Evaluation of bioactive compounds with functional interest from yellow pitahaya (Selenicereus megalanthus Haw)
Evaluación de componentes bioactivos con interés funcional a partir de pitahaya amarilla (Selenicereus megalanthus Haw)
DOI:
https://doi.org/10.15446/rfna.v70n3.66330Keywords:
Antioxidants, bioproducts, constipation, peristalsis, polyphenols (en)Antioxidantes, bioproductos, estreñimiento, peristaltismo, polifenoles (es)
Yellow Pitahaya (YP) is an exotic fruit, cataloged by Colombia International Corporation as a promising fruit to export, due to its sensorial and organoleptic attributes. In addition, this fruit has been associated with the control of constipation, and benefits for health because of its content of antioxidants. Therefore, the purpose of this work was to determine its chemical and biocompounds present in stem, peel, seed, and pulp of yellow pitahaya. The polyphenols were determined with the Folin-Ciocalteu method; the vitamin C content was determined using 2-nitroaniline; the antioxidant capacity was found with the ABTS and DPPH methods; and the peristalsis acceleration by measuring feces in biomodels (golden hamster) fed with various parts of this fruit. The results proved that the fruit composition varies according to its part. It is noteworthy that all parts of the fruit contain bioactive compounds in various proportions; the highest concentration of vitamin C, polyphenols and antioxidant capacity (AC) were found in the seed (22.08 mg ascorbic acid per g dry matter, 1580 mg gallic acid per 100 g dry matter, 79.2±0.2% ABTS, respectively) and peel (20.615 mg ascorbic acid per g dry matter, 1333.33 mg gallic acid per 100 g dry matter, 66.2±0.8% ABTS, respectively); regarding the peristalsis acceleration, the fecal production increased with the consumption of the seeds. Consequently, result showed that yellow pitahaya could be an alternative of a promising product due to its composition, and content of bioactive components of functional interest.
Recibido: 24 de junio de 2016; Aceptado: 15 de enero de 2017
ABSTRACT
Yellow Pitahaya (YP) is an exotic fruit, cataloged by Colombia International Corporation as a promising fruit to export, due to its sensorial and organoleptic attributes. In addition, this fruit has been associated with the control of constipation, and benefits for health because of its content of antioxidants. Therefore, the purpose of this work was to determine its chemical and biocompounds present in stem, peel, seed, and pulp of yellow pitahaya. The polyphenols were determined with the Folin-Ciocalteu method; the vitamin C content was determined using 2-nitroaniline; the antioxidant capacity was found with the ABTS and DPPH methods; and the peristalsis acceleration by measuring feces in biomodels (golden hamster) fed with various parts of this fruit. The results proved that the fruit composition varies according to its part. It is noteworthy that all parts of the fruit contain bioactive compounds in various proportions; the highest concentration of vitamin C, polyphenols and antioxidant capacity (AC) were found in the seed (22.08 mg ascorbic acid per g dry matter, 1580 mg gallic acid per 100 g dry matter, 79.2 ± 0.2% ABTS, respectively) and peel (20.615 mg ascorbic acid per g dry matter, 1333.33 mg gallic acid per 100 g dry matter, 66.2 ± 0.8% ABTS, respectively); regarding the peristalsis acceleration, the fecal production increased with the consumption of the seeds. Consequently, result showed that yellow pitahaya could be an alternative of a promising product due to its composition, and content of bioactive components of functional interest.
Keywords:
Antioxidants, Bioproducts, Constipation, Peristalsis, Polyphenols.RESUMEN
La pitahaya amarilla es una fruta exótica, catalogada por la Corporación Internacional Colombiana como una fruta promisoria para la exportación, debido a sus atributos sensoriales y organolépticos. Además, esta fruta ha sido asociada al control del estreñimiento, y con beneficios para la salud, gracias a su contenido de antioxidantes. Por consiguiente, el propósito de este trabajo fue evaluar las propiedades químicas y los biocomponentes presentes en tallo, cáscara, semilla y pulpa de pitahaya amarilla. La determinación de polifenoles se realizó siguiendo el método de Folin-Ciocalteu; el contenido de vitamina C por el método de 2-nitroanilina; la capacidad antioxidante se determinó por los métodos ABTS y DPPH; y la aceleración del peristaltismo mediante la medición de heces en biomodelos (hámsteres dorados), alimentados con diferentes partes de esta fruta. Los resultados demostraron que la composición de la fruta varía de acuerdo a sus partes. Es de destacar que todas las partes de la fruta contienen compuestos bioactivos en diferentes concentraciones; la concentración más alta de vitamina C, polifenoles y capacidad antioxidante se encontró en la semilla (22,08 mg ácido ascórbico por g materia seca, 1580 mg ácido gálico por 100 g materia seca, 79,2 ± 0,2% ABTS, respectivamente) y cáscara (20,615 mg ácido ascórbico por g materia seca, 1333,33 mg ácido gálico por 100 g materia seca, 66,2 ± 0,8% ABTS, respectivamente); respecto a la aceleración del peristaltismo, la producción de heces incrementó al consumir semillas de pitahaya. De acuerdo a lo anterior, se concluye que la pitahaya amarilla podría ser una alternativa de un producto promisorio, debido a su composición y contenido de compuestos bioactivos de interés funcional.
