion.
Resumen
Se determinan las entalpías de inmersión en soluciones acuosas de fenol y acetaminofén y en agua de tres carbones activados que difieren en el contenido de grupos oxigenados superficiales con el propósito de conocer la intensidad de las interacciones energéticas entre los compuestos y los carbones activados. Los carbones activados presentan valores de área superficial y volúmenes de microporo similares, alrededor de 850 m2.g-1 y 0,35 cm3.g-1, respectivamente, y difieren en el contenido de grupos químicos superficiales que son los que interactúan con los solutos que se encuentran en solución acuosa. Los valores obtenidos para las entalpías de inmersión de los carbones activados en agua están entre -32 y -66 J.g-1. Las entalpías de inmersión de los carbones activados en las soluciones de 100 mg.L-1 y 10 mg.mL-1 de fenol y acetaminofén se encuentran entre -18 y -157 J.g-1, que indican una mayor interacción para los dos solutos con el carbón activado que tiene mayor contenido de grupos básicos superficiales.
Palabras clave: solución acuosa de fenol, solución acuosa de acetaminofén, carbón activado, termogramas, entalpía de inmersión.
Introduction
Currently, many chemical species are found in the water supply due to human activities. Many of these compounds are used for therapeutic purposes (i.e., pharmaceuticals or drugs) and their presence has been studied for many decades [1]. Pharmaceuticals are continuously introduced into the environment and are prevalent at small concentrations [2], which can affect water quality and potentially impact drinking water supplies, the ecosystem and human health [3]. The pollution produced by pharmaceutical products in surface and ground waters has been acknowledged by many countries as an environmental problem and has led to the establishment of a research field known as Pharmaceuticals in the Environment [4]. The pharmaceutical industry uses the designation Active Pharmaceutical Ingredients to describe products that are pharmacologically active, resistant to degradation, highly persistent in aqueous media, potentially able to produce adverse events in water organisms and capable of exerting a negative impact on human health.
Acetaminophen is a drug with wide use as an analgesic and antipyretic, especially in pediatric patients [5]. From the viewpoint of the chemical structure, the molecule is a phenol derivative (Figure 1).
Phenol and its derivatives (including acetaminophen) are pollutants of high priority and concern because of their toxicity and possible accumulation in the environment. Phenols are introduced into surface water from industrial effluents such as those from the coal tar, gasoline, plastic, rubber proofing, disinfectants, the pharmaceutical and steel industries, domestic wastewater, agricultural run-off and chemical spills. Various methods have been proposed for the treatment of wastewaters containing pharmaceutical compounds [6]; one of these is adsorption on porous materials [7].
Adsorption on solids occurs as a result of unsaturated molecular forces present on the surface of the solid. When the surface comes into contact with the solution, an interaction occurs between the forcefield of the surface and the liquid. The surface of the solid tends to satisfy the residual forces for attraction and retention on the surface of the molecules present in the liquid [8].
The adsorption of pharmaceutical compounds can be carried out using adsorbent solids like activated carbon (AC), which has a highly porous structure that confers a large surface area and pore volume compared to other porous solids. These characteristics make these materials excellent adsorbents in processes that involve gas or liquid phases [9, 10].
The interactions present when pharmaceuticals compounds are adsorbed onto the activated carbon can be quantified determining the immersion enthalpy of the solids in the solution. The immersion enthalpy involves the total energy of the interactions between the solid and the solvent, the solid and the solute, and finally the interaction between the solute and the solvent.
A comparison between the immersion enthalpies of different activated carbons in aqueous solutions of pharmaceuticals can be performed and the system with the greatest interaction can be determined [11, 12].
In this work, the immersion enthalpies of water, an aqueous solution of phenol and an aqueous solution of acetaminophen were determined for three activated carbons with different amounts of reduced chemical groups. The experimental results show the amounts of energy generated in the activated carbon-solution interaction. The value of immersion enthalpy reflects the ability to attract the solute to the solid surface under study.
