1-Octanol-water partition coefficient of some cyanopyridine and chalcone compounds
Coeficientes de reparto 1-octanol-agua de algunos compuestos derivados de cianopiridina y chalcona
DOI:
https://doi.org/10.15446/rcciquifa.v46n3.69466Palabras clave:
partition coefficient, cyanopyridines, chalcones, 1-octanol-water system (en)coeficiente de reparto, cianopiridinas, chalconas, sistema 1-octanolagua (es)
Descargas
hydrophobic nature of compounds. There is no regular trend for the variation of log P with different pH.
sustituyente presente en los compuestos. El grupo central también desempeña un papel importante en la naturaleza hidrofílica/hidrofóbica de los compuestos. No hay
una tendencia regular para la variación de log P con el pH.
Recibido: 4 de mayo de 2017; Aceptado: 27 de octubre de 2017
SUMMARY
The partition coefficients (P) of some synthesized cyanopyridine and chalcone compounds have been studied in 1-octanol-water system at different pH. It is observed that log P depends on pH and nature of substitution group present in the compounds. The central moiety also play important role to affect hydrophilic/ hydrophobic nature of compounds. There is no regular trend for the variation of log P with different pH.
Key words:
partition coefficient, cyanopyridines, chalcones, 1-octanol-water system.RESUMEN
Los coeficientes de reparto (P) de algunos compuestos sintetizados derivados de cianopiridina y chalcona se estudiaron en el sistema 1-octanol-agua a diferentes valores de pH. Se observa que log P depende del pH y de la naturaleza del grupo sustituyente presente en los compuestos. El grupo central también desempeña un papel importante en la naturaleza hidrofílica/hidrofóbica de los compuestos. No hay una tendencia regular para la variación de log P con el pH.
Palabras clave:
coeficiente de reparto, cianopiridinas, chalconas, sistema 1-octanol-agua.INTRODUCTION
The knowledge of partition coefficient is highly useful in various branches of chemistry, biology and their associated technologies [1]. In medicinal chemistry and pharmacy, it is of great importance as it is used in QSAR.
The aim of Quantitative Structure-Activity Relationship (QSAR) techniques is to develop correlations between any property or form of activity normally biological activity and the properties, usually physicochemical properties of a set of molecules. There are many physicochemical parameters in QSAR like hydrophobic, electronic, theoretical and steric parameters. Out of these parameters hydrophobic parameter is one of the most important parameter in QSAR study. The ability of drug to permeate across biological membranes has traditionally been evaluated using its distribution in 1-octanol (representing lipid membrane) and water systems. Hydrophobicity governs the partition behavior between aqueous and non aqueous phases in natural, technical, and pharmacological processes.
In Pharmacokinetics, the distribution coefficient has a strong influence on ADME properties (Absorption, Distribution, Metabolism, and Excretion) of the drug [2] and in Pharmacodynamics, the hydrophobic effect is the major driving force for the binding of drugs to their receptor targets [3-7]. Partition coefficients are useful for in estimating distribution of drugs within the body because hydrophobic drugs with high partition coefficients are distributed to hydrophobic compartments of cells such as lipid bilayers while hydrophilic drugs (low partition coefficients) are found in blood serum like hydrophilic compartments.
Many industries which are preparing various consumer products also take into account of distribution coefficient such as in the formulation of cosmetics [7-9], topical ointments [10], dyes [11,12], toxicology of hair colors [13] and many other consumer products. For agro chemicals [14-16], partition coefficient is necessary to measure hydrophobicity because hydrophobic insecticides and herbicides tend to be more active but in general hydrophobic agrochemicals have longer half lives and therefore display increased risk of adverse environmental impact. In metallurgy [17,18], it is an important factor in determining how different impurities are distributed between molten and solidified metal so it is a critical parameter for purification of metals and other study of metals. For environmental study the hydrophobicity of a compound can give the information of how easily a compound might be taken up in groundwater from pollute waterways, and its toxicity to animals and aquatic life [19]. In the field of hydrogeology the 1-octanol water partition coefficient, is used to predict and model the migration of dissolved hydrophobic organic compounds in soil and groundwater [20,21]. Literature survey shows that partition coefficient of various organic compounds have been studied in 1-octanol-water system [22-26]. 1-octanol is an important molecule both for biological and environmental reasons [27].
