Acute disseminated toxoplasmosis in an immunocompetent adult patient. Case report
Toxoplasmosis aguda diseminada en paciente adulto inmunocompetente. Reporte de caso
DOI:
https://doi.org/10.15446/cr.v9n1.94825Palabras clave:
Toxoplasmosis, Cerebral, Toxoplasmosis, Immunocompetence, Multiple Organ Failure, Diagnosis (en)Toxoplasmosis Cerebral, Toxoplasmosis, Inmunocompetencia, Insuficiencia Multiorgánica, Diagnóstico (es)
Descargas
Abstract
Introduction: Toxoplasmosis is a disease of global distribution caused by the parasite Toxoplasma gondii, which develops differently depending on the immunologic status of the patient. In immunocompetent patients, it is usually asymptomatic and complications such as pneumonitis, encephalitis, or multiple organ dysfunction are rare. The following is the case of an immunocompetent patient with acute disseminated toxoplasmosis.
Case report: A 42-year-old man, with no history of immunocompromise, or relevant medical, family or personal history, was transferred to the emergency department of a tertiary care institution in the city of Bogotá D.C. (Colombia) due to a fever that had lasted for a month, headache, and progressive neurological deterioration. Studies looking for other infectious etiologies, as well as for autoimmunity, neoplasms, and metabolic disorders, were negative. Computed tomography (CT) of the skull showed findings of meningoencephalitis. He was considered as a possible case of toxoplasmosis and treatment with trimethoprim/sulfamethoxazole was initiated. During his stay in the intensive care unit (ICU), he developed multiple organ dysfunction syndrome (MODS) and ultimately died. The post-mortem histopathological study of tissues reported the presence of Toxoplasma gondii bradyzoites, which confirmed the diagnosis of acute toxoplasmosis.
Conclusions: Acute disseminated toxoplasmosis is a diagnostic challenge because it can mimic other etiologies. A timely diagnosis may prevent medical complications and increase the patient's chances of recovery. Knowledge about this disease in immunocompetent patients is a subject being developed.
Resumen
Introducción. La toxoplasmosis es una infección de distribución mundial causada por el parásito Toxoplasma gondii, cuyo desarrollo varía según el estado inmunológico del paciente. En inmunocompetentes suele ser asintomática y complicaciones como la neumonitis, la encefalitis o la disfunción multiorgánica son poco frecuentes. A continuación, se presenta el caso de un paciente inmunocompetente con toxoplasmosis aguda diseminada.
Presentación del caso. Hombre de 42 años, sin historia de inmunocompromiso, antecedentes patológicos, familiares o personales de importancia, quien fue trasladado al servicio de urgencias de una institución de tercer nivel de complejidad en la ciudad de Bogotá D.C. (Colombia) por presentar fiebre de un mes de duración, cefalea y deterioro neurológico progresivo. Los estudios en búsqueda de otras etiologías infecciosas, así como para autoinmunidad, neoplasia y enfermedad metabólica fueron negativos. La tomografía computarizada (TC) de cráneo mostró hallazgos de meningoencefalitis. Se consideró un posible caso de toxoplasmosis y se inició tratamiento con trimetoprima/sulfametoxazol. Durante su estancia en la Unidad de Cuidados Intensivos (UCI), presentó síndrome de disfunción multiorgánica (MODS por sus siglas en inglés) y, posteriormente, murió. El estudio histopatológico post-mortem a nivel tisular confirmó presencia de bradizoitos de Toxoplasma gondii, lo cual confirmó el diagnóstico de toxoplasmosis aguda.
Conclusiones. La toxoplasmosis aguda diseminada representa un reto diagnóstico porque puede simular otras etiologías. Su diagnóstico oportuno puede evitar complicaciones médicas aumentando las posibilidades de recuperación del paciente. Los conocimientos acerca de esta patología en el inmunocompetente son un tema en evolución.
https://doi.org/10.15446/cr.v9n1.94825
Acute disseminated toxoplasmosis in an immunocompetent adult patient. Case report
Keywords: Toxoplasmosis, Cerebral; Toxoplasmosis; Immunocompetence;
Multiple Organ Failure; Diagnosis.
Palabras clave: Toxoplasmosis Cerebral; Toxoplasmosis; Inmunocompetencia;
Insuficiencia Multiorgánica; Diagnóstico.
Juan Sebastián Frías-Ordoñez
Whendy Alejandra Mendoza-Acevedo
Universidad de La Sabana - Faculty of Medicine - Department of Internal Medicine -
Chía - Cundinamarca - Colombia.
