The assessment of pharmacoeconomic studies quality of Levodopa’s use for the management of Parkinson’s diseases, 2010-2015
Evaluación de la calidad de estudios farmacoeconómicos del uso de Levodopa para el manejo de la enfermedad de Parkinson, 2010-2015
DOI:
https://doi.org/10.15446/rcciquifa.v46n2.67959Palabras clave:
Levodopa, Parkinson’s disease, cost-utility, methodological quality, QHES (Quality of Health Economic Studies) (en)Levodopa, enfermedad de Parkinson, costo-utilidad, calidad metodológica, QHES (Quality of Health Economic Studies) (es)
Descargas
(PD), since its introduction in the drug market; however, its prolonged use is related to
the occurrence of motor complications, affecting the functionality and quality of life.
Parkinson’s disease is one of the most frequent among the neurodegenerative diseases
in the world, and it is expected that the number of people who suffer it, will increase
due to global population aging. The PD represents, nowadays and for the future, a
high economic burden from all perspectives, including patients, payers, and society.
So it is necessary to know about the use of LD in its treatment and to realize the
quality of pharmacoeconomic studies in the past five years, to identify reliable sources
of information related to the costs and benefits of this medication to contribute in
making decisions. The aim of this paper is to assess the methodological quality of
pharmacoeconomic studies related to the use of LD in PD, specifically those that are
identified as cost-utility studies, applying the QHES instrument. A total of 19 articles
were found, of which 5 met the inclusion criteria and were subjected to examination.
The average overall score obtained after the evaluation was 77.2 out of 100, making
evident a good quality of studies according to the method used.
en el manejo de la enfermedad de Parkinson (EP); sin embargo, su uso prolongado
está relacionado con la aparición de complicaciones motoras, afectándose la funcionalidad
y calidad de vida. La EP es una de las enfermedades neurodegenerativas que
se presenta con mayor frecuencia en el mundo y se espera que el número de personas
que la padecen aumente, debido al envejecimiento poblacional a nivel mundial. La
EP representa, actualmente y a futuro, una alta carga económica desde todas las
perspectivas, incluyendo: pacientes, pagadores y la sociedad; por lo cual es necesario
conocer el uso de la LD en el tratamiento de la misma y lograr determinar la calidad
de los estudios farmacoeconómicos realizados en los últimos cinco años, para identificar
fuentes fiables de información relacionadas con los costos y los beneficios de
este medicamento que aporten en la toma de decisiones. El objetivo de este artículo
es evaluar la calidad metodológica de los estudios farmacoeconómicos relacionados
con el uso de la LD en la EP, específicamente aquellos que se cataloguen como estudios
de costo-utilidad, aplicando el instrumento QHES. Se encontraron en total 19
artículos, de los cuales cinco cumplieron los criterios de inclusión y fueron sometidos
al respectivo análisis. El puntaje global promedio obtenido posterior a la evaluación
fue de 77,2 sobre 100, haciendo evidente una buena calidad de los estudios de
acuerdo con el método utilizado.
Recibido: 5 de noviembre de 2016; Aceptado: 16 de agosto de 2017
SUMMARY
Levodopa (LD) has been the first choice in the management of Parkinson's disease (PD), since its introduction in the drug market; however, its prolonged use is related to the occurrence of motor complications, affecting the functionality and quality of life. Parkinson's disease is one of the most frequent among the neurodegenerative diseases in the world, and it is expected that the number of people who suffer it, will increase due to global population aging. The PD represents, nowadays and for the future, a high economic burden from all perspectives, including patients, payers, and society. So it is necessary to know about the use of LD in its treatment and to realize the quality of pharmacoeconomic studies in the past five years, to identify reliable sources of information related to the costs and benefits of this medication to contribute in making decisions. The aim of this paper is to assess the methodological quality of pharmacoeconomic studies related to the use of LD in PD, specifically those that are identified as cost-utility studies, applying the QHES instrument. A total of19 articles were found, of which 5 met the inclusion criteria and were subjected to examination. The average overall score obtained after the evaluation was 77.2 out of 100, making evident a good quality of studies according to the method used.