In the present study, partition coefficient of some newly synthesized cyanopyridines and chalcones has been studied in 1-octanol-water system by UV spectroscopy at different pH. The partition coefficient is highly affected by pH. So, in the present study, a wide range of pH (0.84 to 8.0) is selected. For 0.84 pH, 0.1 N hydrochloric acid was taken whereas for pH 6.0, 7.4 and 8.0, phosphate buffer was used. These values of pH are selected due to their existence in human body. As hydrochloric acid exists in gastric juice in stomach, 0.1 N HCl is taken. Blood has 7.4 pH, so the study is done at pH 7.4. Further, the middle and upper range of body pH is 6.0 and 8.0 respectively, so study was done at this pH also.
THEORY
Partition coefficient (P) is defined as the ratio of the compounds in organic phase to that present in the aqueous phase 28, i.e.

where Corg and Caq are concentration of solute in organic and aqueous phases respectively.
In the present case, concentrations were determined by UV measurement so, equation (1) written as 29:

where A 0 and Ax are the initial and final absorbance values of organic layer. From equation (2), log P were calculated for each set of experiment.
EXPERIMENTAL
The different chemicals used in the synthesis are purchased from Spectrochem Ltd., Mumbai and are of AR grade. The solvent, 1-octanol was purchased from Spectrochem Ltd., Mumbai and of HPLC grade. It was distilled by usual procedure [30].The purity of solvent was checked by GC (SHIMADZU- GC 14 B) and found to be 99.8%. Distilled water was used throughout for all experiments.
Synthesis of Cyanopyridines
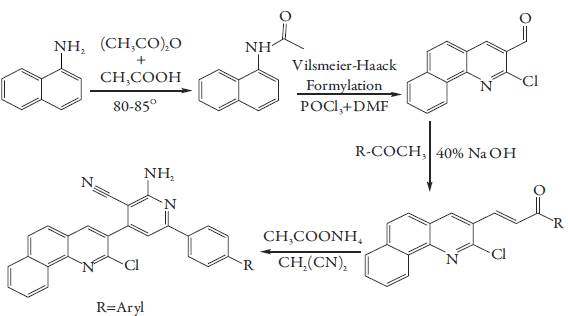
An equimolar mixture of 1-naphthyl amine and acetic anhydride in methanol was refluxed in water bath for 2-3 hrs using CH3COOH as catalyst. The crude product formed was N-(naphthalen-1-yl) acetamide which was isolated and crystallized from absolute ethanol. This synthesized product was added in a mixture of Vilsmeier-Haack reagent (prepared by drop wise addition of 6.5 ml POCl3 in ice cooled 2 ml DMF) and was refluxed for 27 hrs. The reaction mixture was poured into ice and kept for overnight followed by neutralization using sodium bicarbonate. The crude product was isolated and crystallized from ethanol. The product was 2-chloro benzo[h]quinoline-3-carbaldehyde.
To a well stirred equimolar solution of 2-chloro benzo[h]quinoline-3-carbaldehyde and p-methoxy-acetophenone in the binary mixture of ethanol- DMF (75:25), 40% NaOH was added till the solution became basic. The reaction mixture was stirred for 48 hrs. The contents were poured into ice and acidified. The product formed was 3-(2-chlorobenzo[h]quinolin-3-yl)-1-(4-methoxy-phenyl) prop-2-en-1-one, which was filtered and crystallized from ethanol. This is dissolved in ethanol and malononitrile and ammonium acetate was added. The resulting solution was refluxed for 10-12 hrs. The content was poured on crushed ice. The product obtained was filtered, washed with water and crystallized from DMF.