Methodology
Activated carbons
A calorimetric study was performed on three activated carbons. As the starting material, a solid granular activated carbon was prepared from coconut shell by physical activation with CO2 (GAC). This activated carbon was sifted to a particle size between 2-4 mm, washed with 0.1 M hydrochloric acid and dried at 100 °C. The second activated carbon (GACO) corresponds to GAC treated with a boiling solution of 6 M nitric acid for 6 hours in a Soxhlet apparatus; the aim was to generate a greater number of oxygenated groups on the surface. The third activated carbon (GACR) was obtained by heating the starting activated carbon at 900 °C to remove the surface oxygen groups.
Preparation of phenol and acetaminophen solutions
For determine the immersion enthalpies of phenol and acetaminophen solutions, four solutions of the compounds were prepared: acetaminophen at a concentration of 100 mg.L-1, a saturated solution of acetaminophen at 10730 mg.L-1, phenol at a concentration of 100 mg L-1 and phenol at a concentration of 10730 mg.L-1. The latter concentration of phenol corresponded to the concentration of the saturated solution of acetaminophen. The aim of this experiment was to compare the energies at the same concentrations. The difference in the solution concentrations was intended demonstrate a change in the magnitude of the immersion enthalpies.
Textural characterization of the activated carbons
The surface area and the porous volume of the activated carbons was determined by physical adsoption of N2 at 77 K using an Autosorb 3B apparatus (Quantachrome). The apparent surface area and the micropore volume were determined using the Brunauer, Emmett and Teller BET , and Dubinin-Radushkevich models, respectively.
Total acidity and basicity of the activated carbons
The parameters of total acidity and basicity were evaluated by Boehm´s method [13], which consists of a back-titration of oxygen groups on the surface of the activated carbon. To do this, around 1.000 g of the sample was weighed out and added to 50 mL of an NaOH solution to determinate the acidity or to 50 mL a 0.1 M HCl solution to determinate the basicity. Thus, in each mixture, the acidic and basic groups present on the surface of the activated carbon were neutralized. The mixtures were kept at 298 K with constant stirring for five days. At the end of this time period, a 10 mL aliquot of the supernatant of each solution was titrated accordingly with a previously standardized solution of NaOH or HCl.
Determination of immersion enthalpy
The immersion enthalpies of the activated carbons in water and solutions of phenol and acetaminophen at high and low concentrations were determined in a heat conduction microcalorimeter with a stainless steel cell with a capacity of 15 mL; 10 mL of the immersion liquid was used. Around 100 mg of each sample of activated carbon was weighed out and put into a glass vial with a fragile peak, then placed in the calorimetric cell. After this, the registration of electric potential output of sensor was performed for approximately 40 minutes to obtain a stable baseline. The sample was then immersed, and the increased potential due to wetting of the solid was recorded, then the potential was finally allowed to return to baseline [14]. The area under the curve of the electrical potential versus time corresponded to the heat generated by the contact of the activated carbon and the evaluated liquid. Finally, in the electrical calibration, the electrical work was calculated as well as the calorimeter constant K (W.V-1) corresponding to the ratio between the electrical work and the integral of the calibration peak. The heat of immersion ( J) was determined by calculating the product of the calorimeter constant and the immersion peak integral; the enthalpy ( J.g-1) was found by dividing the immersion heat by the mass of the activated carbon placed in the glass ampoule. The immersion of the activated carbon into the phenol and acetaminophen solutions was performed four times.
Statistical treatment of the data determined for immersion enthalpies on the activated carbon in the acetaminophen solution
The influence of the surface chemistry of the activated carbon and its interaction with pharmaceutical compounds [15], especially with acetaminophen [16, 17], is known. Determining the immersion enthalpy of activated carbons with different surface chemistries led to differences in the values, which indicated the effect of surface chemistry on the interaction. To perform the surface chemistry determinations, four immersion enthalpy experiments were done for each treatment. The results were tested by analysis of variance [18].
Results
The results on surface area and the pore volume as well as the acidity and basicity of the samples are presented in Table 1. The results show the changes produced by the different chemical and physical treatments carried out on the samples.