Palabras clave:
Antioxidantes, Bioproductos, Estreñimiento, Peristaltismo, Polifenoles.In the last decade the world trend toward consumption of food with additional benefits as well as their nutrients (Bello, 2000) has increased because they have the ability to prevent and cure certain diseases (Dembitsky et al., 2011), and are associated with the consumers desire for a better quality of life (Diplock et al., 1999). This type of food is named functional foods and are defined as those of vegetal or animal origin that when consumed in daily diet provide biocompounds, which cause therapeutic effects in the body, resulting in benefit for health (Roberfroid, 1999). A functional food may be then a natural one or modified to influence health through addition or modification of any specific component (Howlett, 2008). According to the above, this type of food provide specific effects related to particular components (Labrecque and Doyon, 2008). Some of such components and benefits contained in these foods are polyphenols, vitamin C, antioxidant capacity (AC), and peristalsis acceleration.
Polyphenols are generally found in all vegetal origin foods and form a wide group of substances that include families of compounds with various structures which have the same characteristic of including in their structure various benzenic groups substituted by hydroxylic functions (Peris et al., 1995), they have antioxidant and antimutagenic properties; delay senescence, anticancer, and antimicrobial (Zloch, 1996). These compounds inhibit oxidation of β-carotenes catalyzed by myoglobin and those produced by Fe-ascorbic acid system (Rice-Evans et al., 1996). In addition, vitamin C improves endothelial dysfunction (May, 2000), by stabilizing tetrahydrobiopterin, a cofactor of oxide nitric synthase endothelial enzyme by increasing nitric oxide availability (Huang et al., 2000; Li and Schellhorn, 2007). Vitamin C, likewise, prevents apoptosis of the endothelial cells by intracellularly inhibiting caspase-9 (tumor inhibiting proteins), and the endothelial ET-1 function (endotheline) and preventing IL-6 release (interleucin-6) (Böhm et al., 2007; Dhar-Mascareño et al., 2005).
Taking into account the above reported effects, yellow pitahaya (YP) (Selenicereus megalanthus Haw) may be considered as a source of functional biocompounds, because its peel and even its stem are used to obtain benefits for health (CCI, 2006), and its components include polyphenols (Wu et al., 2006), vitamin C (Serna Cock et al., 2013), among other. Wu et al. (2006) evaluated the polyphenols content and the antioxidant activity of red pitahaya, in addition to its anti-proliferating activity in melanoma cancer cells, finding a high content of these compounds and inhibition of growth of such cells. In addition, YP has effects on peristalsis acceleration (Parra Yambay, 2010) associated with constipation decrease, which is the cause of multiple medical consultations (Fleming and Wade, 2010). Traditionally, the amount of carbohydrate available for colonic bacterial fermentation is determined by the amount of dietary fibre present in foods. However, some of the 'available carbohydrate' (i.e., ''available'' for small intestinal absorption: total carbohydrate minus dietary fibre) in many foods may escape digestion in the small intestine in appreciable amounts and become available for fermentation by the colonic micro flora (Huebner et al., 2007; Greger, 1999) cited by (Mohd et al., 2014).
This fruit is mainly produced in tropical and subtropical countries with a dry climate and 18 - 28 °C temperature; it has very specific features such as its exuberant yellow thorny peel, and white pulp with many small seeds (Nerd et al., 2002); it has been considered by Colombia International corporation - CCI as a promising fruit for export due to its sales price and exotic features (Mosquera et al., 2011). In the world 1083 hectares are cultivated, of which 827 are in Colombia (76.4%), 100 ha in Israel (9.2%), Brazil participates with 3.2% and Ecuador with 1.9%, other countries participate with 9.3% (Betancourt et al., 2010). In addition, Colombia participates with 43.7% of the international exportations (Mosquera et al., 2011).
According to the above mentioned, the purpose of this investigation was to establish chemical and functional characteristics in the various parts of YP, in ripeness state 6, third quality, in order to determine its possible use in the production of functional food. For such purpose, an empirical-analytical research was carried out using experimental units, (YP), provided by the society of producers of yellow pitahaya - Asoppitaya (Colombia).
