Vanillin Schiff bases: Molecular interactions in methanol and THF solutions
Bases de Schiff de vainillina: interacciones intermoleculares en soluciones de metanol y THF
DOI:
https://doi.org/10.15446/rcciquifa.v46n2.67957Palabras clave:
Vanillin Schiff bases, ultrasonic Study, acoustical parameters, methanol, THF (en)bases Schiff de vainillina, estudio ultrasónico, parámetros acústicos, metanol, THF (es)
Descargas
have been studied in methanol and tetrahydrofuran (THF) at 308.15 K. From the
experimental data, various acoustical parameters such as isentropic compressibility
(κs), Rao’s molar sound function (Rm), Van der Waals constant (b), relaxation strength
(r), intermolecular free length (Lf), apparent molar compressibility, etc. have been
evaluated, which helps in understanding the molecular interactions occurring in
these solutions
soluciones de algunas bases de Schiff derivadas de la vainillina en metanol y tetrahidrofurano
(THF) a 308,15 K. A partir de los datos experimentales, se evaluaron
diversos parámetros acústicos, como la compresibilidad isentrópica (κs), la función
acústica molar de Rao (Rm), la constante de Van der Waals (b), la fuerza de relajación
(r), la longitud intermolecular libre (Lf), la compresibilidad molar aparente,
etc., todo lo cual ayuda a comprender las interacciones moleculares que ocurren en
estas soluciones.
Recibido: 4 de mayo de 2017; Aceptado: 4 de agosto de 2017
SUMMARY
Density, ultrasonic velocity and viscosity of some vanillin Schiff bases derivatives have been studied in methanol and tetrahydrofuran (THF) at 308.15 K. From the experimental data, various acoustical parameters such as isentropic compressibility (κ s), Rao's molar sound function (R m ), Van der Waals constant (b), relaxation strength (r), intermolecular free length (L f ), apparent molar compressibility, etc. have been evaluated, which helps in understanding the molecular interactions occurring in these solutions.
Keywords:
Vanillin Schiff bases, ultrasonic Study, acoustical parameters, methanol, THF.RESUMEN
En este trabajo se estudiaron la densidad, la velocidad ultrasónica y la viscosidad de soluciones de algunas bases de Schiff derivadas de la vainillina en metanol y tetrahidrofurano (THF) a 308,15 K. A partir de los datos experimentales, se evaluaron diversos parámetros acústicos, como la compresibilidad isentrópica (k s ), la función acústica molar de Rao (R m ), la constante de Van der Waals (b), la fuerza de relajación (r), la longitud intermolecular libre (L f ), la compresibilidad molar aparente, etc., todo lo cual ayuda a comprender las interacciones moleculares que ocurren en estas soluciones.
Palabras clave:
bases Schiff de vainilla, estudio ultrasónico, parámetros acústicos, metanol, THF.INTRODUCTION
Ultrasonic velocity measurements have been used to study the nature of molecular interactions in various pure liquids 1-3, liquid mixtures 4-10 and in solutions 11-17. However, little work has been done for some organic compound solutions 18-21 especially Schiff bases 22-25.
Some of these bases are known to possess a wide spectrum of biological activities and are used in pharmaceutical science 26-29. The presence of different functional groups cause different type of interactions with different solvents which is an important parameter for the selection of these compounds as starting material, intermediate or product 30-32. Further, binding or interaction of a compound or drug affects their pharmacokinetic and pharmacodynamic properties 33-35. For the prediction of biological activities and transport phenomena also, physiochemical parameters are required 36,37 and one of important parameter is interaction between solute and solvent molecules. The ultrasonic study is one of the non-destructive tools to study different types of interactions occurring in solutions 38,39.
Thus, in present paper, acoustical properties of some vanillin Schiff bases are studied in methanol and THF over entire concentration range at 308.15 K. The results are interpreted in terms of molecular interactions occurring in the solution.
EXPERIMENTAL
The methanol and THF used in the present work were of AR grade and were purchased from Spectrochem Pvt. Ltd (Mumbai) and were purified according to the standard procedure 40. The Schiff bases were synthesized in the laboratory and were recrystallized before use. The common structure of synthesized Schiff bases and their substitution groups (R) are given in Figure 1.
Figure 1: General structure of vanillin Schiff bases. R is: SV-1
: 4-CH3-C6H4;
SV-2
: 3-Cl-4-F-C6H3;
SV-3
: 3-OCH3-C6H4;
SV-4
: 4-F-C6H4;
SV-5
: 2-CH3-C6H4;
SV-6
: 2-Cl,5-Cl-C6H3;
SV-7:
-C6H5;
SV-8
: -C5H6N3O-C6H5.