Johan Fernando Devia-Alvira
María Teresa Ospina-Cabrera
Hospital Universitario de La Samaritana
- Intensive Care Unit -
Critical and Intensive Care Medicine
- Bogotá D.C. - Colombia.
Pedro Pablo Osejo-Diago
Hospital Universitario de La Samaritana
- Department of Pathology -
Bogotá D.C. - Colombia.
Corresponding author
Juan Sebastián Frías-Ordoñez.
Departamento de Medicina Interna,
Facultad de Medicina, Universidad de La Sabana. Chía. Cundinamarca. Colombia.
Email: juanfror@unisabana.edu.co
Received: 21/01/2021 Accepted: 16/07/2021
Resumen
Introducción. La toxoplasmosis es una infección de distribución mundial causada por el parásito Toxoplasma gondii, cuyo desarrollo varía según el estado inmunológico del paciente. En inmunocompetentes suele ser asintomática y complicaciones como la neumonitis, la encefalitis o la disfunción multiorgánica son poco frecuentes. A continuación, se presenta el caso de un paciente inmunocompetente con toxoplasmosis aguda diseminada.
Presentación del caso. Hombre de 42 años, sin historia de inmunocompromiso, antecedentes patológicos, familiares o personales de importancia, quien fue trasladado al servicio de urgencias de una institución de tercer nivel de complejidad en la ciudad de Bogotá D.C. (Colombia) por presentar fiebre de un mes de duración, cefalea y deterioro neurológico progresivo. Los estudios en búsqueda de otras etiologías infecciosas, así como para autoinmunidad, neoplasia y enfermedad metabólica fueron negativos. La tomografía computarizada (TC) de cráneo mostró hallazgos de meningoencefalitis. Se consideró un posible caso de toxoplasmosis y se inició tratamiento con trimetoprima/sulfametoxazol. Durante su estancia en la Unidad de Cuidados Intensivos (UCI), presentó síndrome de disfunción multiorgánica (MODS por sus siglas en inglés) y, posteriormente, murió. El estudio histopatológico post-mortem a nivel tisular confirmó presencia de bradizoitos de Toxoplasma gondii, lo cual confirmó el diagnóstico de toxoplasmosis aguda.
Conclusiones. La toxoplasmosis aguda diseminada representa un reto diagnóstico porque puede simular otras etiologías. Su diagnóstico oportuno puede evitar complicaciones médicas aumentando las posibilidades de recuperación del paciente.
Los conocimientos acerca de esta patología en el inmunocompetente son un tema en evolución.
Abstract
Introduction: Toxoplasmosis is a disease of global distribution caused by the parasite Toxoplasma gondii, which develops differently depending on the immunologic status of the patient. In immunocompetent patients, it is usually asymptomatic and complications such as pneumonitis, encephalitis, or multiple organ dysfunction are rare. The following is the case of an immunocompetent patient with acute disseminated toxoplasmosis.
Case report: A 42-year-old man, with no history of immunocompromise, or relevant medical, family or personal history, was transferred to the emergency department of a tertiary care institution in the city of Bogotá D.C. (Colombia) due to a fever that had lasted for a month, headache, and progressive neurological deterioration. Studies looking for other infectious etiologies, as well as for autoimmunity, neoplasms, and metabolic disorders, were negative. Computed tomography (CT) of the skull showed findings of meningoencephalitis. He was considered as a possible case of toxoplasmosis and treatment with trimethoprim/sulfamethoxazole was initiated. During his stay in the intensive care unit (ICU), he developed multiple organ dysfunction syndrome (MODS) and ultimately died. The post-mortem histopathological study of tissues reported the presence of Toxoplasma gondii bradyzoites, which confirmed the diagnosis of acute toxoplasmosis.
Conclusions: Acute disseminated toxoplasmosis is a diagnostic challenge because it can mimic other etiologies. A timely diagnosis may prevent medical complications and increase the patient’s chances of recovery. Knowledge about this disease in immunocompetent patients is a subject being developed.
Introduction
Toxoplasmosis is an infection with a global distribution caused by the protozoan parasite Toxoplasma gondii. It may be acquired after consuming meat infected with tissue cysts or following ingestion of soil, food or water material contaminated with cat feces containing the oocysts (1,2).
The severity of the infection may vary depending on the immune status of the patient, but it is most often asymptomatic in immunocompetent patients and may persist for life in the host (3). In this type of patient, clinical manifestations derived from the infection —such as meningoencephalitis, myocarditis, hematological alterations, autoimmune disorders, and multiple organ dysfunction triggering a state of shock— are rare. However, these clinical manifestations frequently develop in immunocompromised patients, who usually present with multiple round lesions with a ring-enhancing pattern, similar to central nervous system abscesses (4).