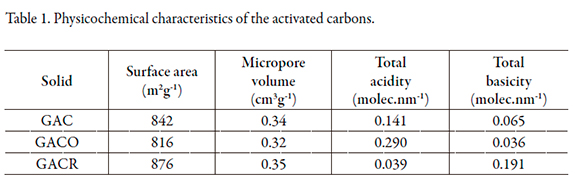
The results show that the activated carbon oxidized with nitric acid (GACO) presented a decreased surface area and micropore volume (Vo) with respect to the activated carbon without treatment (GAC). This behavior was due to oxidation, which promoted the formation of surface oxygen groups located at the edges of the pore openings; this limited the accessibility of nitrogen molecules into the porous structures [19]. Thermal treatment at 900 °C on led to the decomposition of oxygen groups, which led to the difference in surface area with respect to GACO. Table 2 show some oxygenated groups and their decomposition temperatures. At the chosen temperature for the assay, the thermal decomposition of many oxygenated groups generated additional porosity in the activated carbon GACR.
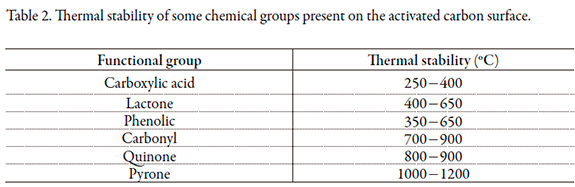
With respect to the total acidity and basicity, it was observed that the oxidized activated carbon in the total acid value was increased due to the formation of acidic groups following treatment with nitric acid. Figure 2 shows the reaction in which the nitric acid forms carbonyl and carboxyl groups on the surface of activated carbon [20]. According to the information provided in Table 2 and the conditions under which the reduction of the GACR sample was performed, some of the basicity of the activated carbon was due to the presence of oxygenated basic groups like pyrone groups, which exhibit high thermal stability, allowing them to remain on the surface of activated charcoal after heat treatment at 900 °C.

The values of immersion enthalpies of solids in different liquids are usually different and provide a characterization parameter of the solid-liquid system. Therefore, the magnitude of the immersion enthalpy depends on the surface area of the solid, so that for a solid-liquid system, the immersion energy increases with the surface area of the solid. If the chemical nature of the surface and the immersion liquid is polar, the immersion energy will increase with the polarity of the functional chemical groups on the surface of the solid [21].
When the calorimetric liquid employed in the immersion of the solid is a solution, the value of the total enthalpy will be related to the interactions between the solid, solvent and solute, as follows:
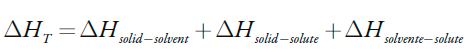 | [1] |
When the immersion enthalpies of activated carbons are determined in aqueous solutions of acetaminophen and phenol, the total energy of these interactions may be obtained to establish the differences between different solutes, considering that acetaminophen has a phenyl ring as part of its structure.
Calculating the immersion enthalpy is done using a graph of the electric potential measured by a heat flow sensor (the thermopile in a heat conduction calorimeter, as was used in this work) as a function of time. Therefore, the thermogram is itself a record of the behavior of the solid-liquid system during immersion.
In the beginning of the experiment, the calorimetric potential was constant since the condition of thermal equilibrium had been established for the system. When the solid and the liquid were brought into contact, a quantity of heat was generated that flowed through the sensor; when the electric potential increased to form a peak, the interaction ceased and the potential returned to the initial potential. Potential readings were recorded for about 15 minutes and electrically calibrated by the introduction of a quantity of electrical work through an electrical resistor. The heat generated by the immersion of the solid in the liquid was proportional to the area under the curve of the peak in the plot of potential versus time, according to the expression:
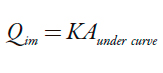 | [2] |
where K is the constant of the calorimeter obtained from electrical calibration. The enthalpy of immersion was obtained by referring to the heat per gram of solid.
Figure 3 shows the thermograms obtained for the immersion of the activated carbons into the phenol solution at a concentration of 100 mg.L-1. This concentration was chosen to determine the interaction between the activated carbon and a solution with a dilute concentration.

Figure 3 shows that most interactions occurred in the sample reduced using heat treatment at 900 °C. In this sample, the oxygen groups on the surface were decomposed, so it presented the highest value of basicity. The oxidized activated carbon showed less interaction, indicating that oxidation increases the number of acidic groups on the surface, which withdraw electron density from the graphene layers, thereby decreasing the amount of solute adsorbed. Thus, a greater number of surface oxygen groups increased the polarity of the activated carbon surface and induced the formation of hydrogen bonds with water, which explains the reduced solid-solution interactions [22, 23].