Keywords:
Levodopa, Parkinson's disease, cost-utility, methodological quality, QHES (Quality of Health Economic Studies).RESUMEN
La Levodopa (LD) ha sido desde su introducción en el mercado la primera elección en el manejo de la enfermedad de Parkinson (EP); sin embargo, su uso prolongado está relacionado con la aparición de complicaciones motoras, afectándose la funcionalidad y calidad de vida. La EP es una de las enfermedades neurodegenerativas que se presenta con mayor frecuencia en el mundo y se espera que el número de personas que la padecen aumente, debido al envejecimiento poblacional a nivel mundial. La EP representa, actualmente y a futuro, una alta carga económica desde todas las perspectivas, incluyendo: pacientes, pagadores y la sociedad; por lo cual es necesario conocer el uso de la LD en el tratamiento de la misma y lograr determinar la calidad de los estudios farmacoeconómicos realizados en los últimos cinco años, para identificar fuentes fiables de información relacionadas con los costos y los beneficios de este medicamento que aporten en la toma de decisiones. El objetivo de este artículo es evaluar la calidad metodológica de los estudios farmacoeconómicos relacionados con el uso de la LD en la EP, específicamente aquellos que se cataloguen como estudios de costo-utilidad, aplicando el instrumento QHES. Se encontraron en total 19 artículos, de los cuales cinco cumplieron los criterios de inclusión y fueron sometidos al respectivo análisis. El puntaje global promedio obtenido posterior a la evaluación fue de 77,2 sobre 100, haciendo evidente una buena calidad de los estudios de acuerdo con el método utilizado.
Palabras clave:
Levodopa, enfermedad de Parkinson, costo-utilidad, calidad metodológica, QHES (Quality of Health Economic Studies).INTRODUCTION
Since the Parkison's disease attributes were outlined in 1817 by English surgeon James Parkinson, there have been advanced to pinpoint what PD exactly is. Today, it is known that the Parkinson's disease is a neurodegenerative pathology, which attributes are tremors, hypokinesia, rigidity, and postural changes. Moreover, with the technology advancements, the PD's neurophysiological deficit is due to an affectation in the dopaminergic neurogenesis without a fully elucidated etiology 1,2.
Since 1960's, Levodopa (LD) is part of the PD's treatment, and it is still a standard drug in the therapeutic arsenal for the disease's management 3-7. Besides, it satisfies two of the most relevant aspect when assessing an anti-Parkinson's medication effectiveness, disability, and mortality. And despite LD's known side effects, its frequent use has not diminished considering the lack of achievement expected of the new medicines 8.
It is estimated that PD's prevalence will double for the year 2050; moreover, the adverse effects of anti-Parkinson's medications will increase in a similar proportion. For this reasons, strategies have been designed to enhance the availability in the cerebral level and to increase the medication's plasma half-life with the passage of the years; for instance, sustained action LD was created, and it was associated with inhibitors of catechol-O-methyltransferase (COMT), like Entacapone and Tolcapone. Another strategy is the alternative dosage forms that have been employed for PD advanced patients, for example, it is used the continuous intraduodenal infusion method to maintain drug stable concentrations, thus, reducing the fluctuations 9,10.
Typically, PD studies have been focused on motor aspects; nevertheless, as new problematics are addressed, other pathologic aspects became relevant, like depression, cognitive deterioration, and behavior disorders. Other factors stir interest, for instance, a patient can be developed dementia in the PD's natural course, and how it affects the caregivers, who developed negative emotions and significant stress as result of caring for a chronically-ill person 11,12.
In Colombia, it is not possible to make a specific estimation of the PD's future in a local level because of the lack of recent epidemiologic data. The most recent is from the midnineties, EPINEURO, a neuro-epidemiologic baseline study, reported a prevalence of 4.7 (IC95%: 2.2 to 8.9) 13.
Accordingly, it is necessary to identify the use of LP in the PD's treatment and to determine the pharmacoeconomic studies' quality that has been done in the last five years. In the literature exists multiple systematic reviews of Parkinson's disease and others associated with the disease treatment 14-21. But, there are not quality evaluations of the publication's methodology focused on cost-utility studies of the LD used in the EP treatment.
The importance of conducting this kind of assessments is based on the fact that health care decisions and their subsequent resource distribution are taken, in many cases, based on the available literature as reference. The objective of this paper is to assess the methodological quality of the pharmacoeconomic studies related to the Levodopa use in Parkinson's disease treatment. Particularly, those studies catalog as a cost-utility study. Being the last one, the author's preference for that kind of study relates the natural course of the disease with its effects on the life quality of both the patients and caregivers.