The following is the case of a male patient with suspected acute toxoplasmosis, no history of immunocompromise or relevant medical history prior to the clinical onset of the symptoms, showing progressive neurological deterioration concomitant with multiple organ failure.
Case presentation
A 42-year-old man from Maracay, Venezuela, who immigrated to Colombia in 2018 due to socioeconomic problems in his home country, residing in Soacha, Cundinamarca (Colombia), was taken by his relatives on October 15, 2019, to the emergency department of a secondary care hospital in the city of Bogotá D.C. (Colombia) due to clinical symptoms consisting of unquantified fever, intense headache, and progressive neurological deterioration over the course of a month. Ten days before his admission, he experienced maculopapular erythema with pruritus, which started in the thorax and abdomen and later spread to the neck, face, and upper and lower limbs. In addition, three days before his admission, he presented deviation of the labial commissure to the left, as well as left hemiparesis and intense headache with a score of 10/10 on the visual analog pain scale (VAS).
On the day of admission, a CT scan of the skull was performed, which showed hyperdensity of the intracerebral region and white matter in the right juxtaventricular region, suggestive of brain abscess vs. brain neoplasm. Likewise, a lumbar puncture was performed with an opening pressure of 15 cmH2O, finding neutrophilic pleocytosis (59/mm3) for which antibiotic therapy was indicated (indefinitely) with intravenous vancomycin at a dose of 1g every 12 hours, metronidazole 500 mg IV every 8 hours, and ceftriaxone 1g IV every 12 hours to treat bacterial meningitis.
On October 17, 2019, he was referred to a tertiary care hospital for further studies and treatment. On October 18, 2019, upon admission to that institution, during physical examination the patient was awake and had the following vital signs: temperature: 37°C, heart rate: 93 bpm (tachycardia), and body mass index (BMI) of 28.56. His neurological examination revealed a slight deviation of the labial commissure to the left, muscle weakness on the left side of the face, and decreased strength on the left side of the body: left upper limb 1/5 on subjective strength rating scale (apparent spasticity on passive movement) and 1/5 on left lower limb, reflexes on the left side of the body (-/++++) —abolished muscle reflexes—, and generalized allodynia. No prior known disease history was reported, but there was a previous history of unquantified weight loss (at least three pant sizes), nocturnal diaphoresis, and loss of appetite. Simultaneously, laboratory tests were requested as shown in Table 1.
Table 1. Laboratory tests performed on admission.
|
Test |
Results |
|
Leucocytes |
3.170/mm3 |
|
Hematocrit |
33.50% |
|
Red blood cell count |
4.14X106/mm3 |
|
Mean corpuscular hemoglobin (MCH) |
26 pg |
|
Platelets |
91.000/mm3 |
|
Acute phase reactants |
|
|
C-reactive protein (CRP) test |
105 mg/L |
|
Coagulation tests |
|
|
Prothrombin time test (PT) |
12.9 seg |
|
INR |
1.26 |
|
Partial thromboplastin time (TPT) |
33.6 seg |
|
Kidney function |
|
|
Creatinine |
6.2 mg/dL |
|
Blood urea nitrogen (BUN) |
70 mg/dL |
|
Liver profile |
|
|
Aspartate aminotransferase (AST) |
194 U/L |
|
Alanine aminotransferase (ALT) |
46 U/L |
|
Total bilirubin |
0.83 mg/dL |
|
Direct bilirubin |
0.6 mg/dL |
|
Indirect bilirubin |
0.23 mg/dL |
|
Creatine phosphokinase test (CPK) |
6.778 UI/L |
|
Complement |
|
|
C3 |
42.3 mg/dL |
|
C4 |
15.9 mg/dL |
|
Urinalysis |
|
|
Appearance |
Cloudy |
|
Color |
Yellow |
|
pH |
5 |
|
Specific gravity |
1.020 |
|
Glucose |
Normal |
|
Proteins |
Negative |
|
Ketones |
10 mg/dL |
|
Blood |
300 mg/dL |
|
Leucocytes |
Negative |
|
Bilirubin |
Negative |
|
Urobilinogen |
Normal |
|
Nitrites |
Negative |
|
Urine sediment |
|
|
Leucocytes |
59/uL |
|
High erythrocytes |
19/uL |
|
Low erythrocytes |
100/uL |
|
Bacteria |
2-5/field |
|
Granular casts |
2-4/field |
|
Hyaline casts |
0-2/field |
Source: Own elaboration.