Similarly, other substituted cyanopyridines have been prepared. The reaction scheme for the synthesis is given in figure 1.
Figure 1: Reaction scheme of cyanopyridine (SP) compounds.
Synthesis of Chalcones
An equimolar mixture of 2-Furaldehyde derivative and substituted acetophenone is stirred for 24 h using NaOH as catalyst. The product was isolated and crystallized from ethanol. Similarly other compounds have also been prepared and crystallized. The purity of compounds was checked by TLC and their structures were confirmed by IR, NMR and mass spectroscopic techniques.
The reaction scheme for the synthesis is given in figure 2.
Figure 2: Reaction scheme of chalcone (SC) compounds.
The physical parameters along with different substitutions of all the synthesized compounds are listed in tables 1 and 2.
Table 1: Physical constants, substitutions groups and pKa values of synthesized Cyano pyridine compounds.
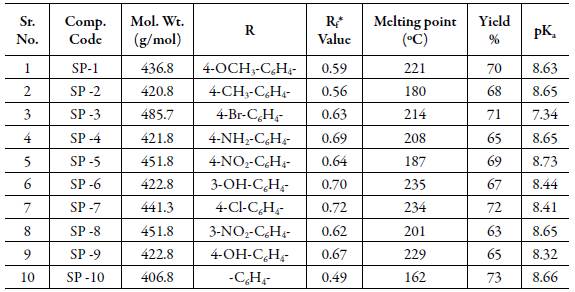
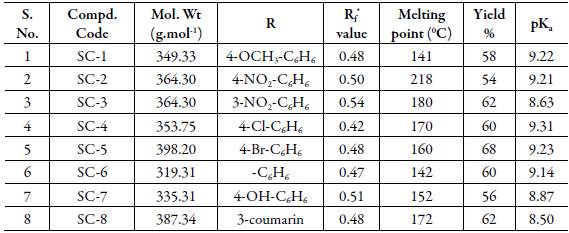
Table 2: Physical constants, substitutions groups and pKa values of synthesized Chalcone compounds.
Preparation of standard solution
10 mg sample was dissolved in 1-octanol to give 100 ml solution of 100 ppm. This solution was known as standard solution. λmax values were measured by using UV spectrophotometer (Shimadzu, UV-1700, Pharmaspec) from this solution at 298.15 K. Suitable dilutions (2 µg to 20 µg) were made from this standard solution. The absorbance of all the solutions was measured. The calibration curve of absorbance versus concentration of compounds was drawn.
Determination of Partition coefficient
A known amount of the compound under investigation was dissolved in 1-octanol at a concentration not higher than 20 µg. Equal volumes of this solution and water is mixed in oven dried stoppered flask and the mixture was stirred for 24 h at room temperature.
After 24 hours, the solution was transferred into 60 ml of separating funnel and allowed to stand in order to separate the aqueous and organic layers. The organic layer will be upper one while lower will be aqueous. The organic layer was then analyzed by UV spectrophotometer. Using calibration curve, the concentration of compounds in organic layer was also then evaluated.
RESULTS AND DISCUSSION
Cyanopyridine derivatives
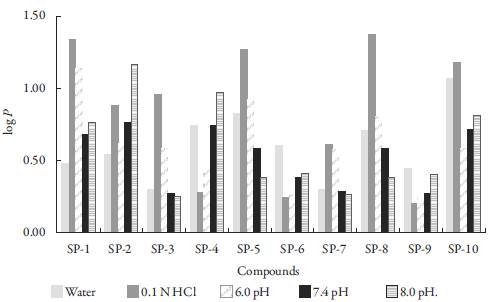
The values of log P for the studied compounds at different pH are given in table 3 and Fig. 3. It is evident that the value of log P varies with pH for each compound. Log P value depends upon the hydrophilic and hydrophobic character of compounds. Log P values have inverse relation with hydrophilic nature of compounds. All the studied compounds have the same moiety but different side chain. Thus, log P depends upon the side chain. Further, the position of substitution affects log P. Thus, different side chains have different hydrophilic or hydrophobic character which affects log P. The compounds with higher log P value are hydrophobic in nature whereas those with lower log P value are hydrophilic.
