Centros de información de medicamentos. Una visión global del concepto
Drug information centers: An overview to the concept
DOI:
https://doi.org/10.15446/rcciquifa.v45n2.59940Palabras clave:
Centro de Información de Medicamentos, uso racional de medicamentos (es)Drug Information Center, rational drug utilization (en)
Los Centros de Información de Medicamentos (CIM) ocupan un lugar importante en el proceso de atención en salud al proporcionar información independiente y actualizada. Con el fin de conocer el estado del arte de los CIMs alrededor del mundo, se desarrolló una búsqueda en las bases de datos Medline, Science Direct, Academic Search Complete, LILACS y en Google Académico. Revisando cronológicamente la creación de CIMs, se encontró que el primero fue fundado en Europa en 1960. Posteriormente, se crearon en Estados Unidos en 1962, Singapur, 1980 y Venezuela, 1981. En India se fundó un CIM en 1997, posteriormente la OMS dictó talleres en ese país para fortalecer la creación de nuevos CIMs en 2006. En Asia se realizó un estudio en 1996 que identificó cuatro CIMs. En cuanto a Latinoamérica, se encontraron estudios de Brasil (2001) y Costa Rica (2003) que describen los CIMs locales. Adicionalmente, en 2011, se creó la red de CIMs de Latinoamérica y el Caribe (REDCIMLAC). En todos los estudios consultados se identificaron características de funcionamiento de los CIMs, como infraestructura, tipo de consultas, personal, entre otras. Algunos de estos estudios incluyeron una comparación con el documento técnico de la OMS.
https://doi.org/10.15446/rcciquifa.v45n2.59940
Drug information centers: An overview to the concept
Centros de información de medicamentos. Una visión global del concepto
Mónica Alexandra Nova Manosalva1*, José Julián López Gutiérrez1**, Martin Cañas2
1 Universidad Nacional de Colombia, Departamento de Farmacia. Carrera 30 N.° 45-03, Bogotá, Colombia. Grupo RAM: Red para el uso Adecuado de Medicamentos.
2 Federación Médica de la Provincia de Buenos Aires, La Plata, Argentina.
* E-mail: manovam@unal.edu.co
*E-mail: jjlopezg@unal.edu.co
Received: July 26, 2015 Accepted: June 22, 2016
Summary
Drug Information Centers (DIC) have an important place in the health care process since they provide independent and updated information. A search was developed in Medline, Science Direct, Academic Search Complete, LILACS and Academic Google in order to know the state of the art of DIC around the world. Regarding to a timeline, the first European DIC was created in 1960 and studies that described local situation were identified in 1996 and 2001. Thereafter, in the United States the first DIC was created in 1962 and 3 studies that describe DIC characteristics and changes trough time were identified between 2003 and 2008. Moreover, DICs were created in Singapore in 1980 and in Venezuela in 1981. In India was created a DIC in 1997. Subsequently, the WHO performed workshops in 2006 in order to create new centers in this country. In Asia was conducted a study in 1996 that identified 4 DIC. Concerning Latin America were found studies that describe local DIC from Brazil (2001) and Costa Rica (2003). Also, the network of Latin American and Caribbean DICs (REDCIMLAC) was created in 2011. In all consulted studies the DICs features were described including type of questions, professionals and infrastructure among others. Some of these studies included a comparison with the WHO technical document.
Keywords: Drug Information Center, rational drug utilization.