Since the patient presented with acute fever and piriformis syndrome, a lumbar puncture was performed, and the results suggested an infectious etiology. Additionally, a chest X-ray was requested, which showed no pathological findings, as well as a computed tomography (CT) scan of the skull (Figure 1), which showed hypodense compressed areas on the right lateral ventricle, the right semioval center, the caudate nucleus, and the anterolateral location of the right thalamus.
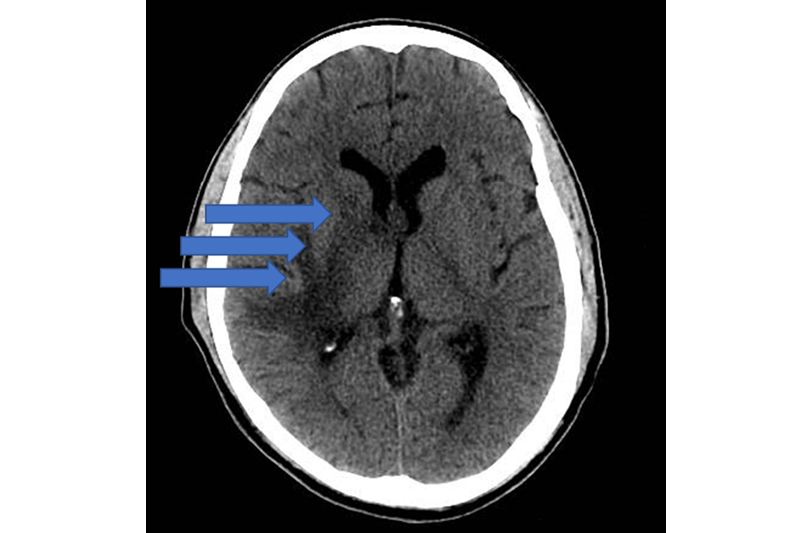
Figure 1. Computed tomography (CT) scan of the skull. The three arrows point to a compressive defect in the brain.
Source: Image obtained while conducting the study.
Taking into account the clinical and paraclinical data, it was considered that the findings suggested a state of sepsis with compensatory anti-inflammatory response. Based on these findings, the group of treating physicians suspected a possible case of toxoplasmosis, so trimethoprim/sulfamethoxazole (TMP/SMX) 80mg/400mg intravenously every 8 hours for three weeks was prescribed, considering that pyrimethamine with sulfadiazine or folinic acid was not available at the institution. Moreover, an IgG immunoglobulin blood test for toxoplasma was requested on the same day. A neoplasm at the central nervous system (CNS) level was also suspected, so a brain MRI was requested, but it could not be performed because, on the second day of admission (October 20, 2019), the patient developed altered state of consciousness and respiratory failure, requiring orotracheal intubation and mechanical ventilation with invasive mechanical ventilation with protective parameters in the intensive care unit (ICU).
Other studies performed on admission to the ICU included gray zone procalcitonin test: 1.02 ng/mL (upper limit: >2 ng/mL; high probability of bacterial infection); blood cultures: negative; serology for hepatitis B and C: negative; autoimmune profile with rheumatoid factor: positive (19. 8; normal values < 8 IU/mL);
and anti-dsDNA, antinuclear antibodies (ANA), and antineutrophil cytoplasmic antibodies (ANCA): negative. Likewise, diabetes was ruled out, and the studies performed for chronic kidney disease were negative.
On the fourth day of hospital stay (October 22, 2019), the patient presented acute renal failure, attributable to hemodynamic instability due to sepsis. Renal replacement therapy (hemodialysis) with standard hemofiltration was immediately started. Given that his condition remained stable, without adequate response to supportive care, and creatine phosphokinase was measured again, yielding a value of 4 563 UI/L, muscle involvement was suspected as the probable origin of the symptoms (infectious myositis with risk of fasciitis). Consequently, treatment was started with clindamycin 600mg intravenous every 6 hours for at least three weeks and metronidazole was discontinued (medication administered since admission to the emergency department). Persistent anemia requiring transfusion (below 7g/dL) and severe thrombocytopenia (below 10x103/mm3), were also documented, requiring repeated blood transfusion.
On October 23, 2019, the result of the Toxoplasma gondii IgG immunoglobulin IgG analysis was received, showing a positive result: 164.5 IU/mL (positive: ≥1.0 IU/mL);
no anti-toxoplasma IgM immunoglobulin IgM analysis, nor avidity test were performed due to limitations in the institutional laboratory at that time. Despite treatment, the patient progressively presented hemodynamic deterioration, requiring vasoactive support, initially with intravenous norepinephrine in continuous infusion with progressive increases in dosage. Antimicrobial therapy was adjusted to meropenem 1g intravenous every 12 hours, clindamycin 600mg intravenous every 6 hours, vancomycin 500mg/day intravenous, and TMP/SMX 80mg/400mg intravenous every 8 hours due to his hemodynamic instability.
