Effect of a low rank coal inoculated with coal solubilizing bacteria for the rehabilitation of a saline-sodic soil in field conditions
Efecto de un carbón de bajo rango inoculado con bacterias solubilizadoras de carbón para la rehabilitación de un suelo salino-sódico en condiciones de campo
DOI:
https://doi.org/10.15446/rfna.v70n3.62478Keywords:
Lignite, humic substances, biotransformation coal, soil salinity (en)Lignito, Sustancias húmicas, Biotransformación del carbón, Salinidad de suelos (es)
Downloads
El objetivo de esta investigación fue evaluar cambios en algunas propiedades químicas, biológicas y físicas, en respuesta mejorada por el tratamiento con carbón de bajo rango (CBR) tipo lignito y bacterias solubilizadoras de carbón (BSC) -Bacillus mycoides, Microbacterium sp y Acinetobacter baumanniique liberan materia orgánica humificada (MOH) mediante la biotransformación de este carbón. En condiciones de campo, se trataron parcelas de 5 m2 con la adición de CBR a una dosis de 5 kg de CBR m-2 y un inóculo de las BSC en una suspensión de 1x108 bacterias mL-1 en una dosis de 100 mL m-2. Se determinaron la respiración del suelo, la actividad microbiológica, la actividad de las enzimas lignino peroxidasa (LiP), manganeso peroxidasa (MnP) y lacasas (Lac). Las variables asociadas a la salinidad sódica del suelo: pH, la conductividad eléctrica (CE), la razón de absorción de sodio (RAS), el porcentaje de sodio intercambiables (PSI), la capacidad de intercambio catiónico (CIC) se midieron cada dos meses, mientras que la densidad aparente (Da) se determinó seis meses después de haber iniciado el experimento. La aplicación de CBR contribuyó a la disminución de la CE, RAS y PSI, pero los niveles de pH no presentaron cambios significativos. Adicionalmente, no se evidenciaron cambios significativos en la Da, sin embargo, el tratamiento logró incrementar la respiración y la actividad microbiológica del suelo, estimuló la actividad de las enzimas LiP, MnP y Lac, y aumento la CIC del suelo. Estos resultados sugieren la posibilidad de utilizar el CBR como fuente de MOH para la rehabilitación de suelos salinos degradados, un problema común en los suelos del Valle del Río Cesar (Colombia) y en las tierras secas del caribe colombiano influenciadas por la minería del carbón a cielo abierto.
Recibido: 6 de febrero de 2017; Aceptado: 19 de abril de 2017
ABSTRACT
The aim of this research was to evaluate changes to several chemical, biological and physical properties of a Salidic Calciustolls, in response to enhancement by treatment with low rank coal (LRC) and coal solubilizing bacteria (CSB) -Bacillus mycoides, Microbacterium sp and Acinetobacter baumannii- that release humified organic matter (HOM) through biotransformation of the coal. Under field conditions, 5 m2 plots were treated with the addition of LRC at a dose of 5 kg m2 and an inoculum of CSB in a suspension of 1x108 bacteria mL-1 at a dose of 100 mL m-2. Soil respiration, microbiological activity, lignin peroxidase (LiP), manganese peroxidase (MnP) and laccase (Lac) enzyme activities were quantified. The variables associated with saline-sodic soils - pH, electrical conductivity (EC), sodium adsorption ratio (SAR), exchangeable sodium percentage (ESP), cation exchange capacity (CEC) were measured every two months bulk density (BD) was determined sixth months after the start of the experiment. The LRC application contributed to the decrease of EC, SAR and ESP, but pH levels did not change significantly. Additionally, no significant changes were found in the BD, however the treatment increased the respiration and microbiological activity of soil, stimulated LiP, MnP and Lac enzyme activity, and increased soil CEC. These results suggest the possibility of using the LRC as an HOM source for the rehabilitation of degraded saline soils - a common problem in soils of the Cesar River Valley (Colombia) and in the dry lands of the Colombian Caribbean influenced by open-pit coal mining.
Keywords:
Lignite, Humic substances, Biotransformation of coal, Soil salinity.RESUMEN
El objetivo de esta investigación fue evaluar cambios en algunas propiedades químicas, biológicas y físicas, en respuesta mejorada por el tratamiento con carbón de bajo rango (CBR) tipo lignito y bacterias solubilizadoras de carbón (BSC) -Bacillus mycoides, Microbacterium sp y Acinetobacter baumannii- que liberan materia orgánica humificada (MOH) mediante la biotransformación de este carbón. En condiciones de campo, se trataron parcelas de 5 m2 con la adición de CBR a una dosis de 5 kg de CBR m-2 y un inóculo de las BSC en una suspensión de 1x108 bacterias mL-1 en una dosis de 100 mL m-2. Se determinaron la respiración del suelo, la actividad microbiológica, la actividad de las enzimas lignino peroxidasa (LiP), manganeso peroxidasa (MnP) y lacasas (Lac). Las variables asociadas a la salinidad sódica del suelo: pH, la conductividad eléctrica (CE), la razón de absorción de sodio (RAS), el porcentaje de sodio intercambiables (PSI), la capacidad de intercambio catiónico (CIC) se midieron cada dos meses, mientras que la densidad aparente (Da) se determinó seis meses después de haber iniciado el experimento. La aplicación de CBR contribuyó a la disminución de la CE, RAS y PSI, pero los niveles de pH no presentaron cambios significativos. Adicionalmente, no se evidenciaron cambios significativos en la Da, sin embargo el tratamiento logró incrementar la respiración y la actividad microbiológica del suelo, estimuló la actividad de las enzimas LiP, MnP y Lac, y aumento la CIC del suelo. Estos resultados sugieren la posibilidad de utilizar el CBR como fuente de MOH para la rehabilitación de suelos salinos degradados, un problema común en los suelos del Valle del Río Cesar (Colombia) y en las tierras secas del caribe colombiano influenciadas por la minería del carbón a cielo abierto.