METHODOLOGY
Identification and selection
A bibliographic search was done in the following Databases: EMBASE, LILACS, and MEDLINE. The same MeSH terms were used in them: Parkinson Disease (Mesh) AND Cost-Benefit Analysis (Mesh) AND Levodopa (Mesh). Four reviewers identified independently the eligible articles, which were selected by a consensus.
Inclusion and exclusion criteria
Internationally indexed articles were selected that comply with the following parameters: Adult population over eighteen years old, main diagnosis of Parkinson's disease, cost-utility analysis (CUA), Levodopa is included among one of the comparators, it must be in English or Spanish, and it was published in the period from January 1st, 2010 to December 1st, 2015.
Quality assessment
The QHES (Quality of Health Economic Studies) instrument was implemented to evaluate the selected articles 22. 16 criteria were established in the instrument, which determined the three types of health care economic analysis: minimizing costs, cost-effectiveness, cost-utility; as shown in Table 1.
Table 1: QHES (Quality of Health Economic Studies) Instrument 22.

The four researchers read independently and then categorize each article according to the 16 items of the instrument. Subsequently, criteria were unified in the points where a disagreement in the rating was evidenced.
Data mining
In the review of each one of the articles, the extracted data was relevant for the analysis of the evidence as comparators, perspective, measuring instrument of life quality, QALY, time horizon, cost per QALY gained, country, among others.
RESULTS AND DISCUSSION
Due to the implications in the life quality of those with Parkinson's disease (PD), only the cost-utility study was selected, which was subjected to the inclusion and exclusion criteria. In total 19 articles were found 23-41, of which 5 met the inclusion criteria and were submitted to the respective analysis (Figure 1). The list of excluded studies is provided in Annex (1).
Figure 1: Flowchart for the evidence selection.
In all the cases, Levodopa was found as a comparator, regardless of whether it was concomitantly administered with another therapy. Two of the studies have a social perspective; three studies include a third-party payer perspective, and only one has a health professionals perspective; clarifying that one of the studies was carried out from two perspectives. It should be noted that four of the five studies included a Markov model, thus simulating in a more 'realistic' way what occurs in the disease process. The articles belonged to the following countries: two from the United Kingdom, one from the United States, one from Netherlands, and one from Norway (Table 2).
*RCT: Randomized clinical trial. **PDQ (Parkinson’s Disease Quality of Life Questionnaire). ***PD: Parkinson’s disease.Table 2: Characteristics of the cost-utility studies.
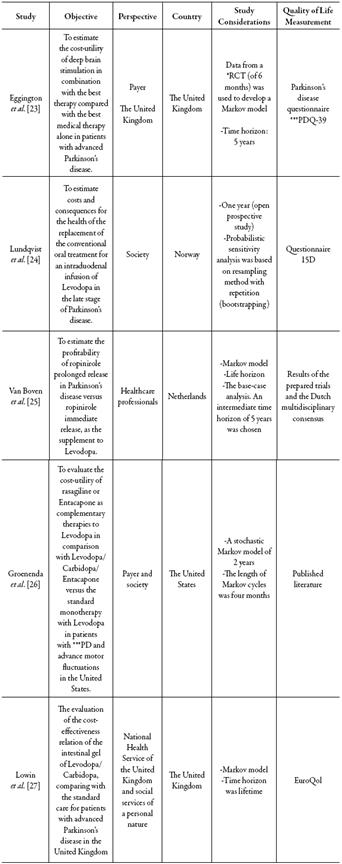
Only two articles have a time horizon of fewer than two years; in both of them, the discount rate was not taken into account, in one of them because the time horizon was less than one year. As a relevant finding, all the studies stated the resources origins that financed the investigation, the great majority done by the pharmaceutical industry.
When evaluating the quality of pharmacoeconomic studies with the QHES (Quality of Health Economic Studies) instrument, it was found that all the studies reached an average score higher than 60, which reveals a good quality in the studies submitted to the qualification (Table 3). The overall detailed analysis of Table 3 allowed to make the following observations:
+ Criteria found in the study. - Article did not fulfill or partially fulfilled the criteria.Table 3: Quality evaluation of studies.