Table 3: Apparent log p values for compounds of cyanopyridine compounds.
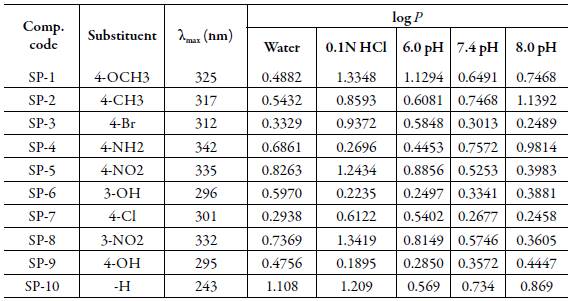
Figure 3: Apparent log P values of compounds of cyanopyridine series.
In water, SP-10 is highly hydrophobic in nature among all the compounds whereas SP-7 is highly hydrophilic. Thus, when there is no substitution, hydrophobicity increases whereas presence of chloro-group at para position increases the hydrophilic character. So, SP-7 will easily be absorbed in blood than SP-10. Due to this reason SP-10 easily spread in body than any other studied compounds. However, it is more likely to absorb in fatty tissues [31,32]. Overall, the decreasing order of hydrophobicity of compounds in water is: SP-10 > SP-5 > SP-8 > SP-4 > SP- 6 > SP-2 > SP-1 > SP-9 > SP-3 > SP-7.
In 0.1N HCl-1-octanol system, SP-8 (containing nitro group at meta-position) is highly hydrophobic whereas SP-9 (containing hydroxyl group at para position) is highly hydrophilic in nature. Thus, in gastric juice also, SP-8 will not be absorbed whereas SP-9 can be easily absorbed. In this case the decreasing order of hydrophobicity of compounds is: SP-8 > SP-1 > SP-5 > SP-10 > SP-3 > SP-2 > SP-7 > SP-4 > SP-6.
At 6.0 pH, SP-1 with methoxyl group at para-position to aromatic ring is highly hydrophobic in nature. While SP-6 is more hydrophilic which contains hydroxyl group at meta-position. Overall the decreasing order of hydrophobicity of compounds is: SP-1 > SP-5 > SP-8 > SP-2 > SP-3 > SP-10 > SP-7 > SP-4 > SP-9 > SP-6.
In 7.4 pH range, among all these compounds SP-7 has minimum log P values máximum is observed for SP-4 which can be considered more hydrophobic in nature. The decreasing order of hydrophobicity of compounds is:
SP-4 > SP-2 > SP-10 > SP-1 > SP-8 > SP-5 > SP-9 > SP-6 > SP-3 > SP-7.
For 8 pH, SP-2 is most hydrophobic whereas SP-7 is most hydrophilic among all the studied compounds. So, due to the hydrophobic nature of SP-2 can be accumulated in lipid material [33,34]. The decreasing order of hydrophobicity of compounds at 8 pH is: SP-2 > SP-4 > SP-10> SP-1 > SP-9 > SP-5 > SP-6 > SP-8> SP-3 > SP-7.
Over all, it is concluded that type of substitution, position of substitution and pH of solution play important role in partition coefficient.
Chalcones
The values of log P for the studied compounds at different pH are given in table 4 and fig. 4. It is observed that SC-7 is highly hydrophobic in nature among all the compounds whereas SC-1 is highly hydrophilic. It is well known fact that plasma of blood constitutes 55% of blood fluid and is mostly water (about 92% by volume). Thus, due to hydrophobic character SC-7 will not be absorbed in blood, and is less likely to spread in the body. However, it is more likely to accumulate in fatty tissues [33,34]. Overall the decreasing order of hydrophobicity of compounds is: SC-7 > SC-6 > SC-8 > SC-5 > SC-3 > SC-4 > SC-2 > SC-1.