Palabras clave:
Lignito, Sustancias húmicas, Biotransformación del carbón, Salinidad de suelos.Currently, soil salinization is an environmental factor of worldwide concern, it has been estimated that 20% of total cultivated and 33% of irrigated soils are affected by high salinity (Shrivastava, 2015). There are around 800 million hectares on the planet affected by salts; of these, 397 million are due to salinity problems and 434 million are affected by conditions associated with sodicity (Munns, 2005; FAO, 2005). The high salt concentrations affect plant growth and crop production by limiting their ability to uptake water and nutrients (Abdul-Qados, 2011), and also contribute to soil degradation processes by increasing the dispersion of aggregates, compaction and soil erosion.
The main source of salt in soils comes from the weathering and erosion of rocks and primary minerals formed in situ or transported by water or wind. The main causes that generate salinization processes are: irrigation with saline water, groundwater level, evapotranspiration, water percolation through salt materials and seawater intrusion (Metternich, 2003). However, the dynamics of salts are so high that they cannot always be directly associated with the materials that precede the soils, but must take into account climatic, topographical, hydrological and anthropic factors (Gómez, 2004).
In sodic and saline-sodic soils, gypsum (CaSO42H2O) is the most commonly used amendment to maintain soil electrolyte levels that improve its physical and hydraulic properties (Wong et al., 2009). The combined application of gypsum-sulfur-compost has also been used, but in most cases has been decreasing due to the high costs of sulfur to farmers. Also the use of new technologies such as biofertilizers (beneficial bacteria, fungi and mycorrhiza), biopolymers and electromagnetism that stimulate microbial activity considerably, improve the soils physical properties affected by salinity due a reduction in compaction and improvements in long-term soil structure (Zúniga et al., 2011).
The addition of humidified organic matter (HOM) to soil has been frequently used to contribute to the rehabilitation of degraded lands (Ros et al., 2003), due to various studies that have demonstrated the positive effect of the HOM on soil properties (Khaled and Fawy, 2011). This has allowed HOM be recognized as fundamental to the performance of fertilization, crop productivity, soil degradation and erosion reduction, as well as the mitigation of soil desertification; because it improves soil structure and aggregation, hydraulic conductivity, promotes high levels of nutrient retention and increases cation exchange capacity (CEC) (Tejada and González, 2007; Sharif, 2002; Hernández, 2000). Several authors have incorporated different wastes and biosolids composted from green residues, vinasse (produced in sugar mills and with California red worms), among others, as amendments to soils affected by sodicity, favorably influencing some physical, chemical and biological properties of the soil , contributing to an increase in carbon immobilized by microorganisms and microbiological activity and plant growth (Duran et al., 2000, Gasca et al., 2011, Wang et al., 2014, Mogollón-Sandoval et al., 2015, Gutierrez et al., 2016). Therefore, the application of organic amendments is an alternative for conditioning soil with these characteristics.
The low rank coals (LRC) such as lignite have a soft, friable consistency, opaque appearance, humidity of 30-45 %, high ash content, low fixed carbon content (low energy content) (Word of coal, 2005) and are considered by-products of open pit mining. LRC asunderstood by its low degree of carbonification is a great source of humic substances (HS) (Peña-Méndez, 2005; Gianoulli et al., 2009) and also has high contents of elements that stimulate microbial growth and development (Hölker et al., 2002; Tao et al., 2009), and, through different mechanisms, its macrostructureallows the release of HS (Peña-Méndez, 2005). Consequently, LRC could be used as an organic amendment for the management of degraded soils (Senesi et al., 1996; Chassapis and Roulia, 2008). Among the microorganisms that have the ability to solubilize LRC to generate substances with similar characteristics to HS obtained from LRC by chemical extraction (Filip and Kubát, 2001) are bacteria isolated from coal samples; some genera and species of Escherichia freundii, Pseudomonas rathonis, Pseudomonas fluorescens, Streptomyces setoni, Pseudomonas putida, Bacillus sp., Staphylococcus, Rhodococcus and others (Laborda et al., 1997; Machnikowska et al., 2002; Pokorný et al., 2005; Valero et al., 2014; David et al., 2017) have been reported.
In a study conducted by Valero et al. (2014), three new LRC biotransformers were reported: Bacillus mycoides, Acinetobacter baumannii and Microbacterium sp.; these were isolated from environmental samples with coal residues, with the ability to solubilize LRC, producing up to 300 mg L-1 of HS in liquid medium. Subsequently Cubillos-Hinojosa et al. (2015) conducted a study where the LRC inoculated with coal solubilizing bacteria (CSB) were evaluated under greenhouse conditions as an organic amendment for a saline-sodic soil and it was found that the effect of the addition of LRC on the biological and chemical properties is greater when applied in conjunction with CSB. The application of LRC 1% and CSB in the Salidic Calciustolls soil promoted short-term biological activity, which was reflected in an increase in soil respiration, hydrolytic enzyme activity in fluorescein diacetate (FDA), ligninolytic enzyme [lignin peroxidase (LiP) and lacsases (Lac) activity associated with LRC biotransformation], and increased cation exchange capacity (CEC). The treatments of saline-sodic soil with LRC and CSB also generated short-term favorable changes in the chemical variables associated with sodium salinity in soil, and showed a decrease in electrical conductivity (EC), sodium adsorption ratio (SAR) and exchangeable sodium percentage (ESP).
In order to give continuity to the experiment conducted by Cubillos et al. (2015) under greenhouse conditions, an experiment was proposed under field conditions with the objective of evaluating the effect of the application of LRC and CSB on several chemical, biological and physical properties of a Salidic Calciustolls soil as a strategy to exploit the use of LRC as a source of humified organic matter (HOM) and CSB as an accelerating agent in the release of HOM from coal, allowing it to mitigate and/or rehabilitate soils with salinity problems, considered a common problem in the soils of the "Cesar" Department, located in an area under the influence of open-pit coal mining in the dry region of the Colombian Caribbean.
MATERIALS AND METHODS
This research was conducted in soil classified by Cubillos (2014) taxonomically as Salidic Calciustolls, which showed the following diagnostic characteristics: mollic epipedon, calcic horizon, base saturation in the profile> 50%, EC> 4, ESP> 15%. At these conditions the soil shows problems of degradation by salinization. This soil is located in the lower part of the alluvial fan of Cesar River Valley (Colombia), near the largest coal mining activity in Colombia. Geographically it is located at latitude 23°55'66'' N, longitude 73°13'47'' W. The area corresponds to tropical dry forest, according to Holdridge classification with an average temperature of 28.4 °C, an altitude of 169 m, annual rainfall of 970 mm and relative humidity between 56-74%. The climate is warm and very dry (IDEAM, 2014).