-
The criteria 1, 2, 5, 6, 7, 13, and 16 were fully satisfied by all the articles. Tose referred to the following considerations: the goal and methodology of the study's data mining were explicit, the analysis perspectives were always mentioned, an incremental analysis and the study model, as well as, the source of funding was always declared; moreover, a sensibility analysis was conducted in all the cases. For these results, a good quality can be partially attributed to the evaluated studies.
-
The estimation of used variables was described except in one article. In two articles, the discount rate was not explicitly given, and in another, the cost measurements were not fully described, the primary outcome measures were only specified in one article, and in only two cases, the measures of outcome were valid and reliable. In two articles, it did not discuss the magnitude of the potential biases and the conclusions were not clear concerning the model results.
Cost-utility study
Eggington et al. 23, compared the deep cerebral stimulation combine with the best medical treatment as a management option for Parkinson's disease versus the best medical treatment independently (Levodopa and other antiparkinsonians) from a third -party perspective. Data of deep cerebral stimulation effects in the progressing PD symptoms from 6-months randomized clinical trial (RCD) were used to develop a Markov model, using a time horizon of 5 years. The total costs were: € 51,499 for deep brain stimulation combined with the best medical treatment, and € 36,023 for the best medical treatment independently (Levodopa and other antiparkinsonians), with QALYs of 2.21 and 1.21 respectively giving an incremental cost-effectiveness relation of € 15,440 per QALY gained.
In the United Kingdom under the perspective used, the results suggest that deep brain stimulation is a cost-effective intervention in patients with advanced Parkinson's disease who are eligible for surgery. However, this information should be taken with caution in the Colombian context, due to the marked differences between the population and the health system of both countries. It is clarified that the costs reported and extracted from the articles were adjusted to the constant prices of October 2015 and were homogenized to a single currency (Euros), considering each country's consumer price index (CPI) and the publication date of each article.
Lowin et al. 27 is another study conducted in the United Kingdom, in which the intestinal gel of Levodopa/Carbidopa was evaluated, compared with the defined standard care as the best available oral medicine as medically determined in patients with advanced Parkinson's disease. A Markov model was used to quantify costs and results, with a lifetime horizon. An incremental cost per AVAC of € 29,048 was obtained for the intestinal gel of Levodopa/Carbidopa compared to standard care. The results were sensitive at the time of the treatment, health status at the start of treatment, and longterm benefit estimates. In this context, the intestinal Levodopa/Carbidopa gel is considered an effective treatment that improves the quality of life in advanced PD and should not be ignored despite the high cost involved, suggesting that this alternative could be considered cost-effective compared to standard care when other options of treatment are ineffective or inadequate. However, it is emphasized that more research is needed to fill gaps in information regarding the current data and to increase the robustness of the model.
In Norway, Lundqvist et al. 24 performed the study of a cohort of ten patients with advanced PD, who from an ineffective conventional (oral) treatment with LD switched to a new form of continuous intraduodenal administration of Levodopa (IDL). They had a follow-up of 12 months, used the unified scale of the PD to measure function and the questionnaire 15D for the quality of life. The objective of the study was to determine the costs and health consequences of the change from a conventional treatment to IDL; all from a social perspective. Although the data on effectiveness, safety, and quality of life results were proprietary, they mention the various limitations from a methodological point of view and conclude that the change in this type of therapy in patients with advanced disease is not cost-effective in their country due to an additional cost of € 88,166,638 per QALY gained.
In the study by Van Boven et al. 25, the profitability of ropinirole prolonged release in Parkinson's disease versus ropinirole immediate release, when used as a complement to Levodopa, was estimated. A Markov model was developed that included the following aspects related to the treatment: (I) the rate of progression of the disease, (II) rates of dyskinesia, and (III) adherence to medication. The base case analysis showed a favorable pharmacoeconomic profile for ropinirole prolonged release versus ropinirole immediate release. Overall, cost savings combined with moderate gains in life quality are considered and profitability remained acceptable within the investigated limits. In this study, it is concluded that ropinirole prolonged release presents a high probability of cost savings or at least being considered cost-effective in the Netherlands when compared to ropinirole immediate release when used as a complement to Levodopa. It should be clarified that the article mentions how the cost per QALY can be calculated and the necessary data to do it; however, it does not present the punctual value, which was calculated by the researchers of this evaluation, obtaining a value of € 113600.