Figure 4 presents the thermograms obtained for the immersion of the activated carbons in saturated solutions of phenol, i.e., 10730 mg.L-1. The behavior was similar to the thermograms for the dilute solution, although the saturated solution generated more heat due the presence of more molecules of phenol. As observed, the character of the interaction was exothermic, corresponding to adsorption processes on the porous surface.

We wanted to compare the interactions between activated carbons and solutions of phenol vs. solutions of acetaminophen at the same concentration, since acetaminophen contains a phenyl group and forms dimers in solution when the pH is close to 5. These dimers are molecules larger than the original molecule, thus decreasing the adsorption on the activated carbon [24]. The results in Figure 5 correspond to the thermograms for the immersion of the activated carbon in acetaminophen solution at a concentration of 100 mg.L-1.
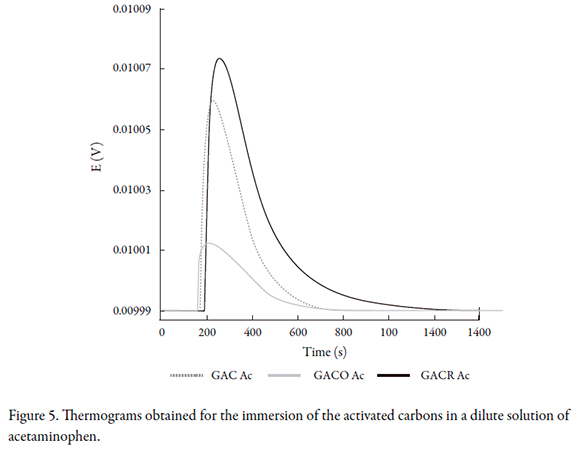
Figure 6 shows the thermograms obtained for the immersion of activated carbons in a saturated solution of acetaminophen. The behavior was similar for the immersion in saturated and dilute phenol solutions; the sample corresponding to the activated carbon reduced with thermal treatment showed the highest interaction with the solution, indicating that the process of adsorption of acetaminophen on the solid was highly influenced by the surface chemistry

When a comparison of the thermograms was performed, we found that there were differences in the maximum potential value obtained for the peaks. While the immersion enthalpy is proportional to the area under curve of the peaks, in qualitative form, the height of the peak indicates that the generation of heat was in the following order from highest to lowest: GACR, GAC, and GACO.
Once the thermograms were determined, the enthalpy values for the activated carbons immersed in solutions of phenol and acetaminophen were calculated. The values of specific enthalpy of immersion and standard deviations are presented in Table 3. The values of the enthalpies of immersion for showed the strongest interaction with the reduced activated carbon, which confirms that the adsorption was carried out mainly by interactions between groups on the carbon surface and the evaluated molecules. These interactions are linked to the amount of solute present in the solution as samples immersed in saturated solutions had higher values than the enthalpies in dilute solutions.

As the interaction between the activated carbon and acetaminophen or phenol was carried out in aqueous phase, one factor that affects the interaction is the pH of the solution because the surface of the activated carbon may have charges that favor or disfavor the interaction with the solute [25]. In this work, the pH of the solution was the one after the compound was dissolved in water without further adjustment since the purpose was to measure the enthalpy produced under these conditions, although the energy of the solid-solution interaction can be modified if the pH of the solution is changed [26].
For the results of the immersion enthalpy of activated carbons in the 100 mg.L-1 acetaminophen solution, analysis of variance was performed in order to confirm that the difference in the surface chemistry of the activated carbon affects the energy of the solid-liquid interaction. The surface chemistry of each activated carbon correspond to the treatment and the four repeats of immersion enthalpy for diluted acetaminophen solution corresponds to observations; the results are summarized in Table 4.