Low rank coal (LRC) samples
In this experiment, the same sample of a lignite type of low rank coal (LRC) was collected and used in the study by Cubillos-Hinojosa et al. (2015) under greenhouse conditions. It was sieved to obtain particles with a diameter of less than 300 μm, before being added to the soil. The characteristics of this LRC were determined in previous research by Valero et al. (2014) and showed a humidity of 28.44%, 11.12% ash, 47.79% volatile substances, a calorific value of 4781 kcal kg-1, 41.09% fixed carbon, and 0.13% S. These characteristics correspond to lignite type LRC due to its high moisture content and volatile materials, and a calorific value lower than 6390 kcal kg-1. The content of C, H, O and N elements in the LRC was 46.04%, 3.26%, 42.95% and 1.38% respectively and the ash minerals were found in values of Fe2O3 4.24%, CaO 69.3%, MnO2 0.14%, MgO 9.37%, SrO 0.89%, K2O 0.05%, and BaO 0.08%. The content of humic substances (HS) was 45% in extractable NaOH (0.5 N), total humic extract 32.91%, humic acid (HA) carbon 24.31%, and fulvic acid (FA) carbon 8.6%. The risk of toxicity from heavy metals in the LRC applied in the soil was considered low and negligible, because the content of As, Co, Pb, V, Cu, Zn, Ni, Cr, B, Mo and Cd [applying the standard methods of the American Section of the International Association for the Testing of Materials (ASTM)], were found in amounts of 0.71, 2.31, 1.73, 1.66, 0.55, 22.43, 3.35, 2.4, 15.11, 2.52 and 0.08 ppm of each metal, respectively.
Coal solubilizing bacteria (CSB)
In this experiment, the same coal solubilizing bacteria (CSB) evaluated in the greenhouse study by Cubillos-Hinojosa et al. (2015) were used. These bacteria (Bacillus mycoides, Acinetobacter baumannii and Microbacterium sp.) had been isolated in a previous study by Valero et al. (2012), from the rhizosphere of plants present in the area of accumulation of coal sediments, sediments from the washing of coal and LRC respectively. The CSB were reported by Valero et al., (2011) for the ability to solubilize LRC in solid medium and liquid releasing humified organic matter (HOM).
These bacteria were conserved in the strains bank of the research group in Agricultural and Environmental Microbiology of the Popular University of Cesar (Colombia), and the inoculum of each of the bacteria were reactivated and prepared in a concentration of 1 x 108 bacteria mL-1 following the methodology used by Cubillos-Hinojosa et al. (2015). A pool (mixture of the three CSB) was also prepared at a concentration of 1 x 108 bacteria mL-1.
The field conditions trial
The area delimited to develop the experiment was prepared by passing a rigid chisel that would break the compacted soil layers as well as subsequently applied irrigation to generate the same moisture conditions.
The experiment was conducted with randomized complete block design with three repetitions per treatment; the experimental unit consisted of a plot of 5 m-2 each separated 2 m from the adjacent plot with a 50 cm high barrier to reduce cross-contamination between treatments by surface flow or wind. In all treatments, the LRC incorporation was performed at a dose of 5 kg m-2 and each bacterial inoculum (B. mycoides, A. baumannii and Microbacterium sp.) separately, and the bacterial pool (mix of three CSB) was applied at the rate of 100 mL m-2 in the concentration of 1x108 bacteria mL-1 (Table 1). The LRC and CSB were mixed with the topsoil to -20 cm depth and star grass (Cynodon plectostachium), adapated to saline-sodic conditions (Más and García-Molinari, 2006), which was previously established in the soil, as a vegetation cover, as well as stimulator of biological activity in the rhizosphere used in all plots.
Table 1: Treatments used in the field conditions trial.

After two, four and six months of treatments had been applied, soil samples were taken from each plot from 0 to -20 cm depth in order to determine the respiration and microbiological activity of the soil, as well as the activity of lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase (Lac) enzymes (associated with the solubilization of LRC) and the variables associated with sodic salinity: pH, electrical conductivity (EC), sodium adsorption ratio (SAR), exchangeable sodium percentage (ESP), and cation exchange capacity (CEC). Bulk density was determined only six months after treatments were applied.
In each plot soil respiration was determined with the closed incubation technique proposed by Celis et al. (2009) with some modifications, - installing an incubation chamber with NaOH (Cubillos-Hinojosa et al., 2015) and after 24 h, the amount of CO2 released from the samples was calculated (Alef, 1995). The soil microbiological activity was determined by the hydrolysis of fluorescein diacetate (FDA) method proposed by Schnürer and Rosswall, (1982) with some modifications for the soil samples (Adam and Duncan, 2001; Greena et al., 2006). The fluorescein produced by FDA hydrolysis was calculated in mg kg−1 of soil per hour (Alvear et al., 2007).
The activity of the enzymes associated with the solubilization of coal LiP and Lac were determined following the protocol used by Cubillos-Hinojosa et al. (2015), while the MnP enzyme was determined following the method of Paszcznsky et al. (1986), where an enzymatic unit was defined as the Mn amount of enzyme required to oxidize 1 µmol of Mn2+ to Mn3+ in one minute.
The chemical properties of soil: pH, EC, SAR, ESP and CEC were determined according to the protocols of the soil laboratory of the Agustin Codazzi Geographic Institute, which are based on Soil Survey Laboratory Methods of the US Department of Agriculture (Burt, 2004) in a saturation paste, and bulk density was performed by the cylinder method.
Statistical analysis
The data were submitted to analysis of variance, significant minimum differences and in some cases, the average Dunnett's test was applied by performing a previous analysis of the of the data normality parameters. Additionally, the data corresponding to the last sampling (sixth month) were submitted to Categorical Principal Components Analysis (CATPCA), using the statistical analysis program SPSS version 18 to determine the association between all variables.