MATERIAL AND METHODS
Fruit samples
The research was performed using third quality YP (Selenicereus magalanthus Haw), in ripeness state 6 (ICONTEC, 1996), from the state of Valle del Cauca, provided by the society of producers of yellow pitahaya -Asoppitaya. The external appearance of the fruit pieces was analyzed; however, fruit pieces affected by mechanical damage or fungi, which constitute third category, were used.
Fruit pieces were cleaned and disinfected, starting by washing them with tap water, using a soft brush to remove thorns from the peel; then they were disinfected by immersion for 10 minutes into a solution with a concentration of 2.5 mL L-1 of citrosan (Diken International, Mexico).
The stem, the peel, the pulp and seeds were manually separated; each part was separately dried with hot air (Binder, USA) at 45 °C for 24 hours. The dry products were pulverized in a blade grinder, and stored in high density polyethylene bags at room conditions (58% HR, 20.5 °C y 3300 lux), for later use in the determination of functional properties.
Chemical analysis
The total soluble solids (TSS), pH and the total titratable acidity (TTA) were determined in the homogenized and filtrated juice extracted from various parts of the pitahaya. The TSS were estimated by using a refractometer (ABBE DR-A1, Atago, Bellevue, Washington), following the AOAC 932.12 method. The pH was estimated with the potentiometric method, using a pH-meter (Hanna Instruments). The TTA was found by using AOAC 942.15A official method, by means of an acid-base titration using a solution of NaOH 0.1N until a pH of 8.1 was reached; acidity was expressed as percentage of citric acid.
Preparation of antioxidant and polyphenol extracts
The extracts were obtained parting from the four parts of YP: pulp, seed, peel, and stem, following the methodology reported by Jara-Palacios et al. (2014), with modifications. 25 mL of ethanol were taken at 80%, and 5 g of dry sample; the mixture was stirred for one hour at 1.26 g in a shaker; then centrifuged for 25 minutes in a centrifuge (Vebmlw T51, USA); the three supernatants were separated, and the process was repeated for three times. The three supernatants were mixed and stored in amber bottles for the later quantification of total polyphenols, vitamin C and AC.
Total polyphenol and vitamin C content
The total polyphenols were quantified with the Folin-Ciocalteu method (Jara-Palacios et al., 2014). 0.25 mL of extract, 0.25 mL of reagent Folin-Ciocalteu (Merck, Colombia), and 3.25 mL of 20% sodium carbonate were used. The mixture was shaked for 90 minutes at 1.26 g, and stored for two hours in dark condition for subsequent determination of polyphenols by spectrophotometry using a Genesys 10 UV-vis HP spectrophotometer (Thermoscientific, USA) at a wavelength of 765 nm. The results were expressed in mg of galic acid (GAE per 100 g of dry sample). Vitamin C was determined with the colorimetric method of the 2-nitrianilin (Bernal, 1993).
Antioxidant capacity
The antioxidant ability was determined by two methods ABTS (acid 2,2'-azino-bis 3-ethylbenzotiazolin-6-sulphonic) and DPPH (2,2-difenil-1-picril hydrazilo) based on the electron transference method, which involves a redox reaction with the oxidant as an indicator of the endpoint of recation (Tovar-del Río, 2013). An ABTS•+ radical cation was produced which directly produced a green-blue ABTS chromophore through the reaction between ABTS•+ and potasium persulfate, which presents a maximum absorption at 734 nm. The ABTS•+ radical cation was produced from a reaction between a solution 7mM of ABTS with potassium persulfate (2.45 mM). The mixture was taken into darkness for 16 hours, then it was diluted by using ethanol (Emsure® ACS, ISO, Reag. Ph. EUr) until achieving an absorbance of 0.715 + 0.005 to 732 nm.
Then, 50 µL de ABTS•+ were taken and mixed with 1450 µL of the solution for analysis; the mixture was kept in darkness for 30 minutes to facilitate the reaction and then the absorbance was read at 732 nm in a Genesys 10 UV-vis HP spectrophotometer (Thermoscientific, USA). The addition of antioxidant caused a decoloration associated with inhibition of the radical in function of concentration and the time; reading was performed at constant time of reaction of 30 minutes. AC expressed on percentage was calculated through equation (1).