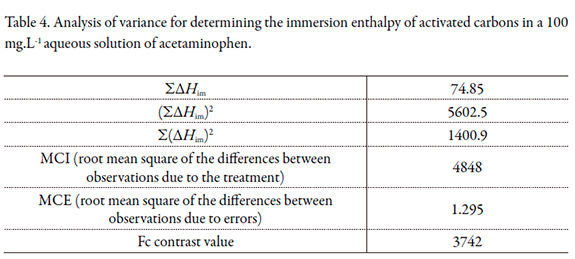
According to the calculated Fc value, 3742, it can be said that the difference in the surface chemistry of the activated carbons caused the difference in the values obtained for immersion enthalpies, since the F value reported in F value tables with a confidence level of 95% for the parameters of the determination is 4.26, which is significantly lower than the value obtained.
The importance of this work was to demonstrate the influence of surface chemistry on the immersion enthalpy with different surface groups in aqueous solutions of acetaminophen. The results indicate that a change in activated carbon surface chemistry affected the solid-solution interaction energy and thus the retention capacity of acetaminophen. For other solutions, the same behavior is assumed. Moreover, a relationship was established between the immersion enthalpy and the content of basic groups on the activated carbon, as it was observed that greater interaction was achieved with the reduced activated carbon. The relationship for the two solutes can be seen in Figure 7.
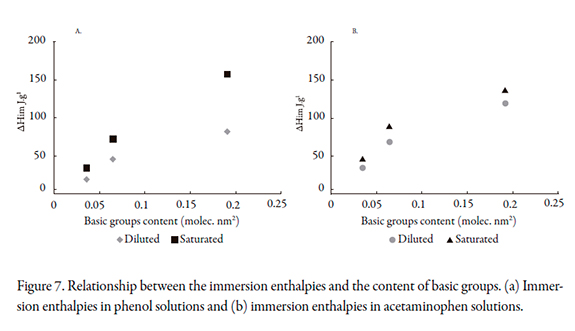
Figure 7(a) presents the immersion enthalpies of the activated carbons in dilute and saturated phenol solutions. It was noted that the enthalpies were exothermic, and increasing the content of basic groups on the activated carbon enhanced the interaction with the solution, reflected as a higher enthalpy value. The values obtained for the immersion enthalpy in saturated solutions were higher due the presence of more molecules of phenol in the medium. The greatest difference between the values of immersion enthalpy was observed for the reduced activated carbon.
For the dilute and saturated acetaminophen solutions, the immersion enthalpies as a function of the content of basic groups on the solid are shown in Figure 7(b). As observed, the values of the immersion enthalpy of activated carbon in the dilute solution and the saturated solutions were similar. Since acetaminophen is a larger compound than phenol, it had lower immersion enthalpy values; the possibility to forming dimers in the saturated aqueous solution of acetaminophen decrease the interactions with the surface on the activated carbons compared to the phenol solutions at the conditions.
The enthalpies of immersion of activated carbon in water were determined with values of 32.4, 49.7 and 66.6 J.g-1 for GACR, GAC and GACO, respectively. These values allowed for using Hess´ law to find the enthalpy of the activated carbon-solute interaction, ΔHac-s. The results are presented in Table 5.

As shown in Table 5, a marked influence of the surface chemistry of the activated carbon was observed on the activated carbon-solution interaction enthalpies. The change in enthalpy between the solid and the solute was obtained by discounting the experimental immersion enthalpy value of the activated carbon in water, which produced a change in the nature of the i nteraction. The results show that for the activated carbons GACO and GAC, when the content of the solute was lower, the interaction was endothermic and higher for the oxidized activated carbon, GACO, indicating energy absorbance by the solid-solute system. The activated carbon GACR exhibited an exothermic interaction enthalpy in all solutions, with higher values for the saturated acetaminophen and phenol solutions.
Conclusions
We determined the immersion enthalpies for activated carbons with modified surface chemistry in water and aqueous solutions of phenol and acetaminophen at two concentration levels, dilute (100 mg.L-1) and saturated (10730 mg.L-1). For the phenol solutions, the value of immersion enthalpy was between 19.4 and -157.2 J.g-1, while for the acetaminophen solutions, the immersion enthalpy was between -18.7 and -102.6 J.g-1. The immersion enthalpy in water was between -32.4 and -66.6 J.g-1.
