Effect of magnesium silicate in cv. ‘ICA Cerinza’ common bean (Phaseolus vulgaris L.) under field conditions
Efecto del silicato de magnesio en frijol común (Phaseolus vulgaris L.) cv. ICA Cerinza bajo condiciones de campo
DOI:
https://doi.org/10.15446/rfna.v70n3.62679Keywords:
Silicon, Leguminous vegetable, Beneficial element, Nutritional dynamics, Amendment (en)Silicio, Leguminosa, Elemento benéfico, Dinámica nutricional, Enmienda (es)
The bean crop is very important in Colombia, being generator of income and employment; and furthermore very importance within the diet of the population due to its high protein content. Currently, the bean production is not sufficient to meet the demand in the country, because of phytosanitary problems, scarce replacement of varieties and inappropriate handling of mineral nutrition of cultivars. Nowadays, the use of products for soils rich in silicon represents an alternative for production increase, because they contribute markedly in nutritional dynamics. So, the purpose of this research was to assess the effect of magnesium silicate on the physiological behavior of ‘ICA Cerinza’ bean cultivar under agro ecological conditions in Tunja (Boyacá, Colombia). For that, a completely random design with 4 treatments each was used, corresponding to increasing doses of magnesium silicate (0, 300, 600 y 900 kg ha-1) with 4 replications. The total chlorophyll content, leaf area, leaf thickness, fresh and dry mass, yield components, silicon and phosphorus leaf content were evaluated. Statistical differences between treatments (P≤0. 05) were found in all variables tested. The application of 900 kg ha-1 of magnesium silicate showed the best results, confirming that silicon as a beneficial element that can submit a favorable response to physiological level in not bio-accumulators crops of this element. However, this response can be linked to agro ecological conditions, the type of product and also the dose used.
El cultivo de frijol es muy importante en Colombia siendo un generador de ingresos y empleo; sumado a su importancia dentro de la dieta de la población debido a su alto contenido de proteínas. Actualmente en el país es insuficiente la producción de fríjol para abastecer la demanda, esto debido a problemas de tipo fitosanitario, escaso recambio de variedades y manejo inadecuado de la nutrición mineral de los cultivares. En la actualidad el uso enmiendas ricas en silicio (Si) representan una alternativa en el incremento de la producción, ya que estas contribuyen de manera notoria en la dinámica nutricional. Por tanto, el objetivo de esta investigación fue el de evaluar el efecto del silicato de magnesio sobre el comportamiento fisiológico del cultivar de frijol ICA Cerinza bajo condiciones agroecológicas del municipio de Tunja-Boyacá. Para ello, se empleó un Diseño Completamente al Azar con cuatro tratamientos correspondientes a dosis crecientes de silicato de magnesio (0, 300, 600 y 900 kg ha-1) con cuatro replicaciones. Se evaluó el contenido total de clorofilas, área foliar, grosor de hoja, masa fresca y seca, componentes de rendimiento, contenido foliar de silicio y fósforo. Se presentaron diferencias estadísticas entre tratamientos (P=0,05) para todas variables. La aplicación de dosis crecientes de silicato de magnesio mostro un efecto positivo, siendo el tratamiento de 900 kg ha-1 el que presentó los mejores resultados, lo anterior indica que el aporte de silicio como elemento benéfico puede presentar una respuesta a nivel fisiológico favorable en cultivos no bio-acumuladores de este elemento. Sin embargo, esta respuesta puede estar ligada a las condiciones agroecológicas, al tipo de fuente empleada y la dosis utilizada.
Recibido: 15 de febrero de 2017; Aceptado: 12 de junio de 2017
ABSTRACT
The bean crop is very important in Colombia, being a generator of income and employment; and furthermore very important within the diet of the population due to its high protein content. Currently, bean production is not sufficient to meet the demand in the country, because of phytosanitary problems, scarce replacement of varieties and inappropriate handling of mineral nutrition of cultivars. Nowadays, the use of products for soils rich in silicon represents an alternative for production increase, because they contribute markedly in nutritional dynamics. So, the purpose of this research was to assess the effect of magnesium silicate on the physiological behavior of ‘ICA Cerinza' bean cultivar under agro-ecological conditions in Tunja (Boyacá, Colombia). For that, a completely random design with four treatments each was used, corresponding to increasing doses of magnesium silicate (0, 300, 600 y 900 kg ha-1) with four replications. The total chlorophyll content, leaf area, leaf thickness, fresh and dry mass, yield components, silicon and phosphorus leaf content were evaluated. Statistical differences between treatments (P≤0.05) were found in all variables tested. The application of increasing doses of magnesium silicate showed a positive effect, being the treatment of 900 kg ha-1 which presented the best results. This indicates that the contribution of silicon as a beneficial element that can submit a favorable response to physiological level in crops which do not use bio-accumulators. However, this response can be linked to agro-ecological conditions, the type of product and also the dose used.