The same day, upon interviewing his relatives, it was confirmed that the patient had no history of renal failure, autoimmunity, or metabolic disease. On the sixth day of hospital stay, tests for HIV, dengue, syphilis and malaria were performed, and the results were negative. There was also no documentation of possible adverse drug reactions to the antimicrobial therapy received throughout his hospital stay; likewise, follow-up blood cultures were negative.
Then, on the seventh day of hospital stay, a transesophageal echocardiogram was performed, which ruled out endocarditis, as well as a new lumbar puncture, which reported lymphocitic pleocytosis (104/mm3), hypoglycorrhachia (39mg/dL), and hyperproteinorrhachia (303mg/dL). Samples were also taken for microbiological examination with culture for common germs, which was negative. Adenosine deaminase analysis (ADA) was also performed, and the result was negative. These results supported the neurological involvement due to toxoplasmosis.
On October 29, 2019, the patient presented with multiple organ failure, with no therapeutic response despite providing renal support therapy, ventilatory support, repeated transfusions, and maximum doses of vasopressors. He finally passed away thirteen days after his admission, on October 31, 2019. At autopsy performed on November 1, 2019, during the cerebral histopathological study, bradyzoites and extracellular tachyzoites of Toxoplasma gondii were observed in the right cerebral hemisphere with massive involvement of the basal nuclei (Figure 2).
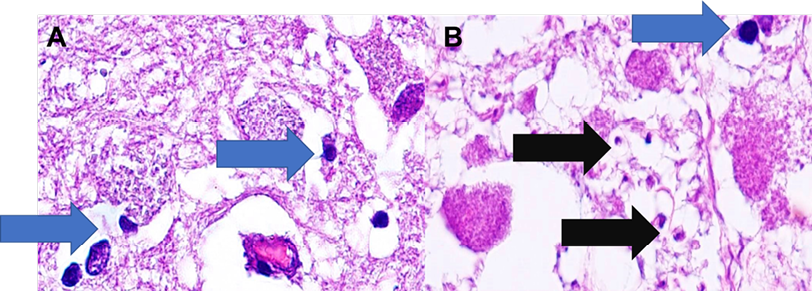
Figure 2. Right lateral ventricle: (A) Right basal ganglia nuclei with bradyzoites (blue arrow). (B) Coagulative necrosis with encysted forms containing bradyzoites (blue arrow) and extracellular tachyzoites (black arrow).
Source: Image obtained while conducting the study.
Discussion
Some features of severe toxoplasmosis cases in immunocompetent patients are striking and should be analyzed in the light of current knowledge, since multiple organ failure and severe or even fatal outcomes are rare in immunocompetent patients with acute toxoplasmosis, although they are common in immunocompromised patients (5-7). Since the patient was admitted to the hospital, different hypotheses appeared, including meningoencephalitis due to any other microbial agent and neoplasm of the central nervous system, as the diagnosis of acute disseminated toxoplasmosis is not expected in a patient with severe meningitis and multiple organ failure (2,6). The association of toxoplasmosis with meningoencephalitis is rare, regardless of immune status; however, fever and headache are common symptoms (8).
As observed in the presented case, antibiotic therapy, proposed based on the patient’s general condition, did not achieve a therapeutic response. For this reason, toxoplasmosis should be considered among the main differential diagnoses of acute febrile syndrome in Colombia due to the high risk of late diagnosis, nonspecific clinical manifestations, and development of complications (5). Significant exposure to the agent has also been described in the country, with a seroprevalence in the general population of 47.1%, with a prevalence of 46.3% among women and 47.9% among men (9-11).
Differentiating between toxoplasma encephalitis and acute disseminated encephalomyelitis is challenging due to the overlapping of clinical and radiological findings; therefore, magnetic resonance imaging is the study of choice for proper diagnosis (1-2). In this case, due to the fatal clinical outcome, it was not possible to perform an MRI of the brain. Cerebrospinal fluid studies revealed pleocytosis and hyperproteinorrachia, findings that can occur in both meningoencephalitis and acute disseminated encephalomyelitis.