An influence of the surface chemistry of the activated carbon was observed, with the highest values of immersion enthalpy found for the reduced activated carbon GACR for both solutes due to the formation of basic groups and π delocalized electrons in the graphene layer of the activated carbon, which acted as electron donors, facilitating the interaction with the carbonyl, phenol and amide groups present in the phenol and acetaminophen molecules.
Acknowledgments
The authors thank the Framework Agreement between the University of los Andes and the National University of Colombia and the act of agreement established between the Chemistry Departments of the two universities for project 21531 "Semilleros de Colciencias".
References
1. A.P. Terzyk, G. Rychlicki, The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro. The temperature dependence of adsorption at the neutral pH, Colloids Surf. A, 163, 135-150 (2000).
2. D.W. Kolpin, E.T. Furlong, M.T. Meyer, E.M. Thurman, S.D. Zaugg, L.B. Barber, H.T. Buxton, Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: A national reconnaissance, Environ. Sci. Technol., 36, 1202-1211 (2002).
3. I. Sirés, E. Brillas, Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review, Environ. Int., 40, 2012-2229 (2012).
4. K. Kümmerer, The presence of pharmaceuticals in the environment due to human use - present knowledge and future challenges, J. Environ. Manag., 90, 2354-2366 (2009).
5. P. Bustamante, S. Romero, A. Reillo, Thermodynamics of paracetamol in amphiprotic and amphiprotic-aprotic solvent mixtures, Pharm. Sci., 1, 505-507 (1995).
6. S.H. Lin, R.S. Juang, Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review, J. Environ. Manag., 90, 1336-1349 (2009).
7. L.M. Cotoruelo, M.D. Marqués, A. Leiva, J. Rodríguez-Mirasol, T. Cordero, Adsorption of oxygen-containing aromatics used in petrochemical, pharmaceutical and food industries by means of lignin based active carbons, Adsorption, 17, 539-550 (2011).
8. R.C. Bansal, M. Goyal, Activated carbon and its surface structure. In: "Activated carbon adsorption", Taylor & Francis Group, New York, 2005, pp. 1-60.
9. I. Cabrita, B. Ruiz, A.S. Mestre, I.M. Fonseca, A.P. Carvalho, C.O. Ania, Removal of an analgesic using activated carbons prepared from urban and industrialresidues, Chem. Eng. J., 163, 249-255 (2010).
10. C. López-Velandia, J.J. Moreno-Barbosa, R. Sierra-Ramírez, L. Giraldo, J.C. Moreno-Piraján, Adsorption of volatile carboxylic acids on activated carbon synthesized from watermelon shells, Adsorpt. Sci. Technol., 32(2-3), 227-242 (2014).
11. L. Giraldo, J.C. Moreno-Piraján, Relation between immersion enthalpies of activated carbons in different liquids, textural properties, and phenol adsorption, J. Therm. Anal. Calorim., 117, 1517-1523 (2014).
12. V. Rakic, V. Rac, M. Krmar, O. Otman, A. Auroux, The adsorption of pharmaceutically active compounds from aqueous solutions onto activated carbons, J. Hazard. Mater., 282, 141-149 (2014).
13. H.P. Boehm, Some aspects of the surface chemistry of carbon blacks and other carbons, Carbon, 32, 759-769 (1994).
14. L. Giraldo, J.C. Moreno, J.I. Huertas, Heats conduction micro-calorimeter with metallic reaction cells, Instrum. Sci. Technol., 30, 177-186 (2002).
15. V. Rakic, V. Rac, M. Krmar, A.A Otman, The adsorption of pharmaceutically active compounds from aqueoussolutions onto activated carbons, J. Hazard. Mater., 282, 141-149 (2015).
16. M. Galhetas, A.S. Mestre, M.L. Pinto, I. Gulyurtlu, H. Lopes, A.P. Carvalho, Chars from gasification of coal and pine activated with K2CO3: Acetaminophen and caffeine adsorption from aqueous solutions, J. Colloid Interface Sci., 433, 94-103 (2014).
17. I. Cabrita, B. Ruiz, A.S. Mestre, I.M. Fonseca, A.P. Carvalho, C.O. Ania, Removal of an analgesic using activated carbons prepared from urban and industrial residues, Chem. Eng. J., 163, 249-255 (2010).