Keywords:
Silicon, Leguminous vegetable, Beneficial element, Nutritional dynamics, Amendment.RESUMEN
El cultivo de frijol es muy importante en Colombia siendo un generador de ingresos y empleo; sumado a su importancia dentro de la dieta de la población debido a su alto contenido de proteínas. Actualmente en el país es insuficiente la producción de fríjol para abastecer la demanda, esto debido a problemas de tipo fitosanitario, escaso recambio de variedades y manejo inadecuado de la nutrición mineral de los cultivares. En la actualidad el uso enmiendas ricas en silicio (Si) representan una alternativa en el incremento de la producción, ya que estas contribuyen de manera notoria en la dinámica nutricional. Por tanto, el objetivo de esta investigación fue el de evaluar el efecto del silicato de magnesio sobre el comportamiento fisiológico del cultivar de frijol ‘ICA Cerinza' bajo condiciones agroecológicas del municipio de Tunja-Boyacá. Para ello, se empleó un Diseño Completamente al Azar con cuatro tratamientos correspondientes a dosis crecientes de silicato de magnesio (0, 300, 600 y 900 kg ha-1) con cuatro replicaciones. Se evaluó el contenido total de clorofilas, área foliar, grosor de hoja, masa fresca y seca, componentes de rendimiento, contenido foliar de silicio y fósforo. Se presentaron diferencias estadísticas entre tratamientos (P≤0,05) para todas variables. La aplicación de dosis crecientes de silicato de magnesio mostro un efecto positivo, siendo el tratamiento de 900 kg ha-1 el que presentó los mejores resultados, lo anterior indica que el aporte de silicio como elemento benéfico puede presentar una respuesta a nivel fisiológico favorable en cultivos no bio-acumuladores de este elemento. Sin embargo, esta respuesta puede estar ligada a las condiciones agroecológicas, al tipo de fuente empleada y la dosis utilizada.
Palabras clave:
Silicio, Leguminosa, Elemento benéfico, Dinámica nutricional, Enmienda.The common bean (Phaseolus vulgaris L.) is one of the most important crops in many parts of the country, providing income, rural employment and a basic product in the diet of the Colombian population due to its high content of proteins and minerals; in this way, helping to improve the poor diet of the rural population (Arias et al., 2007) coming mostly from smallholdings production systems (Ruiz and Rincón, 2000). However, bean production in Colombia is insufficient to meet demand; therefore, imports came from countries like Peru, Argentina, Bolivia, China, Venezuela and Ecuador (FENALCE, 2015). This is because of phytosanitary problems, scarcity in the delivery of new varieties and an inappropriate management of mineral nutrition.
The application of macronutrients and micronutrients for obtaining highly productive and healthy crops, has been widely documented (Marschner, 2012). However, nowadays, elements like silicon (Si), the second most abundant element in soil after Oxygen (Liang et al., 2007; Epstein, 2009), present in silicate minerals (Silicon oxide) and aluminosilicates, representing 90% of all the terrestrial minerals (Datnoff et al., 2007), it's not considered as essential but beneficial in some major crops like rice, being an element which contributes significantly to productivity (Fageria et al., 1997) and healthy crops, because it promotes the synthesis of low molecular weight metabolites with anti-fungal activity like phytoalexins (Fawe et al., 1998). It also generates an increased growth in plants under biotic and abiotic stress conditions (Rodrigues et al., 2003; Ma, 2004) and decreases the oxidative stress caused by heavy metal toxicity (Qinghua et al., 2005).
Nowadays, its beneficial effects have been studied on the growth and development of many plants, particularly Poaceae and some Cyperaceae (Richmond and Sussman, 2003), with the application of mineral sources, especially magnesium silicates or calcium (Álvarez and Osorio, 2014). Likewise, the research should be focused on crops needed for global food security to ensure an increase in their production and observe their beneficial effects with regard to disease control, different types of stress, and mitigation of heavy metals (Epstein, 1999).
Silicon is absorbed by the roots in the form of H4SiO4 and is transported by the apoplast into the xylem and the aerial part of plants, to be accumulated in epidermal cells (Mitani and Ma, 2005). Under field conditions, it can stimulate growth and productivity, because it increases the availability of elements like P, Ca, Mg, K y B, countering antagonistic effects generated in soils with high saturation of Al and Fe (Epstein and Bloom, 2005). One of the most important effects in the application of silicate fertilizers is to improve the availability of phosphorus in the soil and benefit the absorption of the same by cultivated plants, and, as a result, the yield of crops, resulting in their most obvious and valuable effects in tropical soils where phosphorus fixation limits the effectiveness of fertilizers (Álvarez and Osorio, 2014).
It is known that silica gel is deposited between the cell walls of plants and contributes to beneficial effects of the element (Álvarez and Osorio, 2014), forming a double cuticular layer to protect and mechanically reinforce all plants. For this reason, in many countries fertilizers are applied consistently with silicon to increase the productivity and the sustainability of crops (Snyder et al., 2007).