Table 4: Apparent log P values for compounds of Chalcone compounds.

Figure 4: Apparent log P values of compounds of Chalcone series.
In 0.1N HCl-1-octanol system also, SC-7 is again highly hydrophobic whereas SC-5 is highly hydrophilic in nature. Thus, in gastric juice also, SC-7 will not be absorbed whereas SC-5 can be easily absorbed. In this case the decreasing order of hydrophobicity of compounds is:
SC-7 > SC-4 > SC-6 > SC-8 > SC-2 > SC-3 > SC-1 > SC-5.
At 6.0 pH, SC-6 is highly hydrophobic. However SC-7 showed maximum hydrophilicity. Overall the decreasing order of hydrophobicity of compounds is: SC-6 > SC-8 > SC-4 > SC-3 > SC-5 > SC-1 > SC-2 > SC-7.
In 7.4 pH range, among all these compounds SC-2 has minimum log P values whereas maximum is observed for SC-5 which can be considered more hydrophobic in nature. The decreasing order of hydrophobicity of compounds is: SC-5 > SC-4 > SC-7 > SC-3 > SC-1 > SC-6 > SC-8 > SC-2.
Again at 8.0 pH, log P is highest for SC-7 and minimum for SC-2. Further, it is observed from table 4 that at alkaline pH 8.0, most of the compounds have higher log P which suggests that most of these compounds will not be absorbed by the blood but can be accumulated in fatty tissues as observed by Rowe et al. [33] and Fresta et al. [34]. The decreasing order of hydrophobicity of compounds is: SC-7 > SC-6 > SC-5 > SC-8 > SC-1 >SC-4 > SC-3 > SC-2.
Thus, among 8 studied chalcones, SC-7 exhibits maximum hydrophobic nature.
In most of the synthesized cyanopyridine and chalcone compounds, substitution groups are same. So, comparison of log P values of these cyanopyridine and chalcone compounds at different pH shows that log P also depends on the central moiety.
The log P values are also compared with pKa values of compounds reported in Tables 1 and 2. However, there is no regular trend is observed between pKa and log P values at different pH.
Thus, it concluded that in the studied compounds, log P values depend on pH, nature and position of substitution groups as well as on central moiety. No direct relation between pKa and log P values is observed in the studied compounds.
REFERENCES
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Andrea Pinto, Catarina Roma-Rodrigues, Jas S. Ward, Rakesh Puttreddy, Kari Rissanen, Pedro V. Baptista, Alexandra R. Fernandes, João Carlos Lima, Laura Rodríguez. (2021). Aggregation versus Biological Activity in Gold(I) Complexes. An Unexplored Concept. Inorganic Chemistry, 60(24), p.18753. https://doi.org/10.1021/acs.inorgchem.1c02359.
2. Teresa Kaźmierczak, Sylwia Cyboran-Mikołajczyk, Natalia Trochanowska-Pauk, Tomasz Walski, Paulina Nowicka, Dorota Bonarska-Kujawa. (2025). Insights on the Mechanisms of the Protective Action of Naringenin, Naringin and Naringin Dihydrochalcone on Blood Cells in Terms of Their Potential Anti-Atherosclerotic Activity. Molecules, 30(3), p.547. https://doi.org/10.3390/molecules30030547.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Revista Colombiana de Ciencias Químico-Farmacéuticas

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
El Departamento de Farmacia de la Facultad de Ciencias de la Universidad Nacional de Colombia autoriza la fotocopia de artículos y textos para fines de uso académico o interno de las instituciones citando la fuente. Las ideas emitidas por los autores son responsabilidad expresa de estos y no de la revista.
Todo el contenido de esta revista, excepto dónde está identificado, está bajo una Licencia Creative Commons de Atribución 4.0 aprobada en Colombia. Consulte la normativa en: http://co.creativecommons.org/?page_id=13