The densities, ultrasonic velocity and viscosity of pure solvents and their solutions were measured by single capillary pycnometer, single crystal variable path ultrasonic interferometer operating at 2 MHz (Mittal Enterprises) and Ubbelohde viscometer respectively. The accuracy of density, velocity and viscosity are ± 0.0001 g/cm3, ± 0.1% cm/sec and 0.05% respectively. All the measurements were carried out at 308.15 K. The uncertainty of temperature is ± 0.1 K and that of concentration is 0.0001 mol/dm3.
RESULTS AND DISCUSSION
The experimental data of ultrasonic velocity, density and viscosity are given in Table 1.
Table 1: The density (ρ), ultrasonic velocity (U) and viscosity (η) of vanillin Schiff bases in methanol and THF at 308.15 K.

From the experimental data, various acoustical parameters were calculated using equations reported earlier 41.
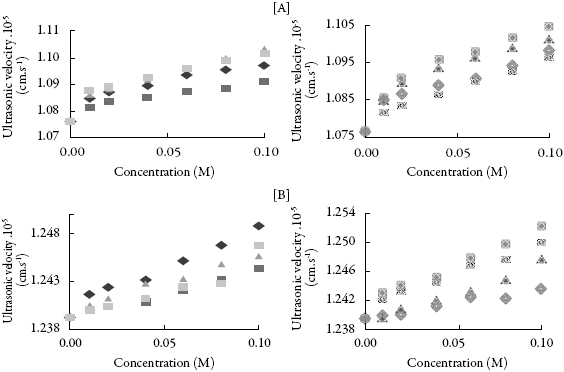
Figure 2 shows the variation of ultrasonic velocity (U) increases with concentration for all the compounds in methanol and THF. It is observed that ultrasonic velocity increases with concentration for all the compounds in both the solvents.
Figure 2: The variation of ultrasonic velocity with concentration in (A) methanol and (B) THF.♦:SV-1,:SV-2,:SV-3,:SV-4,:SV-5,:SV-6,:SV-7,:SV-8.
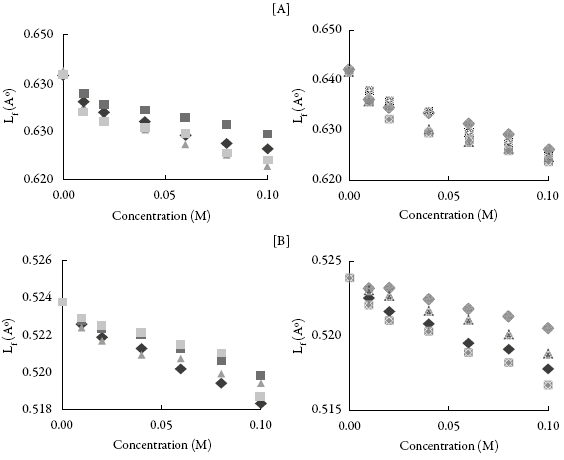
The velocity depends on intermolecular free length (L f ). Figure 3 show that L f decreases continuously with concentration. Thus, intermolecular free length is reverse of velocity. In a solution, when the distance between molecules of solvent and compound decreases, Lf decreases which causes velocity to increase. The decrease in distance suggests interaction between solvent and compound molecules.
Figure 3: The variation of intermolecular free path length (L
f
) with concentration in (A) methanol and (B) THF. : SV-1,: SV-2,: SV-3,SV-4,SV-5,SV-6,: SV-7,: SV-8.
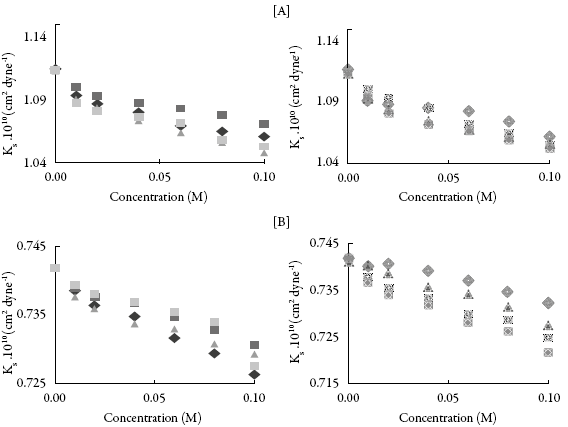
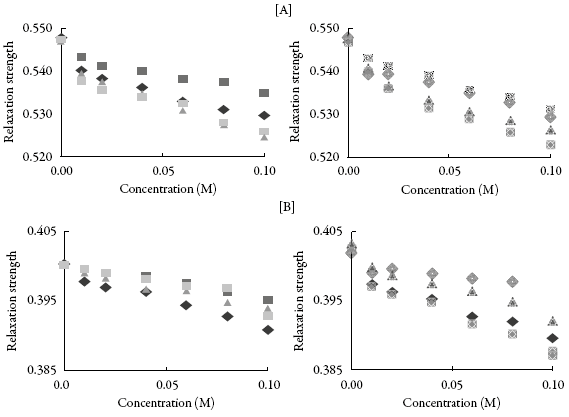
This is further supported by isentropic compressibility (Xs) and relaxation strength (r). The variation of these two parameters with concentration of these compounds is also shown in Figures 4 and 5.