Toxoplasma encephalitis is uncommon in immunocompetent patients; however, in patients with severe immunodeficiency, especially in patients with acquired immunodeficiency syndrome (AIDS), the presence of abscesses in the central nervous system is distinctive (12). Dermatological manifestations of acute disseminated toxoplasmosis are rare, but manifestations such as maculopapular rashes have been reported (7,13-14). In the present case, maculopapular rash was observed from the beginning; this is a finding that should be considered in the clinical manifestations during the diagnosis.
A four-fold increase in serum specific IgG titers between two samples separated by an interval of at least 2 weeks confirms the diagnosis of acquired Toxoplasma gondii infection (2,8). In simple samples, the diagnosis may be suspected due to the presence of IgM, IgA, or IgE antibodies. IgM and IgA antibodies may be present for a few weeks and persist for years. Initially, after primary infection, IgG antibody avidity is usually low (8,13). In the case reported, the measurement of IgM, IgA or IgE antibodies was not possible due to the rapid fatal course, as the patient died in a period shorter than the required interval. Without the possibility of a new measurement of antibodies, the diagnosis was confirmed through post-mortem histopathology.
One of the laboratory findings found is the elevation of liver enzymes during the disease; in previous case reports (15), this finding could be associated with fatal dissemination of the infection. A striking finding in the present report is the marked elevation of creatine kinase at disease onset, but the pathophysiological mechanisms of this alteration are not well understood. Although this finding could increase the spectrum of differential diagnoses to be considered, previous case reports of acute disseminated toxoplasmosis have shown that this alteration, if identified early, is associated with a poor prognosis of the disease and an eminently fatal course (5,16).
The patient in this case report, whose medical condition was later confirmed by the family, had no history or evidence of recurrent infections, metabolic diseases or exposure to immunosuppressive treatments or other risk factors for the development of a secondary immunodeficiency that would facilitate infection and fatal disease progression. During his hospital stay, relevant studies were performed in search of autoimmunity, neoplasms, metabolic disease, or connective tissue disease, which were all negative. In his home, he had access to drinking water and basic services; however, retrospectively, it was found that a close relative, whom he visited frequently, had five cats as pets, which could be considered a risk factor for the transmission of this parasite and the development of an infection that ended in the death of the patient (15,17). This risk factor was not considered from the beginning and, therefore, was not sufficiently investigated, thus preventing treating physicians from taking this possible diagnosis into account at an early stage.
Therapy with pyrimethamine-sulfadoxine (PYR-SDZ) plus folinic acid has been associated with effective clinical response, so it remains the treatment of choice for disseminated toxoplasmosis, as these drugs have the ability to penetrate the blood-brain barrier of the central nervous system (18-19). In patients without tolerance to PYR-SDZ or in cases where PYR-SDZ is not available, TMP/SMX is a reasonable alternative (19). However, in the specific context of toxoplasma encephalitis, randomized controlled studies are required to establish its benefits compared to other treatment systems (18-19). In the case presented here, treatment with TMP/SMX was indicated since PYR-SDZ was not available; however, the patient’s clinical course suggested that this treatment was delayed since he was in a state of sepsis with compensatory anti-inflammatory response before starting targeted therapy. On the other hand, it was not possible to evaluate any possible drug resistance of the parasite using molecular methods, nor was it possible to conclude its genotyping due to the unavailability of such a study at the institution.
Conclusions
Acute disseminated toxoplasmosis poses a diagnostic challenge, especially because it can mimic other etiologies that may be infectious and non-infectious. In cases of acute febrile syndrome, the possibility of acute disseminated toxoplasmosis should be kept in mind and, if possible, the early benefit of the patient should be sought with a timely diagnosis. The understanding of acute disseminated toxoplasmosis in immunocompetent patients remains a developing topic; new clinical and biological findings have emerged in recent years, therefore, more time is needed for a stronger understanding of this disease and its early diagnosis.
Ethical considerations
Informed consent was obtained from the patient’s relatives. Furthermore, the clinical case was approved by the research committee of the Hospital Universitario de La Samaritana, Bogotá D.C. (Colombia).
Conflict of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
To Dr. Lorena Regina Santodomingo, Department of Pathology, Hospital Universitario de La Samaritana, for her assistance in the review of the manuscript and histopathological interpretation.
1.Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264-296. https://doi.org/f3zg7r.
2.Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand J Infect Dis. 2012;44(11):805-814. https://doi.org/gfgp6v.
3.Milne G, Webster JP, Walker M. Toxoplasma gondii: AnUnderestimated Threat? Trends Parasitol. 2020;36(12):959-969. https://doi.org/kfkc.
4.Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999-2004, Decline from the prior decade. Am J Trop Med Hyg. 2007;77(3):405-410. https://doi.org/kd6z.