A study conducted in the United States by Groenendaal et al. 26 aimed to evaluate the cost-utility of rasagiline or Entacapone as an adjuvant to Levodopa versus Levodopa/ Carbidopa/Entacapone (LCE) and also in comparison to standard monotherapy with Levodopa in patients with advanced PE and motor fluctuations in that country. The study was carried out under two perspectives, from the perspective of society and from the perspective of the third payer, using a Markov model in which they performed six cycles of four months for a two-year time horizon with three health states and using the odds of varying from one health condition to another as reported in controlled clinical trials. The results show a higher cost-effectiveness in the use of rasagiline plus Levodopa, with cost-per-QALY data earned € -15,905 and Levodopa-Carbidopa-Entacapone-€ 17,051, when assessed from the perspective of the third-party payer. Moreover, better results are gained with the alternative of Entacapone plus Levodopa (cost per QALY gained € -14,560), assessed from the social perspective.
In all of the articles evaluated, the cost of QALYs gained was recalculated, which is presented underlined in Table 4, to compare it with the value reported by the authors. In all cases, there were minor differences; nonetheless, in the analysis performed by Van Boven et al. 25 as mentioned above, a punctual value was not presented.
* Costs reported and extracted from the articles were adjusted to constant prices of October 2015 and were homogenized to a single currency (Euros); considering each country's consumer price index (CPI) and the publication date of each article.Table 4: Results of the Cost-Utility Studies.

Strength and limitations
The assignment of resources for health is limited in the case of the Colombian system. It is not based solely on economic considerations; it requires the opinion of experts and the review of economic analyses. Therefore, a quality analysis of information such as the one carried out in this research is relevant, especially for a disease, such as, Parkinson's disease whose prevalence is expected to increase over the years, and the decisions taken should take into account the improvement in the life quality of the patient along with the reduction of social burden.
Regarding the evaluation of the studies with the QHES (Quality of Health Economic Studies) instrument, a good quality was generally found because an average score of more than 60 was always obtained. This can be correlated with the detailed description in each article of aspects such as the objective, data mining methodology, analysis perspective, study model, the source of funding, and sensitivity analysis. Regarding applicability, it is important to note that the interventions, the comparator, and the country in which each study was performed, since, the costs reported were clearly described, and in all cases, the study population was appropriate for the subject evaluated 42,43.
It is appropriate to mention the multiple methodological limitations of pharmacoeconomic assessments: results are obtained from assumptions that are supported by probabilities, sensitivity analyses do not necessarily have the capacity to reflect the reality of population behavior nor the different scenarios that arise in the course of the diseases, particularly those of neurological origin. Likewise, the external validity of these articles is low due to the reduced possibility that the results can be extrapolated to different regions from a social, cultural, and economic point of view. Authors such as Welte et al. 44 propose transfer criteria to evaluate, such as perspective, discount rate, cost approach, health system characteristic, variability in clinical practice, availability of technology, epidemiological variables of Illness, life expectancy, preferences for socioeconomic status, productivity, and lost work time, among others.
It is necessary to determine in each case: the correspondence between the country of study and the country of decision, the degree to which the criterion is relevant to the technology investigated and the likely effect of the transfer criterion on the results 43, evidencing this is the need to carry out studies that evaluate the life quality in PE and the costs from different perspectives in Latin America, so as to allow adequate decision making based on methodologically structured studies.
CONCLUSIONS
In this study, a review of the pharmacoeconomic evaluations for the use of Levodopa in Parkinson's disease was carried out, based on the QHES instrument 22, which qualifies methodological quality and has been used by other authors previously 45-49.Only the articles that met inclusion criteria and were considered of cost-utility were taken, finding that the five articles analyzed obtained an overall average score of 77.2 out of 100. This reflects that these studies are of good quality, and this is probably attributable to the fact that they were recently carried out following the current guidelines.
Latin American articles were not found in the systematic search carried out. Terefore, it is necessary to conduct own studies that may be closer to our reality, taking into account the differences in the population, the resources destined for health, and the therapeutic alternatives employed.
ACKNOWLEDGMENTS
Te authors are grateful to Ms. María Jakelin Galvis and Mrs. Judith Otálora, students of the Master of Science in Pharmacology program of the Universidad Nacional de Colombia, for participating in the qualification of the quality of the articles, and also to Ms. Ingrid Carolina Diaz-Becerra for her cooperation in the Spanish-English-languages translation.