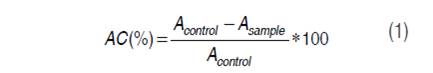 Where: AC = Antioxidant capacity expressed in percentage Acontrol = Absorbance of negative control (solution ABTS) Asample = Absorbance of the sample read at 732 nm
Where: AC = Antioxidant capacity expressed in percentage Acontrol = Absorbance of negative control (solution ABTS) Asample = Absorbance of the sample read at 732 nm
The measurement of AC by DPPH was performed based on the decoloration of the radical, absorbance was measured at 529 nm, and was determined with spectrophotometry, following the method reported by Geng et al. (2015), with some modifications; 1.4 mg of DPPH reagent were used and it was dissolved in 70 mL of methanol until achieving an absorbance of 1.174 measured with a Genesys 10 UV-vis HP spectrophotometer (Thermoscientific, USA) at 529 nm. 2 and 0.5 mL of DPPH reactive and the sample respectively were taken and left in reaction for 30 minutes. AC was quantified similarly to the one reported for ABTS method following equation 1.
The antioxidant activity of extracts evaluated through the two methods (ABTS and DPPH), was expressed in AC in trolox equivalent (TEAC), which represents a trolox solution concentration with the same AC of free radicals as the extract. It was obtained based on the regression equation obtained from a standard curve with the antioxidant reference trolox (equation 2) (Tovar-del Río, 2013; Zhu et al., 2015).

Peristalsis acceleration
Three-month-old golden hamsters with about 100.75 g were used to quantify the peristalsis acceleration. The biotypes were obtained from ICESI University (Colombia), and kept under controlled laboratory conditions of temperature (26 °C) and illumination cycles of 12 hours. The hamsters were fed with 10 + 2 g of sunflower seeds and 70 mL of water approximately every day.
Four groups of 9 hamsters, including male and female, were organized; each group was assigned a part of the YP (seed, peel, stem and pulp), as a dietary complement. The parts of the YP were provided orally, which account for 30% of the daily feeding dose. Immediately after feeding the YP the biotypes were observed in their general aspect, checking health conditions, in compliance with legal rules (Salud, 1993).
The observed patterns were hair appearance, activity, weight, production and characteristics of fecal material, water amount, and consumed food. A numerical scale was applied based on feces consistence, as follows: 1 (Solid), 2 (Semi-solid), 3 (Liquid), and variation in feces production was calculated, by using equation 3.
 Mfi = Mass of initial feces Mff = Mass of final feces
Mfi = Mass of initial feces Mff = Mass of final feces
Statistical analysis and experimental design
A single factor design with 4 levels was used, the evaluated variable was a part of YP with four levels; stem, peel, pulp and seed. The effect of part of YP was determined by the analysis of variance (ANOVA) and mean comparisons with the Tuckey test at 5% probability using Stathgraphics® statistical program. All determinations were performed in triplicate.
RESULTS AND DISCUSSION
Chemical analysis
The pH, total soluble solids (TSS), and total titratable acidity (TTA) of stem, peel, pulp and seed of YP are presented in Table 1. The variations of pH for the various parts of YP, are seen here due to the effect of the part of the fruit, which is higher in the peel (P<0.05); Rodriguez et al. (2005) found that YP in ripeness state 3 has a pH of 4.7, which changes to pH 5.1 during refrigerated and non-refrigerated storage. In a mixture of seeds and pulp of YP under refrigerated storage a pH between 4.1 and 4.8 is reported, with an increase during storage (Serna et al., 2013). The peel of the fruit shows a higher pH which may be associated with a different composition regarding the fruit. These results are correlated with the total titratable acidity, expressed in the percentage of citric acid, since values of a higher pH for the peel show a lower degree of acidity (lower TTA); this case is contrary to the pH in the pulp which is lower and indicates higher amount of acids.
Table 1: Chemical properties and biocompounds of yellow pitahaya.

TTA values are lower than those reported by Serna et al. (2012, 2013) in YP in ripeness state 3; the difference may be caused by the metabolism of organic acids (Davies and Maw, 1972) which are consumed in oxidating reactions during senescence (Neves et al., 2008). Regarding TSS, the values were higher in the pulp, followed by those in the seed, and the lowest ones were found in the peel of the fruit. Statistically significant difference was found between the pulp, seed and peel (P<0.05).
In the pulp of YP values of 17.7 °Brix were found, which are similar to those found in YP in ripeness state 3 after 27 days of refrigerated storage (Serna et al., 2013); such difference may be explained by metabolism of the fruit, associated with the energy consumption in a form of ATP and other compounds to maintain homeostasis of the fruit (Lima et al., 2011); the above include conversion of starch into glucose and fructose, and their use through metabolic routes such as substrate for respiration (Paliyath et al., 2008).
Chemical composition of YP (TSS, TTA, pH), varies according to the part of the fruit. Nerd and Mizrahi (1999) reported difference in water content of the pulp and the peel of YP and, therefore, difference in dry matter.