RESULTS AND DISCUSSION
Soil respiration
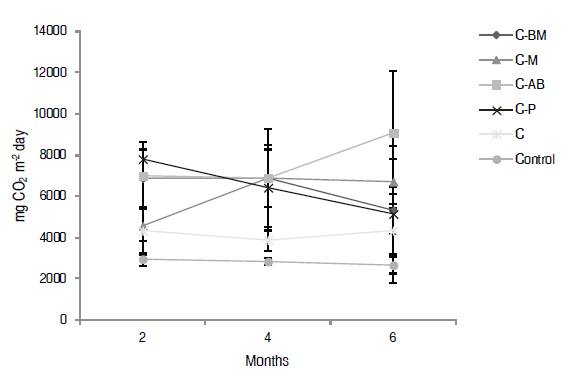
After two months of soil treatment, an increase in soil respiration was found with significant differences (P<0.05) compared to the control in the C-AB, C-BM and C-P treatments. Additionally, it was also observed that C-M and C treatments showed a tendency to increase soil respiration above the control (Figure 1).
Figure 1: Salidic calciustolls soil respiration after treatment with LRC and CSB in field conditions. C coal (low rank coal), BM Bacillus mycoides, M Microbacterium sp, AB Acinetobacter baumannii, P mixture of three CSB.
The addition of B. mycoides, A. baumanii, Microbacterium sp. and the pool of three BSC in conjunction with LRC stimulated the early activity of soil respiration. This result might indicate the possible microbial activity on the LRC or the stimulation of general soil microbial activity of the soil by LRC influence, due to the possible release of HOM mediated by the CSB that have been reported for their ability to solubilize LRC (Valero et al., 2014). The HOM contribute to soil conditioning, aggregate stability of soil (Piccolo and Mbagwu, 1999) and have been used as a coadjuvant in post-mining soil recovery processes (Christanis et al., 2006; Valero, 2013; Valero et al., 2016).
In the fourth month post-treatment the soil respiration remained constant in C-AB, C-BM treatments, increased in treatment C-M but decreased in C-P. However, all treatments present significant differences with respect to the control. In the sixth month only C-P and C-BM treatments managed to maintain and increase soil respiration, while C-P and C-BM treatments began to decrease in soil respiration but maintained differences with respect to the control. This decrease is probably due to intense microbial activity on the LRC at the beginning with a gradual decrease over time.It is also possible that the native soil microorganism have had some activity on the LRC, as this has been described in long term studies of lignite waste incorporation in the organic matter cycle for the rehabilitation of post mining soil (Rumpel and Kogel-Knabner, 2002).
In addition, these results are consistent with those found by Cubillos-Hinojosa et al., 2015 in a previous experiment under greenhouse conditions, where the addition of LRC 1% in conjunction with CSB increased Salidic Calciustolls soil respiration, showing in field studies (Figure 1) a greater effect when the LRC was applied in conjunction with A. baumanii (C-AB treatment), and also showed significant differences with respect to the other treatments and the control. This could occur by the mechanisms and time of solubilization of coal used by the bacteria, indicating a possible effect by the release of HS contributing to the formation of soil aggregates (Piccolo, 2002), which could be influencing the increase in soil respiration.
In the case of treatment C where only LRC was added, there was an increase in soil respiration without the addition of CSB and despite being lower than the other treatments, significant differences from the control were shown. This indicates that LRC, due to its physical properties (high surface area and porosity), stimulates moisture (Levine et al., 1982), which favors the microbial growth present in the LRC and the soil, reflected in the increase in soil respiration. These results agree with those reported by Cubillos-Hinojosa et al. (2015) when adding LRC 1% in a saline-sodic soil under greenhouse conditions and also by Valero et al. (2016) for treating edaphic materials with LRC, where increases in soil respiration without CSB application were observed.
Therefore, the results suggest that the incorporation of LRC as a source of HOM stimulates short-term saline-sodic soil respiration, showing a larger increase when performed in conjunction with CSB, mainly with A. baumanii.
Microbiological activity
The results show that after two months of the LRC and CSB application, all treatments showed an increase in the amount of hydrolyzed FDA, which indicate an increase in microbial enzymatic activity in the soil with significant differences (P<0.05) compared to the control, presenting a greater increase in the C-P, C-M, C-BM and C-AB treatments respectively (Table 2).
Table 2: Microbiological activity of the Salidic Calciustolls soil post-treatment with LRC and CSB in field conditions.

The microbiological activity of soil includes all metabolic reactions, cellular interactions and biochemical processes mediated by soil microorganisms (Siqueira et al., 1994). The FDA hydrolysis technique allows to measurement of the enzymatic activity of microbial populations, although it is not specific because of sensitivity to the activity of other enzymes such as lipases, esterases and proteases. However, this technique can provide information on estimating changes in microbiological activity (Greena et al., 2006), in the saline-sodic soil caused by the addition of LRC and CSB, and because this parameter has been used in several studies as an indicator of soil quality in degraded areas under rehabilitation. In this sense the results suggest that the microbiological activity is stimulated early with the addition of LRC, obtaining a greater increase when LRC is applied in conjunction with CSB. This suggests that the solubilization of LRC by these bacteria result in the release of HS (Valero et al., 2014) that can be used by the native soil and are present in the LRC microorganism, favoring microbiological activity. In addition, these results are similar to those obtained in a previous trial under greenhouse conditions, where an increase in microbiological activity was observed in C-P, C-M, C-BM and C-AB treatments (Cubillos et al., 2015). Valero et al. (2016) found a stimulus of microbiological activity in the soil in the treatment of edaphic materials (soil materials removed during the pre-mining phase of coal) with LRC and CSB, but its effect was independent of the conjoined addition with CSB.
From the fourth to the sixth post-treatment months a decreasing trend in microbiological activity in all treatments was observed, however all treatments maintained microbiological activity above the control except C treatment. This is possibly due to LRC consumption as a substrate by the microorganisms present as in the same LRC as by the native soil microbiota. These results are similar to those obtained in the previous study under greenhouse conditions by Cubillos-Hinojosa et al. (2015).