Figure 4: The variation of isentropic compressibility (Xs) with concentration in (A) methanol and (B) THF : SV-1, SV-2, SV-3,SV-4,SV-5, m:SV-6, SV-7,SV-8.
Figure 5: The variation of relaxation strength with concentration in (A) methanol and(B) THF : SV-1, SV-2, SV-3,SV-4,SV-5, m:SV-6, SV-7,SV-8.
It is observed that both isentropic compressibility and relaxation strength decrease with concentration for all the compounds in both the solvents. The decrease of isentropic compressibility and relaxation strength with increasing concentration might be due to aggregation of solvent molecules around compound molecules which causes interaction between molecules of compound and solvent.
For all the compounds in both the solvents, Rao's molar sound function (Rm), molar compressibility (W) and Van der Waals' constant (b) vary linearly with concentration. Table 2 shows the correlation equation and correlation coefficient values for all these solutions. The linear change indicates that there is no complex formation in solution.
Table 2: The least-square Correlation equations and Correlation coefficients (Υ) for compounds in methanol and THF at 303.15 K. C is the concentration.

The type and magnitude of interactions in solution is further confirmed by apparent molar properties. The apparent molar compressibility's ((Фk) of the solutions is fitted to Gucker's relation (42).
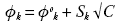
From the plot of Фk verses  and S
k
values are evaluated from the intercept and slope. The isentropic compressibility of all the solutions was also fitted to the following Bachem's relation 43:
and S
k
values are evaluated from the intercept and slope. The isentropic compressibility of all the solutions was also fitted to the following Bachem's relation 43:
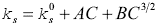
The values of A and B were evaluated from the intercept and slope respectively. k0 s is the isentropic compressibility of pure solvent. All these values of intercept and slopes are given in Table 3.
Table 3: Constants A, B,
Ф
0
k and
S
k
for vanillin Schiff bases in methanol and THF at 308.15 K.

Table 3 shows that in both solvents A and ( Ф 0 k values are negative or very low whereas B and S k values are positive. The low or negative A and Ф 0 k values and positive B and S k values suggest predominance of solute-solvent interactions.
CONCLUSIONS
It is concluded that in studied solutions of vanillin Schiff bases, compound-solvent interactions exist. This suggests that these compounds are more bonded to solvent and some modification is required so that it can easily be absorbed by the target.
ACKNOWLEDGMENTS
Authors are thankful to Head of Chemistry Department, Saurashtra University for providing facilities.
REFERENCES
Referencias
J. Nath, S.N. Dubey, Binary systems of trichloroethylene with benzene, toluene, p-xylene, carbon tetrachloride, and chloroform. Ultrasonic velocities and adiabatic compressibilities at 303.15 and 313.15 K and dielectric properties and refractive indexes at 303.15 K, J. Phys. Chem, 84, 2166 (1980).
C.M. Sehgal, Non-linear ultrasonics to determine molecular properties of pure liquids, Ultrasonics, 33, 155 (1995).
M. Kalidoss, S. Ravi, New method of determining the structure factor of real liquids and their mixtures using ultrasonic velocity, Statist. Mechan. Appl ., 312, 59 (2002).
G.N. Saha, B. Das, D.K. Hazra, Viscosities and excess molar volumes for acetonitrile + methanol at 298.15, 308.15, and 318.15 K, J. Chem. Eng. Data, 40, 1264 (1995).
A. Ali, K. Tiwari, A.K Nain, V. Chakravarthy, Ultrasonic study of molecular interactions in ternary mixtures of dimethylsulphoxide (1) + carbon tetrachloride (2) + aromatic hydrocarbons (3) at 308.15 K, Ind. J. Phys ., 74, 351 (2000).
