Antioxidant activity and GC-MS profile of Conyza bonariensis L. leaves extract and fractions
Actividad antioxidante y perfil CG-EM del extracto y fracciones de hojas de Conyza bonariensis L.
DOI:
https://doi.org/10.15446/rfnam.v73n3.81452Keywords:
bioactive compounds, DPPH, flavonoid, fractionation, FRAP, polyphenol (en)DPPH, flavonóide, fracionamento, FRAP, polifenol (pt)
compuestos bioactivos, DPPH, flavonoide, fractionamiento, FRAP, polifenoles (es)
Downloads
The 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing power (FRAP), and semivolatile compounds of Conyza bonariensis L. leave extract and fractions are discussed. A methanolic crude extract was obtained through maceration, and subsequently, n-hexane, chloroform, and ethyl acetate fractions were collected using a solvent-solvent partition. Total phenols, flavonoids, and antioxidant activity assays were performed in an ultraviolet-visible (UV-Vis) spectrophotometer, and the results were expressed as Gallic Acid, Quercetin, and Trolox equivalents respectively. The findings achieved indicate that ethyl acetate fraction showed the highest DPPH radical scavenging capacity (90.69±3.16%) at 500 µg mL-1, and reduced the ferric tripyridyltriazine complex (Fe3+-TPTZ) with values between 19.68 and 2,355.37 mg Trolox equivalent (TE) g-1. It was identified 28 phytoconstituents through Gas chromatography-mass spectrometry (GC-MS). The scavenging activity of ethyl acetate fraction could be correlated mostly to the presence of eugenol, trans-isoeugenol, lucenin-2, methyl salicylate, and syringic acid. This study reveals that the ethyl acetate fraction could be used as a good source of antioxidants for health benefits.
El 2,2-difenil-1-picrilhidrazil (DPPH), el poder reductor férrico (FRAP) y los compuestos semivolátiles del extracto y fracciones de Conyza bonariensis L. son discutidos. Se obtuvo un extracto crudo en metanol mediante maceración y posteriormente se recogieron fracciones de n-hexano, cloroformo y acetato de etilo usando partición disolvente-disolvente. Los ensayos de fenoles totales, flavonoides y actividad antioxidante se efectuaron en un espectrofotómetro (UV-Vis) y los resultados se expresaron como equivalentes de Ácido gálico, Quercetina y Trolox, respectivamente. Los resultados indican que la fracción en acetato de etilo mostró la mayor capacidad de eliminación del radical DPPH (90.69±3.16%) a 500 µg mL-1 y redujo el complejo de tripiridiltriazina férrico (Fe3+-TPTZ) con valores entre 19.68 y 2355.37 mg equivalente de Trolox (TE) g-1. Se identificaron 28 fitoconstituyentes a través del análisis de cromatografía de gases-espectrometría de masas (CG-EM). La actividad antioxidante presentada puede correlacionarse principalmente con la presencia de eugenol, trans-isoeugenol, lucenina-2, salicilato de metilo y ácido siríngico detectado. Este estudio muestra que la fracción de acetato de etilo podría ser utilizada como una buena fuente de antioxidantes para el beneficio de la salud humana.
Recibido: 2 de agosto de 2019; Aceptado: 9 de abril de 2020
ABSTRACT
The 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing power (FRAP), and semivolatile compounds of Conyza bonariensis L. leave extract and fractions are discussed. A methanolic crude extract was obtained through maceration, and subsequently, n-hexane, chloroform, and ethyl acetate fractions were collected using a solvent-solvent partition. Total phenols, flavonoids, and antioxidant activity assays were performed in an ultraviolet-visible (UV-Vis) spectrophotometer, and the results were expressed as Gallic Acid, Quercetin, and Trolox equivalents, respectively. The findings achieved indicate that ethyl acetate fraction showed the highest DPPH radical scavenging capacity (90.69±3.16%) at 500 µg mL-1, and reduced the ferric tripyridyltriazine complex (Fe3+-TPTZ) with values between 19.68 and 2,355.37 mg Trolox equivalent (TE) g-1. It was identified 28 phytoconstituents through Gas chromatography-mass spectrometry (GC-MS). The scavenging activity of ethyl acetate fraction could be correlated mostly to the presence of eugenol, trans-isoeugenol, lucenin-2, methyl salicylate, and syringic acid. This study reveals that the ethyl acetate fraction could be used as a good source of antioxidants for health benefits.
Keywords:
Bioactivity, DPPH, Flavonoid, Fractionation, FRAP, Polyphenol.RESUMEN
El 2,2-difenil-1-picrilhidrazil (DPPH), el poder reductor férrico (FRAP) y los compuestos semivolátiles del extracto y fracciones de Conyza bonariensis L. son discutidos. Se obtuvo un extracto crudo en metanol mediante maceración y posteriormente se recogieron fracciones de n-hexano, cloroformo y acetato de etilo usando partición disolvente-disolvente. Los ensayos de fenoles totales, flavonoides y actividad antioxidante se efectuaron en un espectrofotómetro (UV-Vis) y los resultados se expresaron como equivalentes de Ácido gálico, Quercetina y Trolox, respectivamente. Los resultados indican que la fracción en acetato de etilo mostró la mayor capacidad de eliminación del radical DPPH (90,69±3,16%) a 500 μg mL-1 y redujo el complejo de tripiridiltriazina férrico (Fe3+-TPTZ) con valores entre 19,68 y 2355,37 mg equivalente de Trolox (TE) g-1. Se identificaron 28 fitoconstituyentes a través del análisis de cromatografía de gases-espectrometría de masas (CG-EM). La actividad antioxidante presentada puede correlacionarse principalmente con la presencia de eugenol, trans-isoeugenol, lucenina-2, salicilato de metilo y ácido siríngico detectado. Este estudio muestra que la fracción de acetato de etilo podría ser utilizada como una buena fuente de antioxidantes para el beneficio de la salud humana.