LiP, MnP and Lac enzyme activity
In Figure 2, an early increase is evident in the activity of lignin peroxidase (LiP) and manganese peroxidase (MnP) enzymes in saline-sodic soil after two months of treatment with LRC and CSB. This is possibly due to the LRC properties, characterized by the presence of element that favor the microbial nutrition and development of CSB (Cubillos-Hinojosa et al., 2015). However, LiP and MnP activity cannot be attributed to the effect of the application of CSB, because peroxidase enzyme activities are not known (LiP and MnP) in bacteria. Therefore, this ligninolytic activity may be being performed by fungi found in the microhabitat formed in the porous spaces of the LRC or by native fungi inthe saline-sodic soil. These microorganisms may be being induced to generate LiP and MnP extracellular enzymes that biotransform LRC as described by Fakoussa and Hofrichter (1999).
Figure 2: Activity of enzymes associated with coal solubilization in the treatment of Salidic calciustolls soil with LRC and CSB under field conditions. A. Lignin peroxidase (LiP), B. Manganese peroxidase (MnP) and C. Laccases (Lac). C coal (low rank coal), BM Bacillus mycoides, M Microbacterium sp., AB Acinetobacter baumannii, P mixture of three CSB.
The activation of ligninolytic enzymes allow fungi to depolymerize the components of the mobile phase of coal, which it is a material of plant origin with a structure very similar to lignin, containing organic matter such as carbon and energy for their metabolism. Also in the process of coal biotransformation some elements important for microbial nutrition such as nitrogen, sulfur and iron can be available. All these phenomena could have been favoring the colonization and growth of inoculated CSB, the native microorganisms of the LRC, and native microorganism of saline-sodic soil, contributing to increase of microbiological activity as described above.
Figure 2B shows that at 4 and 6 months post-treatment of saline-sodic soil with LRC and CSB, the LiP activity was reduced in most treatments and this is probably due to the fact that some microorganisms can induce MnP activity (Figure 2A) and inhibit LiP activity, this phenomenon of enzymatic regulation has been described in studies of the biotransformation of lignin and coal by Hofrichter and Fritsche (1996).
In all treatments, increases in the activity of Lac enzymes were observed in the fourth and sixth month after soil treatment with LRC and CSB, showing significant differences (P<0.05) with respect to the control treatment. The results suggest that the application of C-AB stimulates Lac activity, showing better results in the fourth and sixth month post-treatment with significant differences (P<0.05) compared to the control (Figure 2C), followed by treatment C-P that showed the greatest long-term increase (6 months post-treatment) in Lac activity. Meanwhile, considering that some bacteria have been reported with Lac activity (Madhavi and Lele, 2009; Diamantidis et al., 2000) and as the CSB inoculated into the saline-sodic soil biotransform LRC, the results show evidence that CSB had Lac activity, especially A. baumanni, although it can also be associated with bacteria and fungi native to the LRC used in the experiment or native microorganism in soil.
Chemical variables associated with sodium soil salinity
During the 6 months of the experiment, no significant changes in the pH (P<0.05) were generated according to Dunnett's comparison test (Table 3). After two months post-treatment of saline-sodic soil with the LRC and CSB, the EC of soil decreased in all treatments, showing significant differences (P<0.05) in some treatments (C-P, C, C-AB, C-M and C-BM) with respect to the control. In the fourth month, the results showed that only the C-P, C, and C-AB treatments maintained significant differences with respect to the control, and in the sixth month a greater decrease in soil EC was found when treated only with LRC compared to the control by significant differences (P<0.05). Therefore, the addition of LRC alone or in conjunction with the CSB inoculum have an effect on the EC of saline-sodic soil, and agrees with that found by Vance et al. (1998), that when applying organic matter with gypsum on the surface of a Natrixeralf soil managed to decrease the EC compared to an untreated control soil.
Table 3: Chemical variables associated with sodic salinity in Salidic Calciustulls soil post-treatment with LRC and CSB under field conditions.

The SAR and ESP showed significant changes in all treatments by the addition of LRC and CSB in saline-sodic soil. A decrease was observed after the start of the experiment over the 6 months for the SAR, while the ESP showed a decrease in the second and fourth month, with a greater decrease in the C-AB treatment. Also it was evidenced that when the saline-sodic soil is treated with only LRC a greater decrease in the SAR is obtained, while in a study conducted by Gasca et al. (2011) where organic matter such as vinasse was applied to treat a soil with salinity problems, it did not show changes in SAR and PSI.
In response to the application of LRC and CSB in the saline-sodic soil the CEC increased in the short-term in all treatments and continuously increased during the experiment showing significant differences (P<0.05) compared to the control. This allows the demonstration of the effect of the LRC as a source of HOM that favorably stimulates the CEC (Table 4) and can be explained because the LRC has a high CEC and its application in the soil favors the growth of native soil microorganism and the application of the CSB can solubilize it and give rise to the release of HS which act as polyelectrolytes that stimulate the CEC (Janos et al., 2011).
Table 4: CEC in Salidic Calciustolls soil after being treated with LRC and CSB under field conditions.

Bulk density (BD)
At the sixth month post-treatment mark of saline-sodic soil with LRC and CSB, no changes in any of the treatments compared to the control (P<0.05) were observed, where the BD was 1.62 g cm-3. These results are similar to those obtained by Valero (2013) when treating a soil at the start of post-mining rehabilitation in field conditions with LRC and BSC, where no significant differences regarding the control were found. Therefore, these results suggest that the effect that LRC and BSC could have on BD should be considered in the long term.
Categorical principal components analysis
Figure 3 shows the categorical principal components analysis (CATPCA) that was performed to establish the association between the evaluated variables in the sixth month post-treatment of saline-sodic soil with LRC and CSB. The CATPCA explained 57% of the variability of the data in two dimensions: 39% in dimension 1 and 18% in dimension 2 (Table 5). The most influential variables in dimension 1 were LRC, activity of the enzymes LiP, MNP and Lac, soil respiration, pH, EC, CEC and BD of soil, while in dimension 2 it was the Pool of the three CSB and ESP (Table 6).
Figure 3: Association analysis of the variables evaluated in a Salidic Calciustolls soil after sixth months post-treatment with LRC and CSB in field conditions.
Table 5: Summary table of the model in CATPCA.

Table 6: Saturation in components in CATPCA.