With this in mind, the purpose of this research was to evaluate the effect of silicon application in increasing doses using magnesium silicate as a source on the growth and production of cv.‘ICA Cerinza' (Phaseolus vulgaris L.) common bean under agro ecological conditions in Tunja-Boyacá.
MATERIALS AND METHODS
The research was made under field conditions from October, 2015 to January, 2016 at the "La Maria" farm located at the UPTC in Tunja-Boyacá with coordinates 5°32'25"N 73°21'41"O and a height of 2691 msnm. The climate conditions during the development of the experiment were as follows: Average temperature 13.9°C, relative humidity 70% and an average monthly precipitation of 81.7 mm.
Before the sowing, we made a physicochemical analysis of the soil (Table 1). The analysis in vegetal tissue of phosphorus were realized according to NTC 234, and silicon through technique of atomic emission spectroscopy, internal methodology. The measurement of growth and production variables was made in the Laboratory of Plant Physiology at the UPTC.
Table 1: Physicochemical properties of soil used in the experiment.
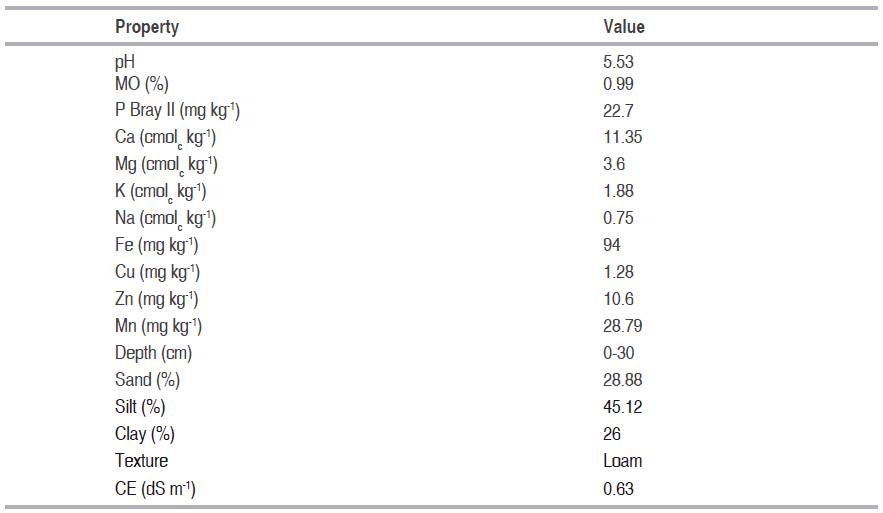
We used a completely random design, with 4 treatments: T1: treatment control (without application); T2: 300 kg ha-1; T3: 600 kg ha-1 y T4: 900 kg ha-1 using Silimag 30-30 Rio Claro® as a source (magnesium silicate MgO-30%; SiO2-30%). Each treatment was replicated 4 times, for a total of 16 experimental units.
Each experimental units corresponded to a plot with dimensions 1.4 x 2 m, for an area of 2.8 m2, the sowing was made with a row spacing of 0.6 m and 0.3 m between plants with a density of 55,555 plants ha-1, placing 2 grains per site, and then, to perform a thinning and having a total of 18 plants per plot. To make the variables measurement, 10 plants were selected from the central furrows to avoid the edge plot effect. We used the cultivar ‘ICA Cerinza' common bean type shrubby seed, well-adapted to the area and with an average yield of 1.6 t ha-1 (FENALCE, 2015).
The application of magnesium silicate was made at the moment of sowing. The fertilization was performed according to the results of the soil analysis obtained one month after sowing. Sprinkler irrigation was applied according the needs of crop, an application of phytosanitary control based on monitoring, and control products of anthracnose and leaf-miners.
Physiological and growth variables evaluated were: Total chlorophyll with a Minolta clorofilometer SPAD 502, taking a total of 10 measurements per plant; sheet thickness with a Mitutoyo digital calibrator precision ± 0.05 mm, leaf area with a CCI-202 meter, fresh and dry weights on an electronic Acculab VIC 612 balance of 0.01 g precision and dried in a Memmert drying oven at 70 °C during 48 h; the P content in plant tissues was performed by the method of calcination at 600 °C, acid digestion and valuation by visible spectrophotometry and the Silicon content was made by closed wet digestion via a microwave oven, quantification by atomic-absorption flame technique. According to the yield, we measured variables such us: number of pods per plant, number of grains per pod, weight of 100 grains to 14% humidity balanced in the Motomco Moisture meter model 919 from the seed laboratory at FENALCE and yield in kg ha-1.
Data obtained were tested for normality and homogeneity of variance by Shapiro-Wilk y Levene testing respectively. Testing the cases, we made the variance analysis, the variables which show statistical differences were tested for comparison means of Tukey (P≤0.05). The analysis was made with the statistical program SAS v.9.2e SAS Institute Inc., Cary, NC.