5.Cortés AD, Aguirre N. Toxoplasmosis aguda diseminada fatal en una paciente adulta inmunocompetente proveniente del Pacífico colombiano. Biomedica. 2018;38:19-23. https://doi.org/kffw.
6.Demar M, Hommel D, Djossou F, Peneau C, Boukhari R, Louvel D, et al. Acute toxoplasmoses in immunocompetent patients hospitalized in an intensive care unit in French Guiana. Clin Microbiol Infect. 2012;18(7):1-11. https://doi.org/fhc8gt.
7.Leal FE, Cavazzana CL, de Andrade HF, Jimenez-Galisteo A, Silva-de Mendonça J, Kallas EG. Toxoplasma gondii pneumonia in immunocompetent subjects: Case Report and Review. Clin Infect Dis. 2007;44(6):e62-e66. https://doi.org/bgvdkz.
8.Neves ES, Bicudo LN, Curi AL, Carregal E, Bueno WF, Ferreira RG, et al. Acute acquired toxoplasmosis: clinical-laboratorial aspects and ophthalmologic evaluation in a cohort of immunocompetent patients. Mem Inst Oswaldo Cruz. 2009;104(2):393-396. https://doi.org/bk7xvw.
9.Pino LE, Salinas JE, López MC. Descripción de un brote epidémico de toxoplasmosis aguda en pacientes inmunocompetentes miembros de las fuerzas militares de Colombia durante operaciones de selva. Infectio. 2009;13(2):83-91. https://doi.org/kff2.
10.Cañón-Franco WA, López-Orozco N, Gómez-Marín JE, Dubey JP. An overview of seventy years of research (1944-2014) on toxoplasmosis in Colombia, South America. Parasit Vectors. 2014;7:427. https://doi.org/kff3.
11.Ramírez AM, Ríos YK, Galvis NF, Entrena E, Mariño NV, Rangel DM, et al. Seroprevalencia y detección molecular de Toxoplasma gondii en donantes de un banco de sangre de Cúcuta, Colombia. Biomedica. 2019;39(2):144-156. https://doi.org/kff5.
12.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965-1976. https://doi.org/bpnb9p.
13.Bossi P, Caumes E, Paris L, Dardé ML, Bricaire F. Toxoplasma gondii-associated Guillain-Barre syndrome in an immunocompetent patient. J Clin Microbiol. 1998;36(12):3724-3725. https://doi.org/kff6.
14.Undseth Ø, Gerlyng P, Goplen AK, Holter ES, von der Lippe E, Dunlop O. Primary toxoplasmosis with critical illness and multi-organ failure in an immunocompetent young man. Scand J Infect Dis. 2014;46(1):58-62. https://doi.org/kff7.
15.Nunura J, Vásquez T, Endo S, Salazar D, Rodriguez A, Pereyra S, et al. Disseminated toxoplasmosis in an immunocompetent patient from Peruvian Amazon. Rev Inst Med Trop Sao Paulo. 2010;52(2):107-110. https://doi.org/c9272j.
16.Bossi P, Paris L, Caumes E, Katlama C, Danis M, Bricaire F. Severe acute disseminated toxoplasmosis acquired by an immunocompetent patient in French Guiana. Scand J Infect Dis. 2002;34(4):311-314. https://doi.org/bxxsq6.
17.Chiang TY, Kuo MC, Chen CH, Yang JY, Kao CF, Ji DD, et al. Risk factors for acute toxoplasma gondii diseases in Taiwan: a population-based case-control study. PLoS One. 2014;9(3):e90880. https://doi.org/kff8.
18.Rajapakse S, MC Shivanthan, Samaranayake N, Rodrigo C, Fernando SD. Antibiotics for human toxoplasmosis: A systematic review of randomized trials. Pathog Glob Health. 2013:107(4).:162-169. https://doi.org/kff9.
19.Wei HX, Wei SS, Lindsay DS, Peng HJ. A Systematic Review and Meta-Analysis of the Efficacy of Anti-Toxoplasma gondii Medicines in Humans. PLoS One. 2015;10(9):e0138204. https://doi.org/kfgc.
Referencias
References
Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264-296. https://doi.org/f3zg7r.
Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand J Infect Dis. 2012;44(11):805-814. https://doi.org/gfgp6v.
Milne G, Webster JP, Walker M. Toxoplasma gondii: AnUnderestimated Threat? Trends Parasitol. 2020;36(12):959-969. https://doi.org/kfkc.
Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999-2004, Decline from the prior decade. Am J Trop Med Hyg. 2007;77(3):405-410. https://doi.org/kd6z.