In Figure 3. an association between the following groups of variables was observed: (1) The activity of the enzymes LiP and MnP, microbiological activity and the pool of the three CSB, indicating that the application of the pool of CSB favors the joint expression of peroxidase ligninolytic enzymes and is reflected in the increase in microbiological activity as described above. This is possible because the CSB can biotransform the LRC resulting in the release of HS that might stimulate the activity of native soil microorganisms or LRC companion microorganisms. (2) The activity of the enzymes Lac, LRC, CEC, soil respiration and A. baumannii inoculation- This association demonstrates that the LRC application in conjunction with A. baumannii favors soil respiration and production of Lac enzymes, which are
generated by bacteria and it is possible that this activity is being performed by A. baumanni because it has the ability to biotransform LRC. The LRC favors the CEC, which in itself has a high CEC, due to the presence of phenolic and carboxylic groups (Janos et al., 2011). (3) The correlation between BD and the salinity parameters EC, SAR, and pH, indicating that the salinity negatively influenced BD.
CONCLUSIONS
The incorporation of LRC and CSB in the Salidic Calciustolls soil in field conditions favorably influences some chemical and biological properties. This is reflected in a decrease in EC, SAR and ESP, and in an increase of microbiological activity, soil respiration and CEC. The activity of ligninolytic peroxidase enzymes of the native microorganism present in LRC and soil are also favored.
The application of LRC as a source of HOM and CSB in the Salidic Calciustolls soil generates a positive effect on chemical and biological soil properties in the short-term when LRC is added in conjunction with CSB, mainly with A. baumannii.
ACKNOWLEDGEMENTS
The authors wish to thanks the Centro Biotecnológico del Caribe of Servicio Nacional de Aprendizaje (SENA) in Colombia for allowing the development of this research and the research group Microbiología Agricola y Ambiental (MAGYA) of Universidad Popular del Cesar in Colombia for technical support.
REFERENCES
References
Abdul Qados, AMS. 2011. Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). Journal of the Saudi Society of Agricultural Sciences 10(1): 7-15. doi: 10.1016/j.jssas.2010.06.002
Adam G, Duncan H. 2001. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biology and Biochemistry 33: 943–51. doi: 10.1016/s0038-0717(00)00244-3
Alef K. Dehydrogenase activity. In: Alef K, Nannipieri P, editors. Methods in applied soil microbiology and biochemistry. USA: Academic Press; 1995. p. 228–31.
Alvear M, Urra C, Huaiquilao R, Astorga M y Reyes F. 2007. Actividades biológicas y estabilidad de agregados en un suelo del bosque templado Chileno bajo dos etapas sucesionales y cambios estacionales. Revista de la Ciencia del Suelo y Nutrición Vegetal 7(3): 38–50. doi: 10.4067/S0718-27912007000300004
Bourbonnais R, Paice MG, Reid ID, Lanthier P and Yaguchi M. 1995. Lignin oxidation by laccase isozymes from trametes versicolor and role of the mediator 2,29-azinobis(3-ethylbenzthiazoline-6-sulfonate) in Kraft Lignin Depolymerization. Applied Environmental Microbiology 61(5): 1876–80.
Burt R. 2004. Soil Survey Laboratory Methods. United States Department of Agriculture, Natural Resources Conservation Service, Natural Soil Survey Center, Lincoln, Nebraska. 503 p.
Celis J, Sandoval M y Zagal E. 2009. Actividad respiratoria de microorganismos en un suelo patagónico enmendado con lodos salmonícolas. Archivos de Medicina Veterinaria 41(3): 275-279. doi: 10.4067/s0301-732x2009000300013
Chassapis K and Roulia M. 2008. Evaluation of low-rank coals as raw material for Fe and Ca organomineral fertilizer using a new EDXRF method. International Journal of Coal Geology 75(3): 185-188. doi: 10.1016/j.coal.2008.04.006
Christanis K, Giannouli A, Kalaitzidis S, Katzur J, Böcker Land Petrakis G. 2006. Application of soil-improving media produced on lignite-basis on the rehabilitation of post-mining sites. Mineral Wealth 140: 43–55.
Cubillos J. 2014. Efecto de la aplicación de bacterias productoras de sustancias húmicas mediante la biosolubilización de carbón de bajo rango en un suelo salino-sódico en el Valle del Cesar. Tesis Magister en Ciencias Agrarias. Facultad de Ciencias Agrarias. Universidad Nacional de Colombia, Medellín. 56 p.
Cubillos-Hinojosa JG, Valero NO and Melgarejo LM. 2015. Assessment of a low rank coal inoculated with coal solubilizing bacteria as an organic amendment for a saline-sodic soil. Chemical and Biological Technologies in Agriculture 2(21). doi: 10.1186/s40538-015-0048-y
David Y, Baylon MG, Pamidimarri SDVN, Baritugo KA, Chae CG, Kim YJ, and Park SJ. 2017. Screening of microorganisms able to degrade low-rank coal in aerobic conditions: Potential coal biosolubilization mediators from coal to biochemicals. Biotechnology and Bioprocess Engineering 22(2): 178–185. doi: 10.1007/s12257-016-0263-9
Diamantidis G, Effosse A, Potier P and Bally R. 2000. Purification and characterization of the first bacterial laccase in rhizospheric bacterium, Azospirillum lipoferum. Soil Biology and Biochemistry 32(7): 919–927. doi: 10.1016/s0038-0717(99)00221-7
Duran R, Garcia J y Amaya R. 2000. Evaluación de varias enmiendas para la corrección de suelos sódicos en el Valle del Cesar. Revista Suelos Ecuatoriares 30(1): 21-28.
Fakoussa RM and Hofrichter M. 1999. Biotechnology and microbiology of coal degradation. Applied Microbiology Biotechnology 52(1): 25–40. doi: 10.1007/s002530051483
FAO. 2005. Global network on integrated soil management for sustainable use of salt-affected soils. FAO, Rome, Italy: Land and Plant Nutrition Management Service. http://www.fao.org/ag/agl/agll/spush.
Filip Z and Kubát J. 2001. Microbial utilization and transformation of humic substances extracted from soils of long-term field experiments. European Journal of Soil Biology 37(3): 167–174. doi: 10.1016/s1164-5563(01)01080-9
Gasca C, Menjivar JC, Torrente-Trujillo A. 2011. Cambios en el porcentaje de sodio intercambiable (PSI) y la relación de adsorción de sodio (RAS) de un suelo y su influencia en la actividad y biomasa microbiana. Acta Agronómica 60(1): 27–38.