RESULTS AND DISCUSSION
Variables of leaf thickness, fresh and dry mass of shoot and root showed significant differences between treatments (P≤0.05), we observed that the higher values were the result of applying 900 kg ha-1 of magnesium silicate and the lower values were in plants without any application (Table 2). This is due possibly, to the Silicon accumulation in the cell walls and intracellular spaces of leaves and root cells (Epstein, 1994), forming a silicon-cellulose membrane which can be associated with pectin and calcium ions (Snyder et al., 2007). Even though the concentration of silicon in plants varies considerably between species of 0.1% to 10% dry weight (Ma et al., 2011).
Table 2: Effect of different doses of magnesium silicate on physiological variables in bean.

Hernández (2002) and Tahir et al. (2010) indicate that the application of silicon helps the root development system and a significant increase in biomass in plants exposed to salt stress. It is shown that silicon can be accumulated in the epidermal root cells (Epstein, 1994). This is associated with the results obtained in this research in which an increasing of mass and leaf thickness was an effect of applying edaphic magnesium silicate.
Meanwhile the leaf area showed significant differences between treatments according to the Tukey Test (P≤0.05). The treatment of 900 kg ha-1 of magnesium silicate presented the higher value to the leaf area with 1287.52 ± 65.91cm2. It showed significant differences versus other treatments; meanwhile, the treatment without any application showed the lower value with 866.68 ± 7.86 cm2 (Table 2). It is reported that the application of edaphic silicon has a positive effect on the growth of plants, because there is an increasing in the leaf area, shoot growth, dry biomass and yield (Gevrek et al., 2012; Tavares et al., 2012). On the other hand, Nwudo and Huerta (2008) indicate that the addition of silicon at 20 days old stimulated the growth of rice plants and inhibits the negative effect of cadmium. According to Liu et al. (2014), the application of silicon increases the photosynthesis rate in sorghum because of the development of the leaf area. Haghighi and Pessarakli (2013) observed that the silicon improved the photochemical efficiency of leaf in S. lycopersicum plants, under conditions of salt stress.
According to Epstein (1999), the silicon absorbed is transferred from roots to the aerial part of the plant through the transpiration stream via xylem, but being inside the plant the phytoliths, which are microscopic mineral particles formed when the silicon is inside and around the cells of the epidermal tissue and in the cell walls remain insoluble until the plant residues return to the soil and begin to decompose (Álvarez and Osorio, 2014). The silicon deposited on the leaf blade allows the silica bodies to act as a window to ease the light transmission to the mesophyll photosynthetic tissue, hypotheses still to be tested (Ma and Takahashi, 2002), easing the transmission of light to leaves. The photosynthesis process will help generate more energy to the plant, increasing its productivity.
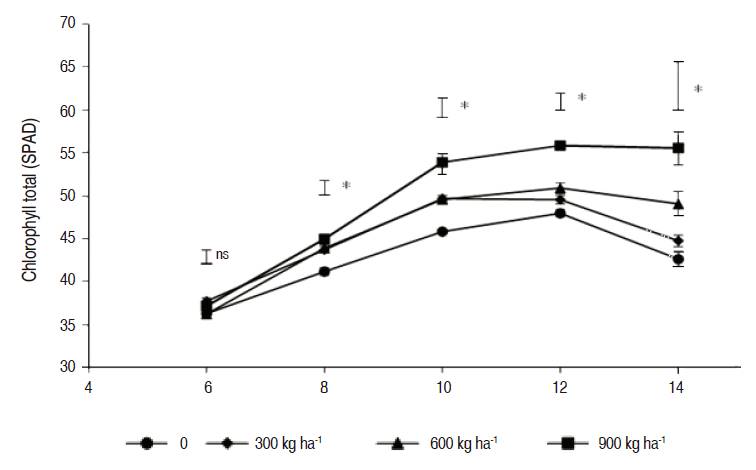
The content of total chlorophyll showed statistical differences between the doses used from week eight after sowing (Figure 1). The treatment of 900 kg ha-1 of magnesium silicate presented the higher value of total chlorophyll (SPAD) during the greater part of the experiment. The accumulation of silicon in the cell walls and intercellular spaces can protect and inhibit morphological and anatomical alterations of the photosynthetic apparatus, of which pigments like chlorophylls make an important part, due to the role of activation of the defense system of the plant (Adrees et al., 2015).
Figure 1: Behavior of the total chlorophyll in cv. ‘ICA Cerinza' (Phaseolus vulgaris L.) common bean under different doses of magnesium silicate. Vertical bars on treatments indicate standard error (n=4). Bars on the sampling points indicate the minimum significant difference in each sampling point according to the Tukey Test (P≤0.05), ns: no statistical difference, *: Significant differences.