Palabras clave:
Bioactividad, DPPH, Flavonoide, Fraccionamiento, FRAP, Polifenoles.Conyza bonariensis L. is a short-lived perennial weed native from South America, extensively spread over tropical and warm temperate areas worldwide (Zambrano-Navea et al., 2016). This species is part of the Asteraceae family, which is popular in the folk medicine, and large number of species are reported to be rich in phenolic compounds and acidic polysaccharides. Besides, some species have been described to possess biological activities such as antiplatelet, anticoagulant, and antioxidant properties (Saluk-Juszczak et al., 2010). C. bonariensis is considered as a glyphosate-resistant weed that causes serious problems to productive crops (Okumu et al., 2019). Nonetheless, C. bonariensis is widely used in traditional medicine for the treatment of rheumatism, gout, and nephritis. Furthermore, antioxidant, antibacterial, and hepatoprotective activities have been reported for C. bonariensis extracts (Thabit et al., 2015). Natural extracts that exhibit antioxidant and antimicrobial activities represent a promising alternative to chemical products because of their high effectiveness, low cost, and non-environmental pollutants (Rodríguez et al., 2000). C. bonariensis tinctures have been shown notably inhibitory activity against fungi that cause skin superficial infections, such as dermatophytes, Candida, and Malassezia (Mussin et al., 2017). In this context, the exploitation of C. bonariensis properties would represent a great benefit for the socio-economic development of countries where the weed interferes with the growth of crops. Even though there is information available about biological activities of C. bonariensis, the species that grow in Ecuador has not been studied yet. Due to a lack of information, this study focuses on determining the chemical profile and antioxidant activity of extracts and fractions of C. bonariensis leaves extract and its fractions in order to support the beneficial use of this weed in Ecuador.
MATERIALS AND METHODS
Plant material
The leaves of C. bonariensis were collected in, Guayaquil (Guayas, Ecuador) in 2014 and authenticated by National Herbarium (Quito, Ecuador). A voucher specimen (no. CIBE002) has been retained at Centro de Investigaciones Biotecnológicas del Ecuador.
Standards and reagents
The chemicals used were of reagent grade or higher. Folin-Ciocalteu, 2,2-diphenyl-1-picrylhydrazy (DPPH), gallic acid, ascorbic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), bis (trimethylsilyl)-trifluoroacetamide, methanol, sodium nitrite, aluminum chloride, and 2,4,6-tripyridyl-s-triazine (TPTZ) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Chloroform, n-hexane, and ethyl acetate were purchased from Fisher Scientific (Pittsburgh, PA, USA). Iron (III) chloride hexahydrate was purchased from Merck (Darmstadt, Germany), hydrochloric acid from Mallinckrodt (St. Louis, MO, USA), sodium acetate trihydrate and acetic acid from J.T. Baker (Phillipsburg, NJ, USAG). Ethanol was purchased from Panreac (Barcelona, Spain). Ultrapure water was purified in a Milli-Q water purification system (Millipore, Bedford, MA, USA).
Extraction procedure
The leaves of C. bonariensis were dried at 37 °C for 48 h in a stove; they were pulverized with a hand mill and passed through a mesh sieve of 0.5 mm. Then, 100 g of dried plant material was macerated with methanol (MeOH) for 48 hours for extraction. The crude methanol extract (ME) was obtained by solvent evaporating, using a rotary evaporator to obtain 10.24 g of total extract (yield 12.24%). This extract was solubilized in H2O:MeOH (300 mL, 1:1) and subsequently partitioned with n-hexane (3×150 mL, HF), chloroform (3×150 mL, CF), and ethyl acetate solvents (3×150 mL, EAF). The remaining water part was freeze dried to obtain an aqueous fraction (AqF).
Total phenolic content
Total phenolic content of the fractions was analyzed using a modification of the Folin-Ciocalteu reagent described by Waterhouse (2002). A calibrated method was used according to the equation y=0.0008x-0.0194 (R2= 0.994) obtained from the standard gallic acid graph. The total phenolic content was expressed as milligram gallic acid equivalents per gram of dry extract or fraction (mgGAE g-1).
Total flavonoid content
Total flavonoid content was estimated using the spectrophotometric method described by Min et al. (2011) using quercetin as a standard. The total flavonoid content was expressed as milligram quercetin equivalents per gram of dry extract or fraction (mgQE g-1). The calibration curve was y=0.0005x-0.0009 (R2=0.9987) obtained from the standard quercetin graph.
DPPH radical scavenging capacity
The DPPH radical scavenging capacity was determined using the method described by Ebada et al. (2008) with minor modifications. Each fraction was reconstituted in ethanol to give concentration ranging from 50 to 500 mg L-1.
The reconstituted fraction in ethanol (0.10 mL) was mixed with 1.6 mL DPPH solution (40 mg L-1 in 100% ethanol). Ethanol and DPPH solution were used as blank and negative control, respectively. The solutions were mixed and incubated in darkness at room temperature for 30 min. The absorbance was measured at 517 nm, and the DPPH radical scavenging capacity (RSC) was calculated according to RSC=((A0-A1)/A0)×100 equation, where A0 is the absorbance of the control and A1 is the absorbance of the fraction.
Ferric reducing antioxidant power (FRAP) assay
The reducing power was determined based on the method used by Li et al. (2012). The FRAP assay measures the ability of the antioxidants in the vegetable extracts to reduce ferric-tripyridyltriazine (Fe3+-TPTZ) complex to the blue colored ferrous form (Fe2+) which absorbs light at 593 nm. FRAP activity were expressed as milligram Trolox equivalents per gram of dry extract/fraction (mgTE g-1). The calibration curve was y=0.0031x+0.0061 (R2=0.9989) obtained from the standard Trolox graph.