Resumen
Los Centros de Información de Medicamentos (CIM) ocupan un lugar importante en el proceso de atención en salud al proporcionar información independiente y actualizada. Con el fin de conocer el estado del arte de los CIMs alrededor del mundo, se desarrolló una búsqueda en las bases de datos Medline, Science Direct, Academic Search Complete, LILACS y en Google Académico. Revisando cronológicamente la creación de CIMs, se encontró que el primero fue fundado en Europa en 1960. Posteriormente, se crearon en Estados Unidos en 1962, Singapur, 1980 y Venezuela, 1981. En India se fundó un CIM en 1997, posteriormente la OMS dictó talleres en ese país para fortalecer la creación de nuevos CIMs en 2006. En Asia se realizó un estudio en 1996 que identificó cuatro CIMs. En cuanto a Latinoamérica, se encontraron estudios de Brasil (2001) y Costa Rica (2003) que describen los CIMs locales. Adicionalmente, en 2011, se creó la red de CIMs de Latinoamérica y el Caribe (REDCIMLAC). En todos los estudios consultados se identificaron características de funcionamiento de los CIMs, como infraestructura, tipo de consultas, personal, entre otras. Algunos de estos estudios incluyeron una comparación con el documento técnico de la OMS.
Palabras clave: Centro de Información de Medicamentos, uso racional de medicamentos.
Introduction
More than 50 years after the development of the concept of Drug Information Center, they occupy an irreplaceable position in the health care process, to be the ideal sources of scientific information for the user through the available database, to receive appropriate information to solve their problem or special need [1].
The Pan American Health Organization (PAHO) defines Drug Information Centers (DIC) as operational units that provide technical and scientific information about drugs in an objective and timely manner. Also states that they constitute an optimal strategy to meet particular needs of information. To do this, the DIC have databases and sources of drug information and trained professionals that generate independent information relevant to the requests that are made or any need identified [2]. Other authors define a DIC as "an institution dedicated to provide objective, independent and current information on drugs and their use, and communicate to the different categories of users for better understanding and benefit of patients" [1]. On the other hand, PAHO defines Drug Information Services (DIS) as part of the activities of pharmaceutical services of a health institution with essential presence of the pharmaceutical professional who provides drug information services supported by scientific sources, updated and independent [1]. Therefore, DIS belongs to an institution and provides information services only within it. The overall objective of the DIC is to promote rational drug use through technical-scientific, objective, current, timely and relevant duly processed and evaluated information [2].
It is generally accepted that the DIC have two basic functions: the development of passive information addressed to solve or contribute to the solution of problems related to drug use in individual cases, and the development of Active Information, represented by education activities, dissemination of information and research in the field of drugs [1]. Within a DIC can specify the following functions: attention to drug information questions, information dissemination, research and education [1-2].
PAHO has raised some basic requirements to run a DIC, which contemplates a physical area used exclusively for the DIC and with sufficient capacity for normal operation (with an area for the reception of requests for information, for the library and work area or to read and assess the information); general office equipment (computer with printer and Internet access, microfiche reader, copier and external hotline and fax services); information resources including primary sources (journals); secondary (indexes, abstracts, databases) and tertiary (books, abstracts, forms), human resource consists of a center director (preferably a pharmacist or a doctor) with expertise in information, clinical pharmacology and therapeutics.
Attendees can be internal, students or interns and secretary, and funding sources that could be agreements between public and private institutions as long as it is never forgotten the preservation of the independence of the information supplied by the center or the collection of services provided by the DIC preferably with differential scales [1- 2].
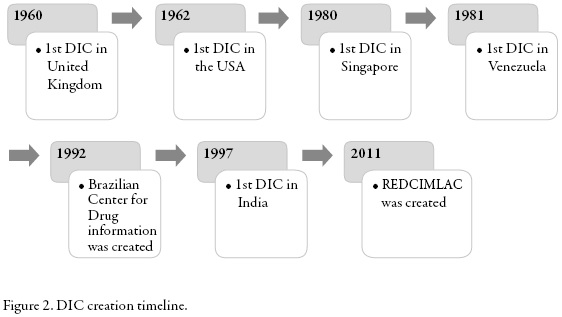
The first Drug Information Centre in Europe was created in 1960 in the UK. Since then they have been developing formal DICs worldwide in order to provide accurate and timely information in response to specific questions on drugs [3].