Giannoulli A, Stavros K, Siavalas G, Chatziapostolou A, Christanis K, Papazisimou S, Papanicolaou C and Foscolos A. 2009. Evaluation of Greek low-rank coals as potential raw material for the production of soil amendments and organic fertilizers. International Journal of Coal Geology 77(3–4): 383–393. doi: 10.1016/j.coal.2008.07.008
Greena VS, Stottb DE and Diacka M. 2006.Assay for fluorescein diacetate hydrolytic activity: optimization for soil samples. Soil Biology and Biochemistry 38(4): 693–701. doi: 10.1016/j.soilbio.2005.06.020
Gómez D. 2004. Recuperación de espacios degradados. Ediciones Mundiprensa, Madrid, España. 585 p.
Gutiérrez MA, Zúñiga O, Ospina-Salazar D. 2016. Effect of three biowastes on the productivity potential of a sodic soil. Agronomía Colombiana 34(2): 250.
Hernández O. 2000. Uso de métodos químicos y biológicos como mejoradores de la conductividad hidráulica de un suelo salino sódico. Tesis Doctorado en Ciencias. Facultad de Ciencias Biológicas y Agropecuarias. Universidad de Colima. Tecomán, México. 126 p.
Hofrichter Mand Fritsche W. 1996. Depolymerization of lowrank coal by extracellular fungal enzyme systems. I. Screening for low rank coal depolymerizing activities. Applied Microbiology Biotechnology 46(3): 220–225.
Hölker U, Schmiers H, Grobe S, Winkerhofer M, Polzakiewicz M, Ludwig S, Dohse J and Hofer M. 2002. Solubilization of lowrank coal by Trichoderma atroviridae: evidence for the involvement of hydrolytic and oxidative enzymes by using 14C-labelled lignite. Journal of Industrial Microbiology and Biotechnology 28(4): 207–212. doi: 10.1038/sj/jim/7000232
Instituto de Hidrología, Meteorología y Estudios Ambientales de Colombia, IDEAM. (2014). Características climatológicas de ciudades principales y municipios turísticos. Recuperado de http://www.ideam.gov.co/documents/21021/21789/1Sitios+turisticos2.pdf/cd4106e9- d608-4c29-91cc-16bee9151ddd#page28
Janos P, Závodská L, Lesný J, Kříženecká S, Synek V, Hejda S and Kub M. 2011. Young brown coals for environmental applications: composition, acid-base, ionexchange, and sorption properties of selected Central European coals. pp. 71–90. In: Stewart J (ed.). Coal extraction. Nova Science Publishers, New York.
Khaled H and Fawy H. 2011. Effect of different levels of humic acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil and Water Research 6(1): 21–29.
Laborda F, Fernandez M, Luna Nand Monistrol IF. 1997. Study of the mechanisms by which microorganisms solubililize and/or liquefy Spanish coals. Fuel Processing Technology 52(1): 95–107. doi: 10.1016/s0378-3820(97)00019-2
Levine DG, Schlosberg RH and Silbernagel BG. 1982. Understanding the chemistry and physics of coal structure (A Review). Proceeding of the National Academy of Sciences 79(10): 3365–3370. doi: 10.1073/pnas.79.10.3365
Machnikowska H, Pawelec Kand Podgorska A. 2002. Microbial degradation of low rank coals. Fuel Processing Technology 77–78: 17–23. doi: 10.1016/s0378-3820(02)00064-4
Madhavi V and Lele SS. 2009. Laccase: properties and applications. BioResources 4(4): 1694–1717.
Más EG and García-Molinari O. 2006. Guía ilustrada de yerbas comunes en Puerto Rico. University of Puerto Rico and USDANRCS. 303 p.
Munns, R. 2005. Genes and salt tolerance: ringing them together. New Phytologist 167(3): 645-663. doi: 10.1111/j.1469-8137.2005.01487.x
Peña-Mendéz EM, Havel J and Patocka J. 2005. Humic substances - compounds of still unknown structure: applications in agriculture, industry, environment and biomedicine. Journal of Applied Biomedicine 3: 13–24.
Piccolo A. 2001. The supramolecular structure of humic substances. Soil Science 166(11): 810–832.
Piccolo A. 2002. The supramolecular structure of humic substances: a novel understanding of humus chemistry and implications in soil science. Advances in Agronomy 57–134. doi: 10.1016/s0065-2113(02)75003-7
Piccolo A and Mbagwu JSC. 1999. Role of hydrophobic components of soil organic matter on soil aggregate stability. Soil Science Society of America Journal 63(6): 1801. doi: 10.2136/sssaj1999.6361801x
Pokorný R, Olejníková P, Balog M, Zifcak P, Holker U, Janssen M and Varecka L. 2005. Characterization of microorganisms isolated from lignite excavated from the Záhorie coal mine (southwestern Slovakia). Research in Microbiology 156(9): 932–943. doi: 10.1016/j.resmic.2005.04.010
Ros M. 2003. Soil microbial activity after restoration of a semiarid soil by organic amendments. Soil Biology and Biochemistry 35(3): 463-469. doi: 10.1016/s0038-0717(02)00298-5
Rumpel C and Kogel-Knabner I. 2002. The role of lignite in the carbon cycle of lignite - containing mine soils: evidence from carbon mineralization and humic acid extractions. Organic Geochemestry
(3): 393–399. doi: 10.1016/s0146-6380(01)00169-3
Schnürer J and Rosswall T. 1982. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Applied and Environmental Microbiology 43(6): 1256–1261.
Senesi M, Miano TM and Brunetti G. 1996. Humic-like substances in organic amendments and effects on native soil humic substances. pp. 531–594 In: Piccolo A (ed.). Humic substances in terrestrial ecosystems. Elsevier, USA.
Sharif M. 2002. Effect of lignite coal derived humic acid on growth and yield of wheat and maize in alkaline soil. Thesis Doctor of Philosophy in Soil and Environmental Sciences. Faculty of Crop Production Sciences. Agricultural University, Peshawar, Pakistan.
Shrivastava P and Kumar R. 2015. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi Journal of Biological Sciences 22(2): 123–131. doi: 10.1016/j.sjbs.2014.12.001
Siqueira JO, Moreira FM de S, Grisi BM, Hungria M and Araujo RS.1994. Microrganismos e processos biológicos do solo: perspectiva ambiental. EMRAPA-SPI. Brasília, p. 7-81.