Gas Chromatography-Mass Spectrometry (GC-MS)
Ethyl acetate fraction of C. bonariensis was analyzed using a gas chromatography-mass spectrometry equipment Agilent Technologies (7890A GC system and 5975C inert XL MSD with triple-axis detector). The procedure used for this process was adapted from the method described by Balladares et al. (2016). A capillary column HP-5MS (30 m×0.25 mm) with phenyl methylpolysiloxane was used as stationary phase (0.25 μm film thickness) and helium as the carrier gas (1.2 mL min-1). Samples dissolved or suspended in a solvent different from the original extraction solvent were filtered or centrifuged to get rid of any insoluble matter. The injection of 2.0 μL of sample (10 mg mL-1) was done at a temperature of 250 °C with splitless mode, the detector temperature was 280 °C and the oven temperature was maintained at 70 °C for 2.0 min, then it was increased up to 285 °C at a rate of 5 °C min-1. The electron ionization was set at 70 eV, 230 °C was used as ion source, and the data compounds were collected with the full scan mode (40-1000 amu). The identification of the components was based on comparison of their retention index using n-alkanes (C7-C40) and mass spectra database of Wiley 9th with NIST 2011 MS Library.
Statistical Analysis
Results were expressed as mean±standard deviation of three repetitions. The assays were compared by using one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test, with statistical significance determined at the P<0.05. All statistical analyses were performed using Minitab 16 software.
RESULTS AND DISCUSSION
Extraction procedure
The maceration process of dried leaf powder (100 g) in methanol showed a yield of 12.80% after solvent evaporation. The liquid-liquid fractionation revealed the following yields: hexane (44.3%), chloroform (13.7%), ethyl acetate (3.0%), and aqueous fraction (37.9%). These results support that alcoholic extracts (MeOH or EtOH) from plant materials contain a wide variety of polar and nonpolar compounds. Several studies have shown that polar solvents are effective for the extraction of polyphenols (Sacchi et al., 2005). Furthermore, it has been reported that the antioxidant activity is attributed to the presence of phenolic compounds and flavonoids in these extracts (Figueroa et al., 2015). In this sense, the yield of the ME (12.8%) was higher than the previously obtained from C. bonariensis by El Zalabani et al. (2012) using 70% ethanol (3.89%), and Kong et al. (2001) using methanol (6.33%).
Total phenolic content
The total phenolic content of C. bonariensis leave extract and fractions ranged from 27.90 to 340.84 mgGAE g-1 for HF and EAF, respectively (Table 1). The phenolic content of EAF presented a significant difference in comparison with the remaining fractions (P<0.05), except for CF, AqF, and ME (P>0.05). Some studies have reported that ethanolic extracts of C. bonariensis obtained from the whole plant showed values between 56.6 and 144.1 mgGAE g-1, informed by Diaz et al. (2012) and Thabit et al. (2015), respectively. Similarly, the research carried out by Daur (2015) showed a total phenolic content of 78.0 mgGAE g-1 in the methanolic extract of the complete plant. Also, Shahwar et al. (2012) described a higher total phenolic content (241.3 mgGAE g-1) for the methanolic extract of the stem. In fact, total phenolic content of EAF obtained by liquid fractionation is slightly higher (395.6 mgGAE g-1) than the other investigations reported.
Table 1: Total phenolic and flavonoid content of ME and its fractions.
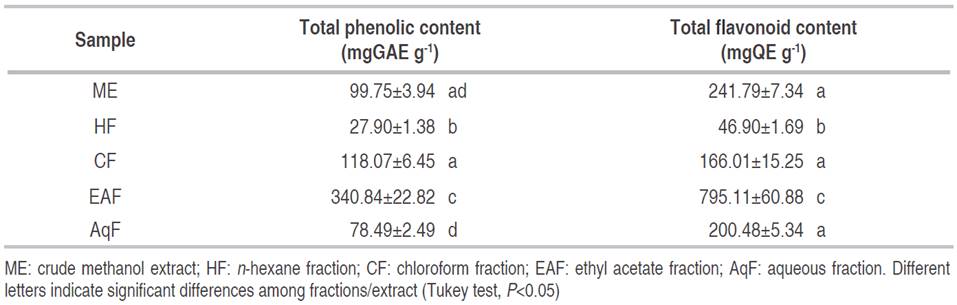
Diaz et al. (2012) also reported a high phenolic content (200.0 mgGAE g-1) from an extract in hot water (autoclaved 121 °C) while El Zalabani et al. (2012) reported a low phenolic content (0.96 mgGAE g-1). This variability of the total phenolic content can be attributed to the geographic site where the plant grows and to the solvent used for the extraction.
Total flavonoid content
The total flavonoid content of C. bonarienesis extract and fractions ranged from 46.90 to 795.11 mgQE g-1 for HF and EAF, respectively (Table 1). The flavonoid content of the EAF showed a significant difference compared with the remaining fractions (P<0.05) except for CF, AqF, and ME (P>0.05).
The total flavonoid content obtained was higher than values reported by Diaz et al. (2012) (73.4 mgQE g-1) and Thabit et al. (2015) (134.0 mgQE g-1) for whole plant extracts in EtOH and EtOH 90%, respectively. In the same study, Diaz et al. (2012) also reported a high flavonoid content (276.4 mgQE g-1) from an extract in hot water (autoclaved 121 °C). This study is comparable to the study informed by Kamdem-Boniface and Pal (2013), which reports a flavonoid content of 199.3 mgQE g-1 in C. sumatrensis, a species from the same family.