Total polyphenol and vitamin C content
Experimental results of total polyphenols for each analyzed part of YP are presented in figure 1a. It may be observed that polyphenols content varied between 600 and 1580 mg GAE per 100 g dry matter, the higher content being in the seed, followed by the peel. Difference found was statistically significant (P<0.05).
Figure 1: Bioactive compounds found in the parts of yellow pitahaya: A. Total polyphenols content. B. Vitamin C content.
The polyphenol content was found in all parts of analyzed YP; however, higher amount of this bio-compound was found in the seed (1580 mg GAE per 100 g dry matter), representing an important source of antioxidants.
As in the YP, other studies have reported the similar values of polyphenol, like apples (Malys pumilla) with 1600 to 1800 GAE per 100 g dry matter (Beltrán-Orozco et al 2009), and strawberry (Fragaria ananassa) with 1600 to 1800 GAE per 100 g dry matter (Beltrán-Orozco et al., 2009). In addition, Daza (2014) reports in the pulp of YP 77.6 + mg GAE per 100 g dry matter; 102.0 + mg GAE per 100 g dry matter in peel, and 202.7 + 1.1 mg GAE per 100 g dry matter in seed (Daza, 2014), these values being lower than those found in this work.
The results of vitamin C for peel, pulp, seed and stem of YP are shown in Figure 1b. Significant difference between the parts of pitahaya were observed (P<0.05). The highest content of ascorbic acid was found in the seed (22.0881 mg Ascorbic Ac. per g dry matter). The results reported by Beltrán-Orozco et al (2009) showed that the content of ascorbic acid in YP is 17.04 + 0.30 mg of Ascorbic acid per g dry matter; in red pitahaya, cherry and white pitahaya the values are, 10.25+0.25, 8.49 + 0.25 and 14.56 + 0.25 mg of Ascorbic acid per g dry matter respectively (Beltrán-Orozco et al., 2009). Muñoz-de-Chavez et al. (2002) reports that 100 g of eatable pulp of YP represents 21% of daily consumption of Vitamin C as recommended for adults (60 mg of Ascorbic Acid per g of dry matter).
Antioxidant capacity
The Table 1 shows the results of analysis of AC performed to the parts of YP (peel, pulp and seed). Highly significant difference was obtained among analyzed parts (P<0.05). It may be observed that AC is higher than 62% for the various parts of YP. High values of AC are found in all parts of the fruit, however these values are higher in the seed, with values of 79.2 and 96% for tests with ABTS and DPPH, respectively. Ayala-Camarillo et al. (2008) reports the AC in yellow pitahaya of 58.96 + 0.55.
Likewise, epidemiological studies have suggested that consumption of a diet rich in biocompounds such as polyphenols provides protection against cancer, diabetes, hypertension, cardiovascular diseases, aging, and other (Pandey and Rizvi, 2009). However, a daily recommended dose of consumption (RDC) for antioxidants has not been defined yet.
The results suggest that the YP is a source of health protection compounds, against free radicals reducing the risk of chronic diseases such as cancer (Ayala-Camarillo et al., 2008).
Acceleration ability of the peristalsis
A statistically significant difference is observed (P<0.05) between pitahaya samples; the seed is the part containing higher acceleration ability of the peristalsis by increasing 55% the amount of feces produced with respect to biomodels fed with a traditional diet (sunflower seed) (Table 2). Laxative ability was also found in pitahaya pulp and stem, otherwise the peel causes decrease of feces produced; therefore, its consumption is not considered suitable to stimulate feces production of the evaluated biomodels.
Table 2: Acceleration ability of peristalsis of yellow pitahaya and appearance of feces according to treatment applied to biomodels.

Regarding feces consistence, the consumption of the pulp and seed changes their appearance to a less solid behavior which could be related to easiness of expulsion; otherwise, the stem and the peel do not produce any changes which may reduce dehydration in the consumers.
The pitahaya pulp consists of highly viscous carbohydrate fibers (cellulose, hemicellulose, fructooligosaccharides and simple sugars), vitamin C and minerals (Nur'aliaa et al., 2010a) cited by (Dasaesamoh et al., 2016). The oligosaccharides as reported by Dasaesamoh et al. (2016) are metabolized by intestinal microbiota and subsequently converted into short chain fatty acids: acetic acid, propionic, butyric acid, lactic acid and butyrate. Butyrate is responsible for some important functions in the intestinal epithelium, such as prevention of certain types of colitis (Scheppach, 1994, cited by Dasaesamoh et al., 2016). In addition, the oligosaccharides present in pitahaya become substances that increase motility by reducing diseases such as colitis and irritable bowel syndrome (Dasaesamoh et al., 2016).