According Shekari et al. (2015) silicon helps the photosynthetic rate, which is directly linked to the content of chlorophyll in leaves. Qinghua et al. (2005), indicate to be an excess of micronutrients such as magnesium biosynthesis is inhibited in the chlorophyll and it causes a decrease in the photosynthesis rate, effects that can be fixed with the application of silicon. Mihaličová et al. (2014) report the positive effects of silicon in the formation of chlorophyll in corn.
Adatia and Besford (1986) indicate that silicon is presented in the chlorophyll in high concentrations per unit area of leaf tissue, representing a positive impact in the plant tolerance with low or high levels of light, making its use more efficient.
In corn silicon improves the levels of chlorophyll in plants under water stress compared with plants without any application (Kaya et al., 2006). The results obtained in this research show that the application of increasing doses of magnesium silicon enhance the content of chlorophyll in the cv. ‘ICA Cerinza' common bean crop, generating a contribution of silicon and magnesium, both, important components of chlorophyll.
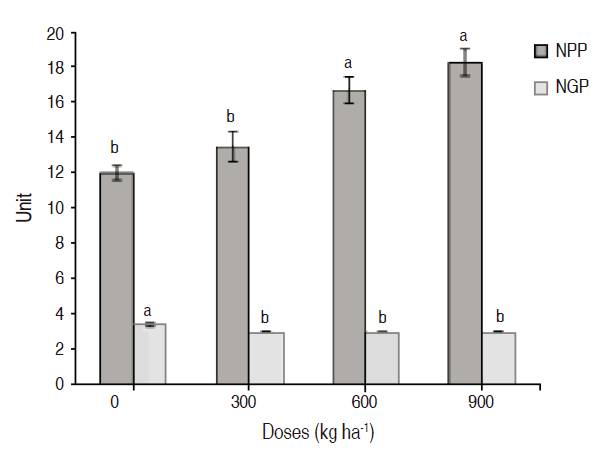
The variables number of pods per plant and grains per pod showed significant differences between treatments (P≤0.05) (Figure 2). The application of 900 kg ha-1 of magnesium silicate resulted in a greater number of pods per plant with a value of 18.25 ± 0.75. The lower value was in the treatment control with 12 ± 0.4 pods per plant. The number of grains per pod showed statistical differences between the control treatment and the other treatments with the application of magnesium silicate, the higher value was observed in the treatment without any application with 3.43 ± 0.09 and the lower value was the treatment of 900 kg ha-1 with 3 ± 0.05 grains per pod without significant differences between treatments 2 and 3 (Figure 2). As was mentioned before, the magnesium silicate generated greater vegetative plant growth, seeing this reflected in the high number of reproductive structures; however, as Delgado et al. (2013) said, there is a negative correlation between the number of pods/plant and the number of grains/pod, it correlates the results obtained before.
Figure 2: NVP: Number of pods per plant, NGP: Number of grains per pod in cv. ‘ICA Cerinza' (Phaseolus vulgaris L.) common bean under different doses of magnesium silicate. Treatments followed by different letters show significant differences according to the Tukey Test (P≤0.05), vertical bars indicate standard error (n=4).
Research made in citric fruits showed that silicified fertilization accelerates their growth and increases maturation and quantity. (Álvarez and Osorio, 2014). In gramineous, the number of tillers is an indicator of productivity, due to the relation that exists between this and the biomass per unit of area; in rice, a considerable increase in the number of pods per plant has been demonstrated, this is possible because of improvement in the nutritional dynamics that allowed the absorption of some nutrients like P, Ca and the contribution of Mg and Si, which generates an increase in the photosynthesis rate and yield.
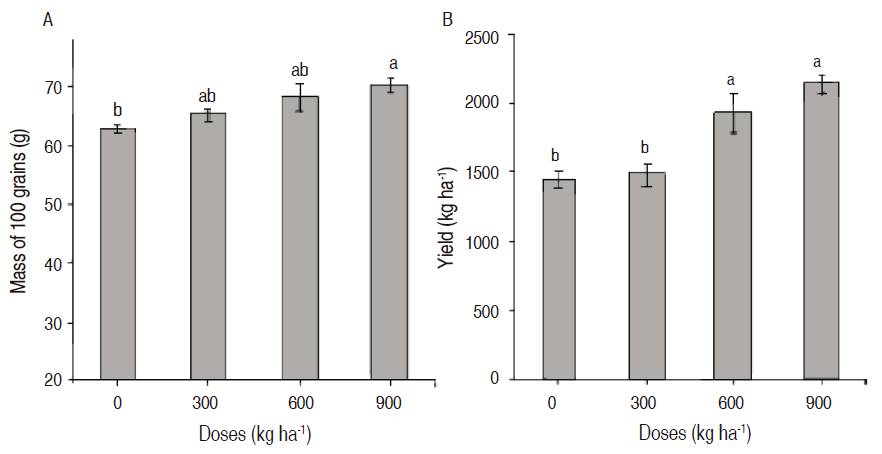
There were some significant differences between treatments in the weight variables of 100 grains (P100) and yield (P≤0.05) (Figure 3). The higher values were in the treatment 900 kg ha-1 with a P100 of 70.36±1.09 g and a yield of 2139.1±59.35 kg ha-1. The treatment without any application showed the lower value with a P100 of 63.11 ± 0.73 g and a yield of 1445.5 ± 58.93 kg ha-1 (Figure 3 A y 3 B). We could observe that the increasing doses of magnesium silicate affect the yield of the bean crop, as it increased by 47%; this is possibly due to the accumulation of this element in the epidermal cells of the grain.