Flavonoids are polyphenolic compounds with great importance that act as potent antioxidants depending on their molecular structures. Quercetin is the most abundant flavonoid with antioxidant properties. Hence, the high content of phenolic compounds and flavonoids in the soluble fraction of ethyl acetate could suggest a good source of bioactive compounds with an interest for human health.
DPPH free radical scavenging capacity
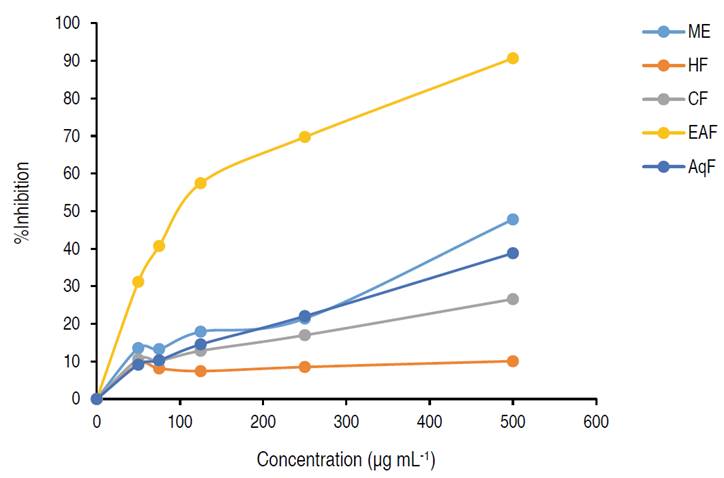
The radical scavenging capacity evaluated between a DPPH concentration range of 50-500 μg mL-1 showed inhibition percentages from 10.07% (HF) to 90.69% (EAF) (Table 2). Figure 1 shows the screening profiles of the ME and its fractions, where it can be observed a dose-dependent behavior. The EAF showed the highest activity with an IC50 value of 146.9 μg mL-1. The results obtained were significantly different (P<0.05).
Table 2: Activity antioxidant of ME and its fractions.
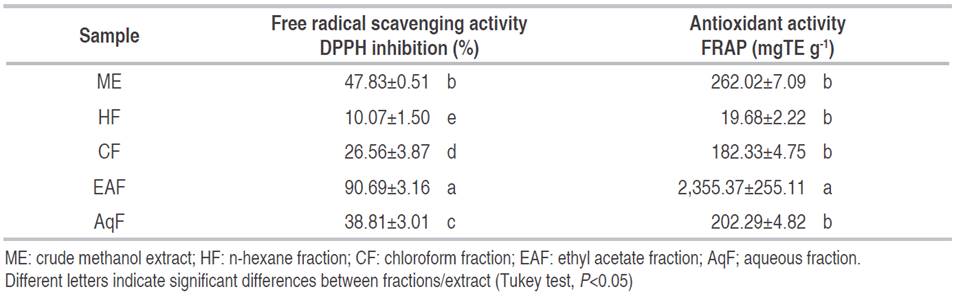
Figure 1: Inhibition effect of ME and its fractions on DPPH
The DPPH scavenging activity of the methanolic extract (47.8%) of the present study was lower than the reported for the ethanolic extract of C. bonariensis whole plant at a concentration of 612 μg mL-1. However, the concentration of the DPPH used in our study (0.1 mM) is higher than the concentration (0.0055 mM) used by Diaz et al. (2012).
This study corroborates the potent antioxidant action of the EAF compared to the other fractions assessed. This behavior is possibly explained by the high phenols and flavonoids content obtained in this fraction. EAF exhibited antioxidant activity value comparable to the result obtained by Shahwar et al. (2012), who showed (90.3%), and IC50 of 89.0 μg mL-1 for the ethyl acetate fraction with a concentration of 500 μg mL-1. This value is lower than the result obtained in the present study (146.9 μg mL-1). The other fractions obtained an IC50>500 μg mL-1.
Hayet et al. (2009) reported a similar result with an IC50 of 150.0 μg mL-1 for the ethyl acetate extract of the aerial parts of Conyza canadensis, assesed with a 0.075 mM DPPH solution. Furthermore, our results are comparable with the Ageratum houstonianum (Asteraceae). In this study, the ethyl acetate extract of the leaves reported a percentage of inhibition of 88.26% with a concentration of 500 μg mL-1 (Tennyson et al., 2012).
Additionally, there is a positive Pearson correlation between DPPH antioxidant activity, total phenol content, and total flavonoid content (r=0.929 for DPPH and totalphenolic content, r=0.962 for DPPH and total flavonoid content). An extract is considered active when IC50 values range from 50 to 100 μg mL-1, and moderate when the values range from 101 to 250 μg mL-1 (Marjoni and Zulfisa, 2017).
Ferric reducing antioxidant power (FRAP) assay
In the FRAP assay of C. bonarienesis extract and fractions ranged from 19.68 to 2,355.37 mgTE g-1 for HF and EAF, respectively (Table 2). EAF gave a high antioxidant power to reduce Fe3+ and was significantly different from the remaining fractions (P<0.05). Therewas not a significant difference in other fractions (P>0.05). Currently, the antioxidant activity of Conyza sp.expressed as Trolox equivalent has not been reported, therefore, it is not possible to make comparisons with the same genus. However, when compared with other plants of the Asteraceae family, the value obtained in C. bonariensis ME (1,015.42 mgTE g-1) is higher. For example, Santos et al. (2015), reported an antioxidant activity from aqueous extract of Tagetes erecta of 24.9 mgTE g-1, hydroethanolic extract of 2.7 mgTE g-1, and the ethanolic extract of 25.9 mgTE g-1 while Tagetes patula showed values of 22.5 and 21.0 mgTE g-1 for hydroethanolic and ethanolic extracts, respectively.