REFERENCES
ANNEX 1. LIST OF STUDIES EXCLUDED FROM THE EVALUATION AND REASONS FOR EXCLUSION.
S. Eggington, A. Brandt, E. Reimer, M. Grifi, J. Nyberg, PMD46 - Cost-effectiveness of deep brain stimulation (Dbs) in the management of advanced Parkinson's disease: A Swedish payer perspective, Value in Health, 18 (7), A352 (2015). Tis study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 28.
J. Lökk, U. Persson, Willingness to pay for a new drug delivery in Parkinson patients, J. Multidiscip. Health, 7, 431 (2014). Tis study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 29.
V. Ryazhenov, Economic evaluation of the treatment compliance in patients with parkinson's disease received different preparations of levodopa, Value in Health, 16, A623 (2013). Tis study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 30.
M. Kamusheva, G. Petrova, Intestinal gel Levodopa + Carbidopa in Parkinson's patients with frequent and prolonged akinesia - an economic evaluation, Int. J. Pharm. Sci. Rev. Res., 22 (1), 244 (2013). This study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 31.
U.J. Kang, Diagnostic biomarkers of Parkinson's disease: What gain at what cost?, J. Neurol. Neurosurg. Psychiatry, 83, 769 (2012). Tis study was excluded in the review, because it didn't include the outcomes considered in the review 32.
F. Valldeoriola, R. Puig, P. González, Cost analysis of the treatments for patients with complicated Parkinson's disease: Scope study, Parkinsonism and Related Disorders, 18, 2 (S46) (2012). This study was excluded in the review, because it didn't include the outcomes considered in the review 33.
M. Jann, Advanced strategies for treatment of Parkinson's disease: The role of early treatment, Am. J. Manag. Care, 17 (Suppl. 12), S315 (2011). Tis study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 34.
F. Valldeoriola, R. Puig, P. González, Cost analysis of deep brain stimulation, apomorphine infusion pumps and continuous duodenal levodopa-carbidopa infusion in patients with advanced Parkinson's disease in Spain: Scope study, Eur. J. Neurol., 18 (Suppl. 2 ), 238 (2011). Tis study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 35.
A. L. Kurtz, D.I. Kaufer, Dementia in Parkinson's disease, Current Treatment Options in Neurology, 13 (3), 242 (2011). This study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 36.
J.J. Adam, H. van Houdt, B. Scholtissen, V. Visser-Vandewalle, A. Winogrodzka, A. Duits, Executive control in Parkinson's disease: Effects of dopaminergic medication and deep brain stimulation on anti-cue keypress performance, Neuroscience Letters, 500 (2), 113 (2011). This study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 37.
M. Willis, U. Persson, Y. Zoellner, B. Gradl, Reducing uncertainty in value-based pricing using evidence development agreements, Appl. Health Econom. Health Policy, 8 (6), 377 (2010). This study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 38.
B. A. Racette, A.W. Willis, Time to change the blind men and the elephant approach to Parkinson disease?, Neurology, 85 (2), 190 (2015). Tis study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 39.
E. Walter, P. Odin, Cost-effectiveness of continuous subcutaneous apomorphine in the treatment of Parkinson's disease in the UK and Germany, J. Med. Economics, 18 (2), 155 (2015). This study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 40.
K.L. Davis, H.M. Edin, J.K. Allen, Prevalence and cost of medication nonadherence in Parkinson's disease: Evidence from administrative claims data, Mov. Disord., 25 (4), 474 (2010). This study was excluded in the review, because it didn't include all the comparisons accounted in the inclusion criteria 41.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2017 Revista Colombiana de Ciencias Químico-Farmacéuticas

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
El Departamento de Farmacia de la Facultad de Ciencias de la Universidad Nacional de Colombia autoriza la fotocopia de artículos y textos para fines de uso académico o interno de las instituciones citando la fuente. Las ideas emitidas por los autores son responsabilidad expresa de estos y no de la revista.
Todo el contenido de esta revista, excepto dónde está identificado, está bajo una Licencia Creative Commons de Atribución 4.0 aprobada en Colombia. Consulte la normativa en: http://co.creativecommons.org/?page_id=13



