Parra Yambay (2010) reported laxative properties of seed and stem of YP, with absence of liquid feces, therefore, the authors recommend its use and incorporation to nutrition. The AC and laxative properties of parts of YP suggest that the seed could be an important source of biocompounds. Due to its high polyphenolic content, it is possible to conclude that the peel of YP could be a source of biocompounds for functional foods, as well as the seed, turning into an alternative for development products with add value.
CONCLUSIONS
Composition of YP varies in function of the part (stem, peel, pulp and seed), although there are bioactive compounds with a functional interest in all parts. The seed may be consumed as a potential alimentary additive. The peel could represent an alternative of use for this byproduct, due to its content of vitamin C and polyphenols that may be extracted and incorporated as functional ingredients to develop new food, for which purpose subsequent research is required.
ACKNOWLEDGEMENTS
The authors express their acknowledgement to the program Jóvenes Investigadores y Semillero de Investigación - Departamento Administrativo de Ciencia, Tecnología e Innovación - Colciencias, for funding this project.
REFERENCES
References
Association of Official Analytical Chemists – AOAC. 2005. Official methods of analysis of AOAC International (17th ed.). AOAC International, Gaithersburg.
Ayala-Camarillo K, Gallardo-Velázquez T and Beltrán-Orozco M. 2008. Determinación del contenido de fenoles totales y de la capacidad antioxidante de tres especies del fruto de la pitaya (Stenocereus griseus H.). En: Memorias V Congreso Internacional de Ingeniería Bioquímica, XVI Congreso Nacional de Ingeniería Bioquímica, VI Jornadas Científicas de Biomedicina y Biotecnología Molecular. Mexico, D.F.
Bello J. 2000. Alimentos con propiedades saludables especiales. pp. 343-356. En: Astiasarán I, Martínez J (eds.). Alimentos: composicion y propiedades McGraw-Hill/Interamericana, Madrid, España.
Beltrán-Orozco M, Oliva-Coba T and Gallardo-Velázquez T. 2009. Ascorbic acid, phenolic content, and antioxidant capacity of red, cherry, yellow and white types of pitaya cactus fruit (Stenocereus stellatus Riccobono). Agrociencia 43(2): 153-162.
Bernal I. 1993. Análisis de alimentos. Academia Colombiana de Ciencias Exactas, Físicas y Naturales, Bogotá. 313,p.
Böhm F, Settergren M and Pernow J. 2007. Vitamin C blocks vascular dysfunction and release of interleukin-6 induced by endothelin-1 in humans in vivo. Atherosclerosis 190(2): 408-415. doi: 10.1016/j.atherosclerosis.2006.02.018
Corporación Colombia Internacional, CCI. 2006. Perfil de producto, pitaya amarilla. Inteligencia de Mercados, Bogotá, 16 p.
Dasaesamoh R, Youravong W and Wichienchot S. 2016. Digestibility , fecal fermentation and anti-cancer of dragon fruit oligosaccharides. International Food Research Journal 23(6): 2581–2587.
Davies J and Maw G. 1972. Metabolism of citric and malic acids during ripening of tomato fruit. Journal of the Science of Food and Agriculture 23(8): 969-976. doi: 10.1002/jsfa.2740230808
Daza L. 2014. Evaluación de propiedades antioxidantes de parte comestible y no comestible de pitahaya, uchuva y mangostino. Biotecnología en el Sector Agropecuario y Agroindustrial 12(1): 98-105.
Dembitsky V, Poovarodom S, Leontowicz H, Leontowicz M, Vearasilp S, Trakhtenberg S and Gorinstein S. 2011. The multiple nutrition properties of some exotic fruits: biological activity and active metabolites. Food Research International 44(7): 1671-1701. doi: 10.1016/j.foodres.2011.03.003
Dhar-Mascareño M, Cárcamo J and Golde D. 2005. Hypoxia–reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Radical Biology and Medicine 38(10): 1311-1322. doi: 10.1016/j.freeradbiomed.2005.01.017
Diplock A, Aggett P and Ashwell M. 1999. Scientific concepts of functional foods in Europe: Consensus document. British Journal of Nutrition 81(1): 1-27.
Fleming V and Wade W. 2010. A review of laxative therapies for treatment of chronic constipation in older adults. The American Journal of Geriatric Pharmacotherapy 8(6): 514-550. doi: 10.1016/S1543-5946(10)80003-0
Geng D, Chi X, Dong Q and Hu F. 2015. Antioxidants screening in Limonium aureum by optimized on-line HPLC–DPPH assay. Industrial Crops and Products 67: 492-497. doi: 10.1016/S1543-5946(10)80003-0
Howlett J. 2008. Functional foods: from science to health and claims. ILSI Europe, Brussels, Belgium 36 p.