In addition, comparisons with medicinal plants from Turkey, the antioxidant activity of this study was higher than the activity obtained for Achillea phrygia (Asteraceae) with ethyl acetate, methanol, and aqueous extract with values of 52.0, 129.9, and 130.6 mgTE g-1, respectively. Bupleurum croceum (Apiaceae) showed values of 64.6, 72.6, and 87.9 mgTE g-1 for the ethyl acetate, methanol, and aqueous extract, respectively. Ceylan et al. (2016) reported lower antioxidant activity of methanolic extract of aerial parts of Cytisopsis dorycniifolia and Ebenus hirsuta, both members of Fabaceae family, 200.6 and 103.4 mgTE g-1, respectively.
The DPPH and FRAP trials were largely in agreement with each other, which is reflected in the high Pearson correlation (r=0.917) between both trials. Therefore, the antioxidant activity for both DPPH and FRAP methods could be due to the presence of phenolic and flavonoids compound with a high correlation value (r=0.973) for both.
Gas chromatography/mass spectrometry analysis
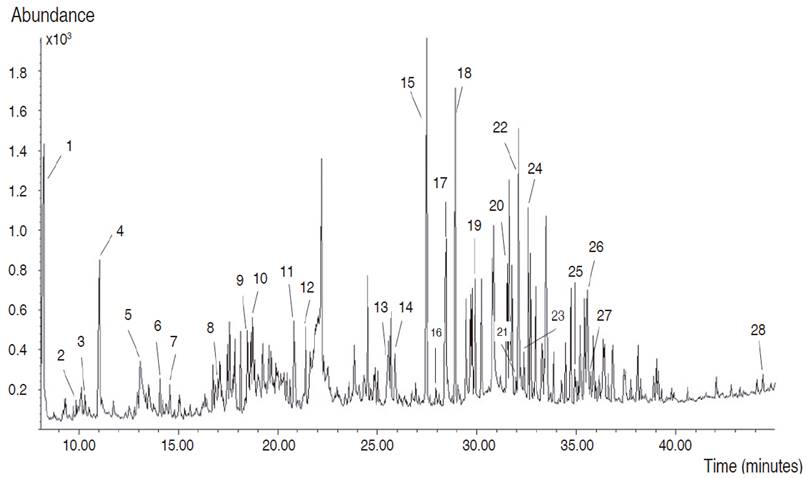
Based on the high phenolic and flavonoid contents and antioxidant activity obtained in the present study, the EAF was subjected to GC-MS analysis. A total of 102 compounds were detected in EAF, but it was only possible to identify 28 corresponding to 36.59% (Table 3). These compounds were mainly esterified fatty acids, free fatty acids, and alcohols, but also the presence of monosaccharides, phenolic, and flavonoid acids were detected (Figure 2).
Table 3: Compounds identified by GC-MS in C. bonariensis ethyl acetate fraction.
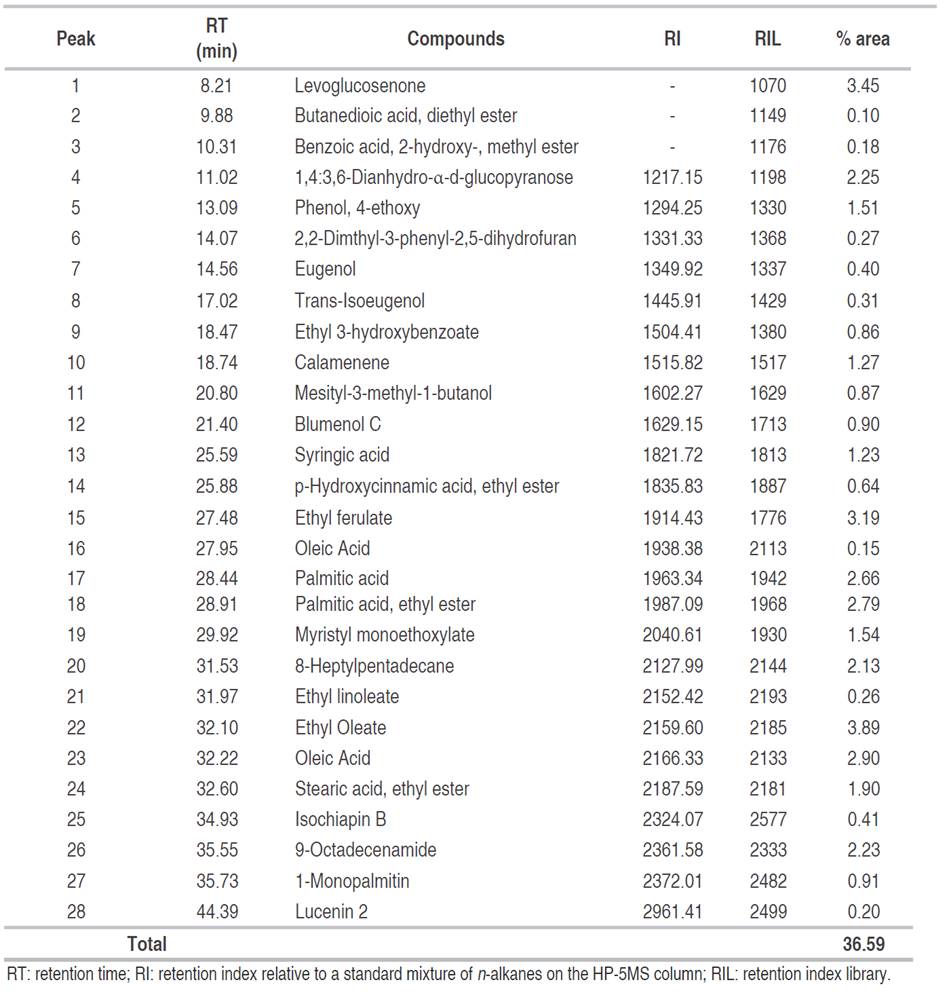
Figure 2: Chromatogram of the GC-MS analysis of C. bonariensis ethyl acetate fraction.