Cortés AD, Aguirre N. Toxoplasmosis aguda diseminada fatal en una paciente adulta inmunocompetente proveniente del Pacífico colombiano. Biomedica. 2018;38:19-23. https://doi.org/kffw.
Demar M, Hommel D, Djossou F, Peneau C, Boukhari R, Louvel D, et al. Acute toxoplasmoses in immunocompetent patients hospitalized in an intensive care unit in French Guiana. Clin Microbiol Infect. 2012;18(7):1-11. https://doi.org/fhc8gt.
Leal FE, Cavazzana CL, de Andrade HF, Jimenez-Galisteo A, Silva-de Mendonça J, Kallas EG. Toxoplasma gondii pneumonia in immunocompetent subjects: Case Report and Review. Clin Infect Dis. 2007;44(6):e62-e66. https://doi.org/bgvdkz.
Neves ES, Bicudo LN, Curi AL, Carregal E, Bueno WF, Ferreira RG, et al. Acute acquired toxoplasmosis: clinical-laboratorial aspects and ophthalmologic evaluation in a cohort of immunocompetent patients. Mem Inst Oswaldo Cruz. 2009;104(2):393-396. https://doi.org/bk7xvw.
Pino LE, Salinas JE, López MC. Descripción de un brote epidémico de toxoplasmosis aguda en pacientes inmunocompetentes miembros de las fuerzas militares de Colombia durante operaciones de selva. Infectio. 2009;13(2):83-91. https://doi.org/kff2.
Cañón-Franco WA, López-Orozco N, Gómez-Marín JE, Dubey JP. An overview of seventy years of research (1944-2014) on toxoplasmosis in Colombia, South America. Parasit Vectors. 2014;7:427. https://doi.org/kff3.
Ramírez AM, Ríos YK, Galvis NF, Entrena E, Mariño NV, Rangel DM, et al. Seroprevalencia y detección molecular de Toxoplasma gondii en donantes de un banco de sangre de Cúcuta, Colombia. Biomedica. 2019;39(2):144-156. https://doi.org/kff5.
Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965-1976. https://doi.org/bpnb9p.
Bossi P, Caumes E, Paris L, Dardé ML, Bricaire F. Toxoplasma gondii-associated Guillain-Barre syndrome in an immunocompetent patient. J Clin Microbiol. 1998;36(12):3724-3725. https://doi.org/kff6.
Undseth Ø, Gerlyng P, Goplen AK, Holter ES, von der Lippe E, Dunlop O. Primary toxoplasmosis with critical illness and multi-organ failure in an immunocompetent young man. Scand J Infect Dis. 2014;46(1):58-62. https://doi.org/kff7.
Nunura J, Vásquez T, Endo S, Salazar D, Rodriguez A, Pereyra S, et al. Disseminated toxoplasmosis in an immunocompetent patient from Peruvian Amazon. Rev Inst Med Trop Sao Paulo. 2010;52(2):107-110. https://doi.org/c9272j.
Bossi P, Paris L, Caumes E, Katlama C, Danis M, Bricaire F. Severe acute disseminated toxoplasmosis acquired by an immunocompetent patient in French Guiana. Scand J Infect Dis. 2002;34(4):311-314. https://doi.org/bxxsq6.
Chiang TY, Kuo MC, Chen CH, Yang JY, Kao CF, Ji DD, et al. Risk factors for acute toxoplasma gondii diseases in Taiwan: a population-based case-control study. PLoS One. 2014;9(3):e90880. https://doi.org/kff8.
Rajapakse S, MC Shivanthan, Samaranayake N, Rodrigo C, Fernando SD. Antibiotics for human toxoplasmosis: A systematic review of randomized trials. Pathog Glob Health. 2013:107(4).:162-169. https://doi.org/kff9.
Wei HX, Wei SS, Lindsay DS, Peng HJ. A Systematic Review and Meta-Analysis of the Efficacy of Anti-Toxoplasma gondii Medicines in Humans. PLoS One. 2015;10(9):e0138204. https://doi.org/kfgc.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2023 Case reports

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Los autores al someter sus manuscritos conservarán sus derechos de autor. La revista tiene el derecho del uso, reproducción, transmisión, distribución y publicación en cualquier forma o medio. Los autores no podrán permitir o autorizar el uso de la contribución sin el consentimiento escrito de la revista.
El Formulario de Divulgación Uniforme para posibles Conflictos de Interés y los oficios de cesión de derechos y de responsabilidad deben ser entregados junto con el original.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons 4.0 que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).