Figure 3: A. Mass of 100 grains at 14 % of humidity; B. Yield in kg ha-1 in beans (Phaseolus vulgaris L.) cv. ‘ICA Cerinza', under different doses of magnesium silicate. Treatments followed by different letters show significant differences according to the Tukey Test (P≤0.05), vertical bars indicate standard error (n=4).
According to Hernández (2002) and Quero (2008), the silicon is essential for tomato and cucumber crops; this is also found in oat, barley and bean seeds in concentrations of 4.25; 2.42; and 1.20 g kg-1 dry mass respectively. In the chili crops, the production and quality of the harvest increased with the application of fertilizers, irrigation water and other elements rich in silicon (Quero, 2008).
The role of silicon in plant metabolism has got great attention (Kaya et al., 2006). The silicon has many functions like the stimulation of photosynthesis, increasing the strength of tissues and reducing the rate of transpiration, functions that help increase the production of dry mass and the resistance of plants to physical, chemical and biological stress (Álvarez and Osorio, 2014). It's important to know that silicon can be utilized in metabolic, physiological and/ or structural activities in higher plants, mainly when they are exposed to biotic and abiotic stress (Liang et al., 2007).
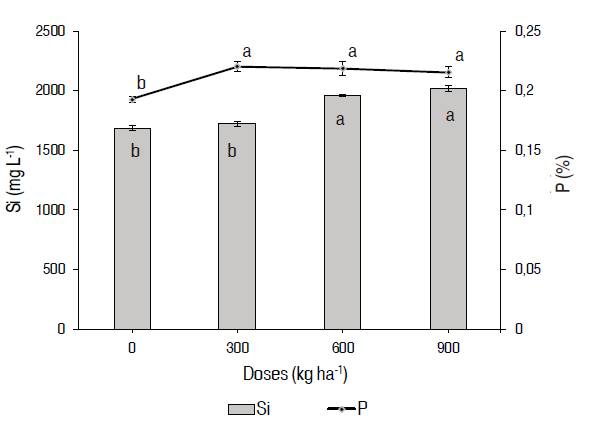
According to Álvarez and Osorio (2014), one of the most important effects in the application of silicate fertilizers is to improve the availability of phosphorus in the soil, thus, improving its absorption by the cultivated plants, and, of course, the yield of crops. In the variables of silicon and phosphorus concentration at plant tissue levels (leaves), there were significant statistical differences (Figure 4). For the concentration of silicon, the higher value used a dose 900 kg ha-1 of magnesium silicate with 2013±27.26 mg L-1, without any statistical difference with treatment 3, but similar to treatments 1 and 2. For the variable of phosphorus concentration in a tissue, there were no statistical differences between the treatments with application of magnesium silicate, but they were related to the treatment control. The highest concentration of phosphorus was for treatment 2 with a value of 0.22±0.002%. The application of 300 kg ha-1 of magnesium silicate generated an increase in phosphorus absorption and its accumulation in tissue (leaves); similar results were obtained with the application of treatments 600 and 900 kg ha-1.
Figure 4: Concentration of phosphorus (mg kg-1) in plant tissue (leaves) and silicon (mg kg-1) in cv. ‘ICA Cerinza' (Phaseolus vulgaris L.) common bean under different doses of magnesium silicate. Bars indicate concentration of Si and dispersion line a concentration of P. Treatments followed by different letters show significant differences according to the Tukey Test (P≤0.05), vertical bars indicate standard error (n=4).
Roy et al. (1971) found that applying calcium silicate (500 mg kg-1 of silicon) in four tropical soils, significantly reduced the phosphorus fixation of the soil. This beneficial effect of silicon makes that the quantity of soluble phosphorus greater, reducing the application of phosphorus fertilizers and the prices of production. According to the same author, the application of phosphorus on tropical soils can be reduced with the application of calcium silicate and lime at the same time.
According to the species, plants accumulate an amount of silicon in their tissues, mainly Poaceae which accumulate a higher content of this element and dicotyledonous species, which do not greatly benefit from silicon; so, highly silicate fertilizers will provide a greater absorption of this element, showing its beneficial effects in plants.