The above guidelines PAHO Drug Information Centers, which were raised in 1995 and 1997 [1-2] define the fundamental aspects of a DIC operation. However, some of these requirements may be obsolete or need an update.
Methodology
In order to identify whether the concept of DIC varied in different countries or whether there were differences in their functioning, a narrative review by mean search in Medline, Science Direct and Academic Search Complete, available on the platform SINAB (Universidad Nacional de Colombia Library) was performed, as well as in the LILACS database. Descriptors were used as search terms "drug information center", "drug information service" and "rational use of medicines drugs utilization". In the case of databases searched through SINAB platform, only articles with full text available were included.
Additionally a search in Google Academic and the Virtual Health Library, NLM were performed in order to complement the information on the state of the art of DIC in Latin America.
Results and discussion
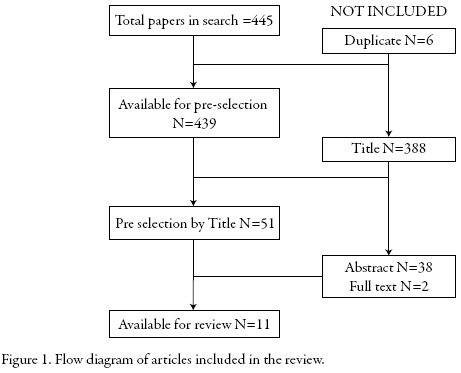
The search strategy found 445 articles. After removing duplicate papers and those papers that do not contain the term "Drug Information Center" in the title, it was proceeded to review the abstracts to identify those papers related to definition and functioning of DIC. Finally, 11 papers were available for review. Figure 1 shows the flow diagram.
This search allowed finding previous overviews about DIC. However, the scope of the found papers was national or about a specific region. This review sought to provide a description worldwide of DIC.
The first European DIC was created in United Kingdom in 1960. Since then, it had been developing formal DIC around the world [3].
In 1996 Müllerová and Vlcek implemented a survey of activities of 129 drug information centers in 18 European countries. The response rate was 71.3% (n=92) and the information from 84 DICs that met the inclusion criteria was analyzed. The results of the study show that DICs are affiliated mainly with European hospitals (68%), and rarely to pharmacy (6%) or medical schools (8.3%).
The activities of the DICs included service response questions (98%), publishing newsletters (68%), participation in committees of Pharmacy and Therapeutics (F & T) (63%), tutorials (61%) and use evaluation drug (52%). The most frequently employees of DICs are the pharmacists, usually 1 or 2 full time or part time. By analyzing the query resolution service it was found that 56% of the DICs offer their services only to health professionals and 43% to both these professionals and the general public. The questions were mainly related to adverse effects, indication / therapeutic use and dosage of drugs. Most DICs (91%) frequently recorded their activities in a computer database. Quality assurance activities were developed in 75% of centers, usually by means of reviews (58%) or feedback questionnaires (32%). The most frequently used information sources were Martindale-The Extrapharmacopeia, journals such as Lancet and databases such as Medline and Micromedex. The DICs are generally funded by the organizations they are affiliated with. The 9.5% of DICs charged fees for special activities [4].
In 2001 Scala et al. published a study of Italian Information Centers. The results resembled European and American trends [3]. It was found that the number of DICs in Italy grew rapidly since 1985, but then stalled in the 90s. According to the authors, this behavior is due to the availability of computerized drug information designed to be user friendly, many of them online, which could facilitate to satisfy the users information needs without consulting a formal DIC. Comparing the results with those found in a survey conducted in Italy in 1997, a greater number of DICs acquired direct Internet access (60%), allowing pharmacists to use online resources. Another finding was an increase in the number of DICs produced newspapers and newsletters (48% in 1997 vs. 90% in 1999), reflecting the increased interest in active information [3].