Tao XX, Pan LY, Shi KY, Chen H, Ying S Dand Luo ZF. 2009. Bio-solubilization of Chinese lignite I: extra-cellular protein analysis. Mining Science and Technology (China) 19(3): 358–62. doi: 10.1016/s1674-5264(09)60067-3
Tejada M and González JL. 2007. Influence of organic amendments on soil structure and soil loss under simulated rain. Soil and Tillage Research 93(1): 197–205. doi: 10.1016/j.still.2006.04.002
Vance WH, Tisdell JM and McKenzie BM. 1998. Residual effects of surface application of organic matter and calcium salts on the sub-soil of a red–brown earth. Australian Journal of Experimental Agriculture 38(6): 595. doi:10.1071/ea97102
Valero N. 2013. Transformación microbiana de carbón de bajo rango para inducir cambios en las propiedades del suelo. Tesis Doctorado en Ciencias Agropecuarias. Facultad de Agronomía. Universidad Nacional de Colombia, Bogotá. 121 p.
Valero N, Beleño J y Mancilla S. 2011. Biotransformación de carbón de bajo rango por bacterias aisladas de microhábitats influenciados por residuos de carbón. Revista Colombiana de Biotecnología 13(1): 58–65.
Valero N, Gómez L, Pantoja M and Ramírez R. 2014. Production of humic substances through coal-solubilizing bacteria. Brazilian Journal of Microbiology 45(3): 911–918. doi: 10.1590/s1517-83822014000300021
Valero N, Rodríguez LN, Mancilla S y Contreras L. 2012. Obtención de bacterias biotransformadoras de carbón de bajo rango a partir de microhábitats con presencia de residuos carbonosos. Acta Biológica Colombiana 17(2): 335–48.
Wang L, Sun X, Li S, Zhang T, Zhang W and Zhai P. 2014. Application of Organic Amendments to a Coastal Saline Soil in North China: Effects on Soil Physical and Chemical Properties and Tree Growth. PLoS ONE 9(2) e89185. doi: 10.1371/journal.pone.0089185
World Coal Institute. 2005. The coal resource, a comprehensive overview of coal. WCI, London. 44 p.
Wong VNL, Dalal RC and Greene RSB. 2009. Carbon dynamics of sodic and saline soils following gypsum and organic material additions: A laboratory incubation. Applied Soil Ecology 41(1): 29-40. doi: 10.1016/j.apsoil.2008.08.006
Zúñiga O, Osorio J, Cuero R y Peña J. 2011. Evaluación de tecnologías para la recuperación de suelos degradados por salinidad. Revista Facultad Nacional de Agronomía 64(1): 5769 – 5779.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Collins Amoah-Antwi, Jolanta Kwiatkowska-Malina, Steven F. Thornton, Owen Fenton, Grzegorz Malina, Ewa Szara. (2020). Restoration of soil quality using biochar and brown coal waste: A review. Science of The Total Environment, 722, p.137852. https://doi.org/10.1016/j.scitotenv.2020.137852.
2. Fengyuan Jin, Qilin Hu, Yingxu Zhao, Xiaoyu Lin, Jianfeng Zhang, Jiejing Zhang, Durgesh Kumar Jaiswal. (2022). Enhancing quinoa growth under severe saline-alkali stress by phosphate solubilizing microorganism Penicillium funicuiosum P1. PLOS ONE, 17(9), p.e0273459. https://doi.org/10.1371/journal.pone.0273459.
3. Lily Shylla, Santa Ram Joshi. (2025). Mineral Transformation and Bioremediation by Geo-Microbes. Interdisciplinary Biotechnological Advances. , p.303. https://doi.org/10.1007/978-981-96-3033-2_12.
4. Anna Detman, Michał Bucha, Bernd R.T. Simoneit, Damian Mielecki, Cezary Piwowarczyk, Aleksandra Chojnacka, Mieczysław K. Błaszczyk, Mariusz Orion Jędrysek, Leszek Marynowski, Anna Sikora. (2018). Lignite biodegradation under conditions of acidic molasses fermentation. International Journal of Coal Geology, 196, p.274. https://doi.org/10.1016/j.coal.2018.07.015.
5. Xing-Feng Huang, Paul H. Fallgren, Song Jin, Kenneth F. Reardon. (2025). A Low-Rank Coal-Derived Soil Amendment Promotes Plant Growth and Shapes Rhizosphere Microbial Communities of Lettuce (Lactuca sativa). Agriculture, 15(21), p.2310. https://doi.org/10.3390/agriculture15212310.
6. Nuraly S. Akimbekov, Ilya Digel, Kuanysh T. Tastambek, Dinara K. Sherelkhan, Dariya B. Jussupova, Nazym P. Altynbay. (2021). Low-Rank Coal as a Source of Humic Substances for Soil Amendment and Fertility Management. Agriculture, 11(12), p.1261. https://doi.org/10.3390/agriculture11121261.
7. Manuel Pantoja-Guerra, Ramiro Ramirez-Pisco, Nelson Valero-Valero. (2019). Improvement of mining soil properties through the use of a new bio-conditioner prototype: a greenhouse trial. Journal of Soils and Sediments, 19(4), p.1850. https://doi.org/10.1007/s11368-018-2206-x.
8. Nuraly S. Akimbekov, Ilya Digel, Kuanysh T. Tastambek, Adel K. Marat, Moldir A. Turaliyeva, Gulzhan K. Kaiyrmanova. (2022). Biotechnology of Microorganisms from Coal Environments: From Environmental Remediation to Energy Production. Biology, 11(9), p.1306. https://doi.org/10.3390/biology11091306.
9. Liangliang Guo, Zhaoyang Nie, Jie Zhou, Fenghua An, Lu Zhang, Shixin Zhang, Tibor Tóth, Fan Yang, Zhichun Wang. (2023). Effects of organic amendments on soil bacterial community structure and yield in a saline‐sodic soil cropped with rice. Land Degradation & Development, 34(17), p.5514. https://doi.org/10.1002/ldr.4861.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2017 Revista Facultad Nacional de Agronomía Medellín

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.