Antioxidant activity of ethyl acetate fraction of C. bonariensis could be explained by the presence of the flavonoid lucenin-2, methyl salicylate, the aromatic compound eugenol, trans-isoeugenol, and the sesquiterpene lactone isochiapin B due to phenyls groups and double bonds in their chemical structures able to stabilize free radicals (Choe and Min, 2009). Lucenin-2 has been reported as remarkable antihepatotoxic activity against CCl4 and galactosamine cytotoxicity in primary cultured rat hepatocytes (Hoffmann-Bohm et al., 1992). Besides this c-glycosyl compound has been isolated from the leaves of Passiflora edulis f. flavicarpa, contributing to the positive anxiolytic activity of leaf ethanol extracts evaluated, and it has been shown to induce dose-dependent alterations on germination and growth of Tortula muralis increasing spore germination, protonemal length, seed germination, and length of ipocothyl-root until 18 days of inoculation (Basile et al., 2003). Several biological activities have been attributed to eugenol such as anticonvulsant, analgesic, anti-inflammatory, antifungal, antioxidant, and radical-scavenging activity (Singh and Chaudhuri, 2018). Furthermore, levoglucosenone has been demonstrated to have antitumor activity (Giri et al., 2016). Besides, syringic acid has exhibited anti-inflammatory, antioxidant, antimicrobial, anticancer properties (Kumar et al., 2012). On the other hand, the sesquiterpene lactone isochiapin B has been used to treat arthritis, tonsillitis, and other ailments by Chinese medicine (Krishnan and Murugan, 2014). It is important to note that sesquiterpene lactones are characteristic secondary metabolites of the Asteraceae family derivative of sesquiterpenoids which have been shown to possess antimalarial, antimicrobial, antitumor, anti-inflammatory and antioxidant activity (Shoaib et al., 2017).
CONCLUSIONS
This study reports that ethyl acetate fraction of C. bonariensis leaves showed a relevant antioxidant activity, which could be correlated to the presence of eugenol, trans-isoeugenol, lucenin-2, methyl salicylate, and syringic acid. These results confirm that this plant species is a good source of antioxidants that could be medicinally useful.
ACKNOWLEDGMENTS
This work was financed by Escuela Superior Politécnica del Litoral (ESPOL).
REFERENCES
References
Balladares C, Chóez-Guaranda I, García J, Sosa D, Pérez S, González JE, Viteri R, Barragán A, Quijano-Avilés M and Manzano P. 2016. Physicochemical characterization of Theobroma cacao L. sweatings in Ecuadorian coast. Emirates Journal of Food and Agriculture 28(10): 741-745. doi: 10.9755/ejfa.2016-02-187
Basile A, Sorbo S, López-Sáez JA, and Castaldo Cobianchi R. 2003. Effects of seven pure flavonoids from mosses on germination and growth of Tortula muralis HEDW. (Bryophyta) and Raphanus sativus L. (Magnoliophyta). Phytochemistry. 62(7): 1145–1151. doi: 10.1016/S0031-9422(02)00659-3
Ceylan R, Katanić J, Zengin G, Matić S, Aktumsek A, Boroja T, Stanić S, Mihailović V, Guler GO, Boga M and Yılmaz MA. 2016. Chemical and biological fingerprints of two Fabaceae species (Cytisopsis dorycniifolia and Ebenus hirsuta): Are they novel sources of natural agents for pharmaceutical and food formulations? Industrial Crops and Products 84: 254–262. doi: 10.1016/j.indcrop.2016.02.019
Choe E and Min DB. 2009. Mechanisms of Antioxidants in the Oxidation of Foods. Comprehensive Reviews in Food Science and Food Safety 8(4): 345–358. doi: 10.1111/j.1541-4337.2009.00085.x
Daur I. 2015. Chemical composition of selected Saudi medicinal plants. Arabian Journal of Chemistry 8(3): 329–332. doi: 10.1016/j.arabjc.2013.10.015
Diaz P, Jeong SC, Lee S, Khoo C and Koyyalamudi SR. 2012. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chinese medicine. 7(1): 26. doi: 10.1186/1749-8546-7-26
Ebada SS, Edrada RA, Lin W and Proksch P. 2008. Methods for isolation, purification and structural elucidation of bioactive secondary metabolites from marine invertebrates. Nature Protocols 3(12): 1820–1831. doi: 10.1038/nprot.2008.182
El Zalabani SM, Hetta MH and Ismail AS. 2012. Genetic Profiling, Chemical Characterization and Biological Evaluation of Two Conyza Species Growing in Egypt. Journal of Applied Pharmaceutical Science 2(11): 54–61. doi: 10.7324/JAPS.2012.21110
Figueroa L, Navarro L, Vera M and Petricevich V. 2015. Antioxidant activity, total phenolic and flavonoid contents, and cytotoxicity evaluation of Bougainvillea xbuttiana. International Journal of Pharmacy and Pharmaceutical Sciences 6(5): 497–502.