P. Vasantharani, V. Pandiyan , A.N. Kannappan, Ultrasonic velocity, viscosity, density and excess properties of ternary mixture of N-methylcyclohexylamine+benz ene+1-propanol, Asian J. Appl. Sci, 2, 169 (2009).
S.T. Awad, Ultrasonic studies of the crystallization behavior of two palm fats O/W emulsions and its modification, Food Res. Int ., 37, 579 (2004).
V. Krishna, S. Sastry, Dielectric and thermodynamic studies on the hydrogen bonded binary system of isopropyl alcohol and aniline, J. Solution Chem ., 39, 1377 (2010).
K.R. Reddy, G.S Rao, P.V.S. Sairam, P. Anila, C Rambabu, Study of molecular interactions in the binary liquid mixtures of NMP with alkoxyethanols, J. Atoms Mol., 4, 734 (2014).
J.D. Pandey, V. Sanguri, D.K. Dwivedi, K.K. Tiwari, Computation of isothermal compressibility, thermal expansivity and ultrasonic velocity of binary liquid mixtures using hole theory, J. Mol. Liq ., 135, 65 (2007).
S. Rajagopalan, S.J. Sharma, Adiabatic compressibility and solvation studies of cellulose acetate in cyclohexanone and in carbon tetrachloride, J. Pure. Appl. Ultra ., 24, 87 (2002).
D. Das, B. Das, D.K. Hazra, Ultrasonic velocities and isentropic compressibilities of some symmetrical tetra alkyl ammonium salts in N, N-dimethyl acetamide at 298.15 K, J. Mol. Liq, 111, 15 (2004).
J.D. Pandey, K. Mishara, A. Shukla, R.D. Rai, Ultrasonic and thermodynamic studies of tetracyclines in solutions, Can. J. Chem ., 65, 303 (1987).
R. Hagen, R. Behrends, U. Kaatze, Acoustical properties of aqueous solutions of urea: Reference data for the ultrasonic spectrometry of liquids, J Chem. Eng. Data, 49, 988 (2004).
M. Hasan, D.F. Shirude, A.P. Hiray, U.B. Kadam, A.B. Sawant, Densities, viscosities and ultrasonic velocities of binary mixtures of methylbenzene with hexan-ol, heptanol and octanol at T = 298.15 and 308.15K, Fluid Phase Equilib ., 252, 89 (2007).
V. Singh, P.K. Chhotaray, P.K. Banipal, T.S. Banipal, R.L. Gardas, Volumetric properties of amino acids in aqueous solutions of ammonium bases protic ionic liquids, Fluid Phase Equilib ., 385, 258 (2015).
T.S. Banipal, D. Kaur, P.K. Banipal, G. Singh, Interactions of some peptides with sodium acetate and magnesium acetate in aqueous solutions at 298.15 K, J. Mol. Liq., 140, 54 (2008).
R. Kumar, R. Mahesh, B. Shanmugapriyan, V. Kannappan, Volumetric, viscometric, acoustic and refractometric studies of molecular interactions in certain binary systems of o-chlorophenol at 303.15 K, Ind. J. Pure Appl. Phys ., 50, 633 (2012).
A.U. Mandakmare, M.L. Narwade, D.T. Tayade, A.B. Naik, Intermolecular interactions in dioxane-water solutions of substituted coumarins according to ultrasonic data, Russ. J. Phys. Chem ., 88, 2334 (2014).
S.S. Aswale, S.R. Aswale, R.S. Hajare, Adiabatic compressibility, intermolecular free length and specific acoustic impedance of antibiotic ampicillin sodium, Int. J. Pharm. Pharm. Sci., 5, 76 (2013).
S. Chauhan, K. Kumar, B.S. Patal, Study of acoustical parameters of proline in lecithin-ethanol mixture at varying temperature, Ind. J. Pure Appl. Phys ., 51, 531 (2013).
S. Baluja, S. Oza, Ultrasonic studies of some derivatives of sulphonamide in dimethyl formamide, Fluid Phase Equilib ., 200, 11 (2002).
S. Baluja, R. Bhalodia, Study of molecular interactions in solutions of azomethines of sulfamethoxazole in N, N-dimethyl formamide and tetrahydrofuran, Russ. J. Phys. Chem. A, 87, 62 (2013).
S. Baluja, A. Shah, Acoustical studies of some derivatives of 4-amino antipyrine in 1,4-dioxane and dimethyl formamide at 318.15 K, Fluid Phase Equilib ., 215, 55 (2004).