Huang A, Vita J, Venema R and Keaney J. 2000. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. Journal of Biological Chemistry 275(23): 17399-17406. doi: 10.1074/jbc.M002248200
Instituto Colombiano de Normas Técnicas y Certificaciones - ICONTEC. 1996. Norma técnica colombiana 3554: Frutas frescas, pitahaya amarilla. NTC, Bogotá, Colombia.
Jara-Palacios M, Hernanz D, González-Manzano S, Santos-Buelga C, Escudero-Gilete M and Heredia F. 2014. Detailed phenolic composition of white grape by-products by RRLC/MS and measurement of the antioxidant activity. Talanta 125: 51-57. doi.10.1016/j.talanta.2014.02.065
Labrecque J and Doyon M. 2008. Functional foods: a conceptual definition. British Food Journal 110(11): 1133-1149. doi: 10.1108/00070700810918036
Li Y and Schellhorn H. 2007. New developments and novel therapeutic perspectives for vitamin C. Journal of Nutrition 137(10): 2171-2184.
Lima F, Saavedra J, Marcos E and Kluge R. 2011. Pós-colheita de lichia ‘Bengal’ tratada com etileno e 1-metilciclopropeno. Ciência Rural 41(7): 1143-1149.
May J. 2000. How does ascorbic acid prevent endothelial dysfunction?. Free Radical Biology and Medicine 28(9): 1421-1429.
Ministerio de Salud. 1993. Título V. La investigación biomédica con animales. En: Resolución 008430 de 1993, El Ministerio, Bogotá.
Mohd Adzim R, Che Abdullah A and Abdul Manaf A. 2014. Isolation and characterization of oligosaccharides composition in organically grown red pitaya, white pitaya and papaya. International Journal of Pharmacy and Pharmaceutical Sciences 6 (Suppl. 2): 131–136.
Mosquera H, Betancourt B, Castellanos J and Perdomo L. 2011. Vigilancia Comercial de la cadena productiva de la pitaya amarilla. Cuadernos de Administración 27(45): 75-93.
Muñoz-de Chavez M, Ledesma Solano J, Chávez Villasana A. 2002. Tablas de valor nutritivo de alimentos: los alimentos y sus nutrientes. McGraw Hill Interamericana, México. 203 p.
Nerd A and Mizrahi Y. 1999. The effect of ripening stage on fruit quality after storage of yellow pitaya. Postharvest Biology and Technology 15(2): 99-105. doi: 10.1016/S0925-5214(98)00080-5
Nerd A, Tel-Zur N, Mizrahi Y. 2002. Fruits of vine and columnar cacti. Chapter 12. In: P.S. Nobel (ed.). Cacti: Biology and uses. University of California Press, Berkeley. 280 p.
Neves L, Moreno R, da Silva V, Lopes R and Ruffo S. 2008. Dano de frio em limas-ácidas Tahiti, colhidas em diferentes épocas e submetidas a tratamentos térmicos e bioquímicos. Revista Brasileira de Fruticultura 30(2): 377-384. doi: 10.1590/S0100-29452008000200019
Paliyath G, Murr D, Handa A and Lurie S. 2008. Postharvest biology and technology of fruits, vegetables and flowers. Wiley-Blackwell, New Delhi. 498 p.
Pandey K and Rizvi S. 2009. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity 2(5): 270-278. doi: 10.4161/oxim.2.5.9498
Parra Yambay M. 2010. Tamizaje fitoquímico y determinación de la actividad laxante de tallos y semillas de pitahaya (Hylocereus triangularis). Tesis de grado Bioquímico Farmacéutico. Facultad de Ciencias, Escuela Superior Politécnica de Chimborazo, Riobamba, Ecuador. 123 p.
Peris J, Stubing G and Vnaglosha B (eds.). 1995. Heterósidos, pp. 61-73. In: Fitoterapia aplicada. Primera edición, Editorial MICOF, Valencia, 1995. 678 p.
Roberfroid M. 1999. Concepts in functional foods: The case of inulin and oligofructose. Journal of Nutrition 129(7): 1398-1401.
Rice-Evans C, Miller N and Paganga G. 1996. Structureantioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine 20(7): 933-956. doi: 10.1016/0891-5849(95)02227-9
Rodríguez D, Patiño M, Diego M, Fischer G and Galvis J. 2005. Efecto de dos índices de madurez y dos temperaturas de almacenamiento sobre el comportamiento en poscosecha de la pitahaya amarilla (Selenicereus megalanthus Haw). Revista Facultad Nacional de Agronomía 58(2): 2837-2857.
Serna L, Torres L and Ayala A. 2012. Effect of pre- and postharvest application of 1-methylcyclopropene on the maturation of yellow pitahaya (Selenicerus megalanthus Haw). Vitae 19(1): 49-59.