Giri GF, Danielli M, Marinelli RA and Spanevello RA. 2016. Cytotoxic effect of levoglucosenone and related derivatives against human hepatocarcinoma cell lines. Bioorganic & Medicinal Chemistry Letters 26(16): 3955–3957. doi: 10.1016/j.bmcl.2016.07.007
Hayet E, Maha M, Samia A, Ali MM, Souhir B, Abderaouf K, Mighri Z and Mahjoub A. 2009. Antibacterial, antioxidant and cytotoxic activities of extracts of Conyza canadensis (L.) Cronquist growing in Tunisia. Medicinal Chemistry Research 18(6): 447–454. doi: 10.1007/s00044-008-9141-0
Hoffmann-Bohm K, Lotter H, Seligmann O and Wagner H. 1992. Antihepatotoxic C-glycosylflavones from the leaves of Allophyllus edulis var. edulis and gracilis. Planta Medica 58(6): 544–548. doi: 10.1055/s-2006-961546
Kamdem-Boniface P and Pal A. 2013. Substantiation of the ethnopharmacological use of Conyza sumatrensis (Retz.) E.H.Walker in the treatment of malaria through in vivo evaluation in Plasmodium berghei infected mice. Journal of Ethnopharmacology 145(1): 373–377. doi: 10.1016/j.jep.2012.10.025
Kong LD, Abliz Z, Zhou CX, Li LJ, Cheng CHK and Tan RX. 2001. Glycosides and xanthine oxidase inhibitors from Conyza bonariensis. Phytochemistry 58(4): 645–651. doi: 10.1016/S0031-9422(01)00176-5
Krishnan R and Murugan K. 2014. Comparison of GC-MS analysis of phytochemicals in the ethanolic extracts of Marchantia linearis Lehm & Lindenb. and Marchantia polymorpha L. (Bryophyta). International Journal of Pharmaceutical Sciences and Research. 49(5): 1981–1987. doi: 10.13040/IJPSR.0975-8232.5(5).1981-87
Kumar S, Prahalathan P and Raja B. 2012. Syringic acid ameliorates l-NAME-induced hypertension by reducing oxidative stress. Naunyn-Schmiedeberg's Archives of Pharmacology 385(12): 1175–1184. doi: 10.1007/s00210-012-0802-7
Li H, Deng Z, Zhu H, Hu C, Liu R, Young JC and Tsao R. 2012. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Research International 46(1): 250–259. doi: 10.1016/j.foodres.2011.12.014
Marjoni MR and Zulfisa A. 2017. Antioxidant Activity of Methanol Extract/Fractions of Senggani Leaves (Melastoma candidum D. Don). Pharmaceutica Analytica Acta 8(8). doi: 10.4172/2153-2435.1000557
Min B, McClung AM and Chen MH. 2011. Phytochemicals and Antioxidant Capacities in Rice Brans of Different Color. Journal of Food Science 76(1): C117–C126. doi: 10.1111/j.1750-3841.2010.01929.x
Mussin JE, Manzano P, Mangiaterra M and Giusiano G. 2017. Inhibitory activity of Conyza bonariensis (L.) Cronquist tincture against fungi and bacteria causing superficial infections. Revista Cubana de Plantas Medicinales 22(3).
Okumu MN, Vorster BJ and Reinhardt CF. 2019. Growth-stage and temperature influence glyphosate resistance in Conyza bonariensis (L.) Cronquist. South African Journal of Botany 121: 248–256. doi: 10.1016/j.sajb.2018.10.034
Rodríguez AT, Morales D and Ramírez MA. 2000. Efecto de extractos vegetales sobre el crecimiento in vitro de hongos fitopatógenos. Cultivos Tropicales. 21(2): 79–82.
Sacchi KL, Bisson LF and Adams DO. 2005. A review of the effect of winemaking techniques on phenolic extraction in red wines. American Journal of Enology and Viticulture 56(3): 197–206.
Saluk-Juszczak J, Pawlaczyk I, Olas B, Kołodziejczyk J, Ponczek M, Nowak P, Tsirigotis-Wołoszczak M, Wachowicz B and Gancarz R. 2010. The effect of polyphenolic-polysaccharide conjugates from selected medicinal plants of Asteraceae family on the peroxynitrite-induced changes in blood platelet proteins. International Journal of Biological Macromolecules 47(5): 700–705. doi: 10.1016/j.ijbiomac.2010.09.007
Santos PC, Santos VHM, Mecina GF, Andrade AR, Fegueiredo PA, Moraes VMO, Silva LP and Silva RMG. 2015. Phytotoxicity of Tagetes erecta L. and Tagetes patula L. on plant germination and growth. South African Journal of Botany 100: 114–121. doi: 10.1016/j.sajb.2015.05.013
Shahwar D, Raza MA, Saeed A, Riasat M, Chattha FI, Javaid M, Ullah S and Ullah S. 2012. Antioxidant potential of the extracts of Putranjiva roxburghii, Conyza bonariensis, Woodfordia fruiticosa and Senecio chrysanthemoids. African Journal of Biotechnology 11(18): 4288–4295. doi: 10.5897/AJB11.2564
Shoaib M, Shah I, Ali N, Adhikari A, Tahir MN, Shah SWA, Ishtiaq S, Khan J, Khan S and Umer MN. 2017. Sesquiterpene lactone! a promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complementary and Alternative Medicine 17(27): 1–11. doi: 10.1186/s12906-016-1517-y
Singh D and Chaudhuri PK. 2018. A review on phytochemical and pharmacological properties of Holy basil (Ocimum sanctum L.). Industrial Crops and Products 118: 367–382. doi: 10.1016/j.indcrop.2018.03.048
Tennyson S, Balaraju K, Park K, Ravindran KJ, Eapen A and William SJ. 2012. In vitro antioxidant activity of Ageratum houstonianum Mill. (Asteraceae). Asian Pacific Journal of Tropical Disease 2 Suppl. 2: S712–S714. doi: 10.1016/S2222-1808(12)60249-7
Thabit RA, Cheng XR, Tang X, Sun J, Shi YH and Le GW. 2015. Antioxidant and antibacterial activities of extracts from Conyza bonariensis growing in Yemen. Pakistan journal of pharmaceutical sciences. 28(1): 129–134.