It has been reported that in tomato crops the most accumulation of silicon is found in roots and not in stems, a typical effect in plants which do not accumulate silicon (Menzies et al., 1991). In the bean leaves, a concentration that varies from 0.14% silicon without any application of silicified source to 0.2% of silicon in treatments with application of magnesium silicate was found; it allows to catalog the bean cv ‘ICA Cerinza' as a species which does not accumulate silicate, but in which it has important benefits, because of a significant increase in the growth variables and in the yield components. Therefore, the application of magnesium silicon has become an important alternative at the level of nutritional dynamics for plants which do not accumulate of silicon like the common bean.
CONCLUSIONS
The application of increasing doses of magnesium silicate significantly helped the growth variables and the yield components, because the productivity increased by 47% per hectare. The application of magnesium silicate helped to improve the absorption of phosphorus and increased the concentration of silicon in leaves, indicating that the product used did not have a high bio-accumulation of silicon, but on the contrary, had a beneficial effect.
ACKNOWLEDGMENTS
This research was carried out with the support of Cales and Calcareous Derivatives Rio Claro Naranjo and Compañía S C A and the Pedagogical and Technological University of Colombia (UPTC) Tunja.
REFERENCES
References
Adatia M and Besford R. 1986. The effects of silicon on cucumber plants grown in recirculating nutrient solution. Annals of Botany 58: 343-351. doi: 10.1093/oxfordjournals.aob.a087212.
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum M and Irshad M. 2015. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicology and Environmental Safety 119: 186–197. doi: 10.1016/j.ecoenv.2015.05.011.
Álvarez C and Osorio W. 2014. Silicio Agronómicamente esencial. Mejisulfatos S.A.S., Itagüí, Colombia y Universidad Nacional de Colombia, Medellín, Colombia. 108 p.
Arias JH, Jaramillo M and Rengifo T. 2007. Manual Técnico: Buenas Prácticas Agrícolas (BPA) en la producción de fríjol voluble. CORPOICA – MANA – FAO. C.I. La Selva, Medellín. 168 p.
Datnoff L, Rodrigues F and Seebold K. 2007. Silicon and Plant Disease. pp.233-246. In Datnoff L, Elmer W and Huber DM (eds.). Mineral nutrition and plant disease. The American Phytopathological
Society, St. Paul, MN.
Delgado, H, Pinzón E.H, Blair M, y Izquierdo P.C. 2013. Evaluación de líneas de fríjol (Phaseolus vulgaris L.) de retrocruce avanzado entre una accesión silvestre y radical cerinza. Revista U.D.C.A Actualidad & Divulgación Científica 16(1): 79-86.
Epstein E. 1994. The anomaly of silicon in plant biology. Proceedings of the National Academy of Sciences 91: 11-17.
Epstein E. 1999. Silicon. Annual Review of Plant Physiology and Plant Molecular Biology 50: 641-664. doi: 10.1146/annurev.arplant.50.1.641.
Epstein E. 2009. Silicon: its manifold roles in plants. Annals of Applied Biology 155: 155–160. doi:10.1111/j.1744-7348.2009.00343.x.
Epstein E and Bloom A. 2005. Mineral nutrition of plants, principles and perspectives. Second edition. Sinauer Associates, Sunderland. 400 p.
Fageria NK, Baligar VC and Jones CH. 1997. The role of essential nutrients on plant diseases. Growth and mineral nutrition of field crops. Marcel Dekker Inc., New York. 199-218p.
Fawe A, Abou-Zaid M, Menzies JG and Belanger RR. 1998. Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 88: 396-40. doi: 10.1094/PHYTO.1998.88.5.396.
Federación Nacional de Cultivadores de Cereales y Leguminosas (FENALCE). 2015. Área, producción y rendimiento cereal y leguminosas 2014 B. http://www.fenalce.org/nueva/plantillas/arch_down_load/APR_2014_B.pdf; consulta: Febrero 2016.
Gevrek MN, Atasoy GD and Yigit A. 2012. Growth and yield response of rice (Oryza sativa L.) to different seed coating agents. International Journal of Agriculture and Biology 14: 826–830.
Haghighi M and Pessarakli M. 2013. Influence of silicon and nano-silicon on salinity tolerance of cherrytomatoes (Solanum lycopersicum L.) at early growth stage. Scientia Horticulturae 161: 111–117. doi: 10.1016/j.scienta.2013.06.034
Hernández GR. 2002. Nutrición mineral de las plantas. Facultad de Ciencias Forestales y Ambientales. Universidad de los Andes-Mérida, Venezuela. En www.forest.ula.ve/-rubenhg, consulta: febrero 2016.
Kaya C, Tuna L and Higgs D. 2006. Effect of Silicon on Plant Growth and Mineral Nutrition of Maize Grown Under Water-Stress Conditions. Journal of Plant Nutrition 29(8): 1469-1480. doi: 10.1080/01904160600837238.
Liang Y, Sun W, Zhu Y and Christie G. 2007. Mechanisms of silicon mediated alleviation of abiotic stresses in higher plants: a review. Environmental Pollution 147(2): 422–428. doi: 10.1016/j.envpol.2006.06.008
Liu P, Yin L, Wang S, Zhang M, Deng X, Zhang S and Tanaka K. 2014. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environmental and Experimental Botany 111: 42–51. doi: 10.1016/j.envexpbot.2014.10.006.