S. Baluja, P. Inamdar, M. Soni, Acoustical studies of some Schiff bases in 1,4 dioxane and dimethyl formamide at 308.15 K, Acta Phys. Chim. Sinica, 20, 1104 (2004).
P. Przybylski, A. Huczynski, K. Pyta, B. Brzezinski, F. Bartl, Biological properties of Schiff bases and azo derivatives ofphenols, Curr. Org. Chem ., 13, 124 (2009).
C.M. Da Silva, D.L. Da Silva, L.V. Modolo, R.B. Alves, M.A. de Resende, C.V.B. Martins, A. de Fatima, Schiff bases: A short review of their anti-microbial activities, J. Adv. Res ., 2, 1 (2011).
T.M. Tamer, K. Valachova, S.M. Mohyeldin, L. Soltes, Free radical scavenger activity of cinnamyl chitosan Schiff base, J. Appl. Pharm. Sci, 6, 130 (2016).
D.S. Kundariya, B.M. Bheshdadia, N.K. Joshi, P.K. Patel, Synthesis, characterization and pharmacological evaluation of some novel Schiff bases containing 1H-pyrazolo [3,4-b] pyridine moiety, Int. J. ChemTech. Res. (USA), 3, 238 (2011).
E. Benoist, J.F. Gestin, P. Blanchard, M. Jubault, J.P. Quintard, Spectroscopic and electrochemical studies of new copper (II) and nickel (II) tetradentate complexes of potential interest to nuclear medicine, Trans. Metal Chem ., 24, 42 (1999).
J. Wang, Y. Song, X. Gao, Synthesis of amino antipyrine chromones Schiff base in ionic liquid, Hech. Hua ., 16, 225 (2008).
V.V. Mulwad, J.M. Shirodkar, Synthesis and biological activity of some new Schiff bases, thiazolidinones and azetidinones of 4-hydroxy coumarin, Ind. J. Heterocycl. Chem ., 11, 199 (2002).
N. Fathima, T. Mamatha, H.K. Qureshi, N. Anitha, J.V. Rao, Drug-excipient interaction and its importance in dosage form development, J. Appl. Pharm. Sci ., 1 , 66 (2011).
P. Imming, C. Sinning, A. Meyer, Drugs, their targets and the nature and number of drug targets, Nature Rev. Drug Disc ., 5, 821 (2006).
F. Poureshghi, P. Ghandforoushan, A. Safarnejad, S. Soltani, Interaction of an antiepileptic drug, lamotrigine with human serum albumin (HSA): Application of spectroscopic techniques and molecular modeling methods, J. Photochem. Photobiol. B:Biology, 166, 187 (2017).
D.E. Clark, Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 1. Prediction of intestinal absorption, J. Pharm. Sci, 88, 807 (1999).
T. Loftsson, M.E. Brewster, Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization, J. Pharm. Sci ., 85, 1017 (1996).
S. Thirumaran, D.M.C. Gardilya, Volumetric and ultrasonic studies on interionic interactions of some amino acids in aqueous magnesium acetate medium at 306.15 K, Rec. Res. Sci. Tech, 3, 56 (2011).
V. Natchimuthu, K.A. Jayalatha, S. Ravi, Characterizing the molecular interaction of perfluorocarbons with carbamazepine and benzodiazepine using photo- acoustic studies, J. Mol. Liq, 218, 120 (2016).
J.A. Riddick, W.B. Bunger, T. Sakano, "Organic solvents-physical properties and methods of purification", Fourth Edition, Techniques of Chemistry, II, A Wiley-Interscience Publication, John Wiley, New York, 1986.
K. Bhesaniya, S. Baluja, Molecular interactions in some synthesized pyrimidine derivatives in methanol and DMF solutions at 308.15 K, J. Mol. Liq ., 191, 116 (2014).
F.T. Gucker, Jr., The apparent molal heat capacity, volume and compressibility of electrolytes, Chem. Rev ., 64, 111 (1993).
C. Bachem, The compressibility of electrolytic solution, Z. Electrochem ., 41, 570 (1935).
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2017 Revista Colombiana de Ciencias Químico-Farmacéuticas

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
El Departamento de Farmacia de la Facultad de Ciencias de la Universidad Nacional de Colombia autoriza la fotocopia de artículos y textos para fines de uso académico o interno de las instituciones citando la fuente. Las ideas emitidas por los autores son responsabilidad expresa de estos y no de la revista.
Todo el contenido de esta revista, excepto dónde está identificado, está bajo una Licencia Creative Commons de Atribución 4.0 aprobada en Colombia. Consulte la normativa en: http://co.creativecommons.org/?page_id=13