Serna L, Torres L and Ayala A. 2013. Physical, chemical and sensory changes of refrigerated yellow pitahaya treated preharvest with 1-MCP. Dyna 80(178): 11-20.
Tovar-Del Río J. 2013. Determinación de la actividad antioxidante por DPPH y ABTS de 30 plantas recolectadas en la ecoregión cafetera. Trabajo de grado Químico Industrial. Facultad de Tecnología. Universidad Tecnológica de Pereira, Pereira. 150 p.
Wu L, Hsu H, Chen Y, Chiu C, Lin Y and Ho J. 2006. Antioxidant and antiproliferative activities of red pitaya. Food Chemistry 95(2): 319-327. doi: 10.1016/j.foodchem.2005.01.002
Zhu Q, Nakagawa T, Kishikawa A, Ohnuki K and Shimizu K. 2015. In vitro bioactivities and phytochemical profile of various parts of the strawberry (Fragaria × ananassa var. Amaou). Journal of Functional Foods 13: 38-49. doi: 10.1016/j.jff.2014.12.026
Zloch Z. 1996. The role of dietary plants polyphenols in health maintenance. US National Library of Medicine 135(3): 84-88.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. M.P. Díaz-Trujillo, D.S. Villa-Fonseca, O. Álvarez-Solano, I. Hernando Hernando, V. Larrea, M. Hernández-Carrión. (2023). Formulación de un producto lácteo a partir de pitahaya amarilla y sus subproductos. Investigación y Desarrollo en Ciencia y Tecnología de Alimentos, 8(1), p.697. https://doi.org/10.29105/idcyta.v8i1.91.
2. Ives Yoplac, Carla Julissa Canicoba-Rubio, River Chávez-Santos, Marilu Mestanza, Jorge L. Maicelo-Quintana, Alyssa Hidalgo, Cong Wang. (2025). Influence of Maturity Stage and Storage Time on Physicochemical and Bioactive Properties of Yellow Pitahaya (Hylocereus megalanthus). Journal of Food Processing and Preservation, 2025(1) https://doi.org/10.1155/jfpp/2494113.
3. Jessica Sanmiguel, Valdemar Andrade, Yadira Vargas-Tierras, Iván Samaniego, Fernando Paredes-Arcos, Wilson Vásquez-Castillo, William Viera-Arroyo. (2025). Physical-Chemical Characterization of Fruit Harvested at Different Maturity Stages of Grafted Yellow Pitahaya (Selenicereus megalanthus Haw.). Plants, 14(2), p.178. https://doi.org/10.3390/plants14020178.
4. Alejandra Sanín, Diana P. Navia, Johanna A. Serna-Jiménez. (2020). Functional Foods from Crops on the Northern Region of the South American Andes: The Importance of Blackberry, Yacon, Açai, Yellow Pitahaya and the Application of Its Biocompounds. International Journal of Fruit Science, 20(sup3), p.S1784. https://doi.org/10.1080/15538362.2020.1834894.
5. Miguel Ángel Castillo Reina, Nely Pérez Martínez, Iván David Ruiz Rosas . (2023). Pitahaya amarilla (Selenicereus megalanthus) en Miraflores, Boyacá, Colombia. Una perspectiva de capital social en una asociación de productores . Acta Agronómica, 71(4) https://doi.org/10.15446/acag.v71n4.100152.
6. Antonio Obregón-La Rosa, Eliana Contreras-López, Carlos Elías-Peñafiel, Ana Muñoz-Jauregui, Ricardo Yuli-Posadas, Edwin Cóndor-Salvatierra. (2021). Nutritional and physicochemical profile of the pitahaya cultivated in the central coast of Peru. Revista de la Facultad de Agronomía, Universidad del Zulia, 39(1), p.e223911. https://doi.org/10.47280/RevFacAgron(LUZ).v39.n1.11.
7. Daniel Valero, Alex Erazo-Lara, María Emma García-Pastor, Pedro Antonio Padilla-González, Vicente Agulló, Fátima Badiche El-Hiali, María Serrano. (2025). Yellow Pitahaya (Selenicereus megalanthus Haw.): The Less Known of the Pitahayas. Foods, 14(2), p.202. https://doi.org/10.3390/foods14020202.
8. Frank L. Romero-Orejon, Ana María Muñoz, Luciana de la Fuente-Carmelino, Diana Jimenez-Champi, Eliana Contreras-López, Ivan Best, Luís Aguilar, Fernando Ramos-Escudero. (2022). Secondary Metabolites - Trends and Reviews. https://doi.org/10.5772/intechopen.102419.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2017 Revista Facultad Nacional de Agronomia

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.