Waterhouse AL. 2002. Determination of Total Phenolics. Current Protocols in Food Analytical Chemistry 6(1):1-8. doi: 10.1002/0471142913.fai0101s06
Zambrano-Navea CL, Bastida F and González-Andújar JL. 2016. A cohort-based stochastic model of the population dynamic and long-term management of Conyza bonariensis in fruiting tree crops. Crop Protection 80(6): 15–20. doi: 10.1016/j.cropro.2015.10.023
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Armel-Frederic Namkona, Rami Rahmani, Xavier Worowounga, Jean-Laurent Syssa-Magalé, Hubert Matondo, Jalloul Bouajila. (2024). Copaifera mildbraedii Desf.: Phytochemical Composition of Extracts, Essential Oil, and In Vitro Biological Activities of Bark. Plants, 13(6), p.877. https://doi.org/10.3390/plants13060877.
2. Imtiaz Mustafa, Shahzad Irfan, Ghulam Hussain, Muhammad Umar Ijaz, Muhammad Irfan Ullah, Jaweria Nisar, Tahir Maqbool, Haseeb Anwar. (2024). Conyza bonariensis (L.) Impact on Carbohydrate Metabolism and Oxidative Stress in a Type 2 Diabetic Rat Model. Bioinformatics and Biology Insights, 18 https://doi.org/10.1177/11779322241292239.
3. Methodius S. Lahngong, Thomas M. Schaefer, Bertrand Y. Wanyu, Carla Hamann, Lais P. de Carvalho, Mbua J. Vekima, Olivia Jansen, Allison Ledoux, Robert A. Shey, Smith B. Babiaka, Jana Held, Moses N. Ngemenya, Germain S. Taiwe, Stephen M. Ghogomu, Gisèle E. Loe, Michel Frédérich. (2026). Toxicity profile, antiplasmodial and gametocytocidal properties of Erigeron bonariensis L. (anc. Conyza bonariensis (L.) Cronquist). Journal of Ethnopharmacology, 355, p.120609. https://doi.org/10.1016/j.jep.2025.120609.
4. Chabaco Armijos, Jorge Ramírez, Melissa Salinas, Giovanni Vidari, Alírica Suárez. (2021). Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals, 14(11), p.1145. https://doi.org/10.3390/ph14111145.
5. Weam M. A. Khojali. (2025). Phytochemical Characterization and Bioactivity Evaluation of Teucrium polium Methanolic Extract: Antioxidant, Antibacterial, and In vitro Anticancer Activities. Asian Journal of Pharmaceutical Research and Health Care, 17(1), p.67. https://doi.org/10.4103/ajprhc.ajprhc_49_25.
6. Farha Aijaz, Mo Shadab, Nazish Akhtar, Uzma Parveen, M. B. Siddiqui. (2025). Erigeron bonariensis: phytochemistry, allelopathy, and potential for sustainable pest management and healthcare. Vegetos, https://doi.org/10.1007/s42535-025-01376-x.
7. Husam Qanash, Reham Yahya, Marwah M. Bakri, Abdulrahman S. Bazaid, Sultan Qanash, Abdullah F. Shater, Abdelghany T. M.. (2022). Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Scientific Reports, 12(1) https://doi.org/10.1038/s41598-022-09993-1.
8. Salwa M. Abdel Rahman, Maher A. Kamel, Mennatallah A. Ali, Badriyah S. Alotaibi, Ohud Muslat Aharthy, Mustafa Shukry, Hala Mohamed Abd El-Bary. (2023). Comparative Study on the Phytochemical Characterization and Biological Activities of Azolla caroliniana and Azolla filiculoides: In Vitro Study. Plants, 12(18), p.3229. https://doi.org/10.3390/plants12183229.
9. Mohammad Saleem, Valerie B. Schini-Kerth, Khalid Hussain, Syed H. Khalid, Muhammad Asif, Mahmoud Alhosin, Muhammad F. Akhtar, Bashir Ahmad, Atif Raza, Mahrukh. (2022). Molecular Mechanisms Responsible for In Vitro Cytotoxic Attributes of Conyza bonariensis Extract against Lymphoblastic Leukaemia Jurkat Cells. Anti-Cancer Agents in Medicinal Chemistry, 22(9), p.1793. https://doi.org/10.2174/1871520621666210906092314.
10. Fulgence Ntangere Mpenda, Simeon P. Nsindagi, Cyprian B Mpinda. (2023). Chemical profiling and antimicrobial potential of crude extract and essential oil from Conyza bonariensis leaves. Baghdad Journal of Biochemistry and Applied Biological Sciences, 4(03), p.136. https://doi.org/10.47419/bjbabs.v4i03.244.
11. Omer H.M. Ibrahim, Ahmed A.H. Issa, Wafa Mohammed Al-Otaibi, Hatim M. Al-Yasi, Luluah M. Al Masoudi, Osama H. Tawfik, Ahmad I. Alqubaie, Fayez Althobaiti, Esmat F. Ali. (2025). Biological activity of Sansevieria cylindrica Bojer ex Hook. grown at a high-altitude region: Phytochemical screening of leaf and rhizome extracts and their in vitro antimicrobial and anticancer activities. Industrial Crops and Products, 223, p.120169. https://doi.org/10.1016/j.indcrop.2024.120169.
12. K.J. Al-Sallami, K. Shalabi, A.S. Fouda. (2021). Impact of Conyza bonariensis extract on the corrosion protection of carbon steel in 2 M HCl solution. International Journal of Electrochemical Science, 16(9), p.210929. https://doi.org/10.20964/2021.09.40.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2020 Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.