18. E.L. Bauer, "Manual de estadística para químicos", Madrid: Ed. Alhambra, 1982.
19. F. Rodríguez-Reinoso, M. Molina-Sabio, M.A. Muñecas-Vidal, Effect of microporosity and oxygen surface groups of activated carbon in the adsorption of molecules of different polarity, J. Phys. Chem., 96, 2707-2713 (1992).
20. J. Collins, T. Ngo, D. Qu, M. Foster, Spectroscopic investigations of sequential nitric acid treatments on granulated activated carbon: Effects of surface oxygen groups on π density, Carbon, 57, 174-183 (2013).
21. R. Denoyel, F. Rouquerol, J. Rouquerol, Porous texture and surface characterization from liquid-solid interactions: Immersion calorimetry and adsorption from solution. In: "Adsorption by carbon", Elsevier, San Diego, 2008, pp. 273-297.
22. J.C. Moreno, L. Giraldo, A. Gómez, A batch-type heat conduction microcalorimeter for immersion heat determinations: Design and calibration, Thermochem. Acta, 290, 1-12 (1996).
23. L. Li, P. Quilivan, D. Knape, Effects of activated carbon surface chemistry and pore structure on adsorption of organic contaminants from aqueous solution, Carbon, 40, 2085-2100 (2002).
24. C. Moreno-Castilla, Adsorption of organic molecules from aqueous solutions on carbon materials, Carbon, 42, 83-94 (2004).
25. M. Galhetas, A.S. Mestre, M.L. Pinto, I. Gulyurtlu, H. Lopes, A.P. Carvalho, Carbon-based materials prepared from pine gasification residues for acetaminophen adsorption, Chem. Eng. J., 240, 344-351 (2014).
26. D.A. Blanco, L. Giraldo, J.C. Moreno, Effect of the pH in the adsorption and in the immersion enthalpy of monohydroxylated phenols from aqueous solutions on activated carbons, J. Hazard. Mater., 169, 291-296 (2009).
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Valentina Bernal, Alessandro Erto, Liliana Giraldo, Juan Moreno-Piraján. (2017). Effect of Solution pH on the Adsorption of Paracetamol on Chemically Modified Activated Carbons. Molecules, 22(7), p.1032. https://doi.org/10.3390/molecules22071032.
2. Astrid R. Moreno-Marenco, Liliana Giraldo, Juan Carlos Moreno-Piraján. (2019). Dataset of the immersion enthalpy of activated carbons chemically modified in methylparaben aqueous solution: Relation with adsorption. Data in Brief, 25, p.104100. https://doi.org/10.1016/j.dib.2019.104100.
3. Astrid Roxanna Moreno-Marenco, Liliana Giraldo, Juan Carlos Moreno-Piraján. (2019). Parabens Adsorption onto Activated Carbon: Relation with Chemical and Structural Properties. Molecules, 24(23), p.4313. https://doi.org/10.3390/molecules24234313.
4. Juan Carlos Moreno-Piraján, Carlos A. Guerrero-Fajardo, Liliana Giraldo. (2025). Determination of the immersion enthalpy of graphene oxide in aqueous solutions of emerging organic compounds with pharmaceutical activity. Journal of Molecular Liquids, 421, p.126846. https://doi.org/10.1016/j.molliq.2025.126846.
5. Yesica Vicente-Martínez, Manuel Caravaca, Antonio Soto-Meca, Rubén Solana-González. (2020). Magnetic core-modified silver nanoparticles for ibuprofen removal: an emerging pollutant in waters. Scientific Reports, 10(1) https://doi.org/10.1038/s41598-020-75223-1.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2015 Revista Colombiana de Ciencias Químico-Farmacéuticas

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
El Departamento de Farmacia de la Facultad de Ciencias de la Universidad Nacional de Colombia autoriza la fotocopia de artículos y textos para fines de uso académico o interno de las instituciones citando la fuente. Las ideas emitidas por los autores son responsabilidad expresa de estos y no de la revista.
Todo el contenido de esta revista, excepto dónde está identificado, está bajo una Licencia Creative Commons de Atribución 4.0 aprobada en Colombia. Consulte la normativa en: http://co.creativecommons.org/?page_id=13

