Ma J and Takahashi E. 2002. Soil, Fertilizer, and Plant Silicon Research in Japan. First edition. Elsevier, Amsterdam. 281 p.
Ma J, Yamaji N and Mitani-Ueno N. 2011. Transport of silicon from roots to panicles in plants. Review. Proceedings of the Japan Academy. Series B, Physical and biological sciences 87(7): 377-385. doi: 10.2183/pjab.87.377.
Ma JF. 2004. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Journal Soil Science and Plant Nutrition 50(1): 11–18. doi: 10.1080/00380768.2004.10408447.
Marschner, P. 2012. Mineral nutrition of higher plants. 3nd. Edition. Elsevier. Oxford, UK. 645 p.
Menzies JG, Ehret DL, Glass AD, Helmer T, Koch C and Seywerd F. 1991. Effects of soluble silicon on the parasitic fitness of Sphaerotheca fuliginea on Cucumis sativus. Phytopathology 81: 84-88.
Mihaličová S, Ducaiova Z, Maslanakova I and Backor M. 2014. Effect of silicon on growth, photosynthesis, oxidative status and phenolic compounds of maize (Zea mays L.) grown in cadmium excess. Water, Air, & Soil Pollution 225: 1–11. Doi: 10.1007/s11270-014-2056-0.
Mitani N and Ma JF. 2005. Uptake system of silicon in different plant species. Journal Experimental Botany 56: 1255–1261. doi: 10.1093/jxb/eri121.
Nwugo C and Huerta A. 2008. Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice plants exposed to low-level cadmium. Plant Soil 311: 73–86.
Qinghua S, Zhiyi B, Zhujun Z, Yong H, Qiongqiu Q and Jingquan Y. 2005. Silicon-mediated alleviation of Mn toxicity in Cucumis sativus in relation to activities of superoxide dismutase and ascorbate peroxidase. Phytochemistry 66: 1551–1559. doi:10.1016/j.phytochem.2005.05.006.
Quero E. 2008. Silicio en la producción de chile. La biosilicificacion: Proceso biológico fundamental en la productividad vegetal. De riego: Protección y Nutrición de Hortalizas y Frutas 39: 72-76.
Richmond KE and Sussman M. 2003. Got silicon? The nonessential beneficial plant nutrient. Current Opinion in Plant Biology 6: 268–272. doi: 10.1016/S1369-5266(03)00041-4.
Roy A, Ali MV, Fox R and Silva J. 1971. Influence of calcium silicate on phosphate solubility and availability in Hawaiian Latosols. pp.757–765 In: Proceedings of the International Symposium on Soil fertility Evaluation, vol. 1, New Delhi, 815 p.
Rodrigues FA, Vale FCR, Korndorfer GH, Prabhu AS, Datnoff LE, Oliveira AMA and Zambolim L. 2003. Influence of silicon on sheath blight of rice in Brazil. Crop Protection 22: 23–29. doi: 10.1016/S0261-2194(02)00084-4.
Ruiz R and Rincón O. 2000. Cultivo de Maíz y Frijol. Temas de Orientación Agropecuaria. Tercera Edición. Bogotá-Colombia. 181 p.
Shekari F, Abbasi A and Mustafavi S. 2015. Effect of silicon and selenium on enzymatic changes and productivity of dill in saline condition. Journal of the Saudi Society of Agricultural Sciences. 1-8. doi: 10.1016/j.jssas.2015.11.006.
Snyder GH, Matichenkov VV, Datnoff LE. 2006. Silicon. pp 551–568. In: Barker A, Pilbeam D (eds.). Handbook of plant nutrition. Taylor and Frances, Boca Raton. 773 p.
Tahir M, Rahmatullah A, Aziz T and Ashraf M. 2010. Wheat genotypes differed significantly in their response to silicon nutrition under salinity stress. Journal Plant Nutrition 33: 1658–1671. doi: 10.1080/01904167.2010.496889.
Tavares LC, Rufino CA, Dorr CA, Barros AS and Peske ST. 2012. Performance of lowland rice seeds coated with dolomitic limestone and aluminum silicate. Revista Brasileira de Sementes 34: 202–211. doi: 10.1590/S0101-31222012000200003.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. David Fernando Torres-Hernández, Elberth Hernando Pinzón-Sandoval, Helber Enrique Balaguera-López, Amanda Silva-Parra, Jesús Hernando Galvis-Quintero. (2023). Responses of growth and yield of 'Diacol Capiro' potatoes to application of silicate fertilizer amendments . Revista Colombiana de Ciencias Hortícolas, 17(3) https://doi.org/10.17584/rcch.2023v17i3.16450.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2017 Revista Facultad Nacional de Agronomía Medellín

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.