In the United States the first Drug Information Center was created in 1962 at the University of Kentucky and since then some studies have been published which review various aspects of U.S. DICs. Rosenberg et al. have studied the national scene DICs using surveys since 1974, in 2003 it was conducted a study that aimed to make a diagnosis of the current state of the centers and compare the results with those of similar surveys applied in the past 30 years. 151 DICs were contacted, and 119 of them responded to the survey (79%); 81 met the inclusion criteria.
The U.S. centers were located in 33 states, the District of Columbia and Puerto Rico. The results showed that most DICs are affiliated with hospitals or medical centers (72%) and pharmacy schools (61%). The staff was composed mainly of pharmacists, students, interns, residents, secretaries and administrative staff. Only 12% of the DICs reported not having at least one full-time pharmacist, 71% of pharmacists were doctors of pharmacy (PharmD) and 24% had bachelor's degree, plus 9% had a master's degree in science, master's degree in business administration, master of public health, or doctoral (Ph.D.), 94% of reported DICs had within its staff at least 1 pharmacist PharmD title.
The services offered by DICs, from the U.S, in addition to answering questions about medications, included the preparation of newsletters (80%), participation in activities of the pharmacy and therapeutics committees (79%), training or education (79%), development of administrative tasks not directly related to the DIC (66%), literature search queries not related to daily living (65%), reporting of adverse reactions (65%), and development of medicines use reviews (63%). The main consultants were pharmacists (40.6%), doctors (24.4%) and nurses (15%). The questions were related more often with therapeutic issues (17%), adverse reactions (16.2%), identification of U.S. or foreign products (14.3%) and dosage (10.1%). For the system used to access, store and retrieve the questions and answers, 38% of the DICs used a computerized database, 32% use a paper system and 30% used both. A formal program of quality assurance had been implemented in 51% of the DICs and in 18% it was being developed, the consultation service drug information is most commonly evaluated using criteria such as accuracy, that the answer is complete, documentation references used, timeliness, clarity, objectivity and impact on patient care.
In the U.S. centers, the most common method for judging was an internal review by a person (70%), followed by feedback from the applicant (36%), and internal review by a committee (21%). The most commonly reported source acknowledged as useful was Micromedex Healthcare Series, followed by Medline and AHFS Drug Information. The DICs funding came primarily from U.S. hospitals and medical centers (73%) and schools and universities (37%), other sources including charging fees for certain activities (19%), federal and state aid (5%), pharmaceutical companies (4%) and non-governmental organizations and foundations (1%). When comparing these results of 2003 with those of similar surveys applied in the past 30 years, it was found that the number of DICs decreased in the last decade and the number of pharmacists and other staff was the lowest in the last 30 years. Pharmacists were better trained then than in the past and a large percentage had high educational qualifications. The services remained consistent with previous findings, except for the increased participation in training and education of pharmacy students and residents. The percentage of DICs system with formal quality assurance did not change significantly in the past decade and funding resources and service fees remained the same [5].
In 2008, Rosenberg et al. conducted a study that surveyed 89 U.S. DICs previously identified in 2003 to determine if they still existed and if they had experienced changes. We found that 75 (84%) of the 89 DICs remained active. The most notable change in the activities was the increase in the time spent on education to students in the area of health (53%), in supporting the pharmacovigilance program of the institution (44%), and to provide system support information (36%). No changes in the number of employees were observed. With respect to the number of questions received, 29% of the DICs reported an increase, 42% a decrease and 29% no change, 70% reported having had an increase in the number of complex questions, while 53% reported an increase the time required to answer each question [6].
In the United States, FDA sponsored two DICs, one located in the (Center for Biologics Evaluation and Research [CBER]) and another in the Center for Drug Evaluation and Research (CDER). Each DIC provided product information and regulatory guidance. Representing the regulatory agency, these DICs provided services to a wider range of applicants than those DICs belonging to hospitals, universities and the pharmaceutical industry. In essence, served as a liaison between the public and FDA [7].
The CBER had divided his work into two branches DIC: the first was in charge of consumer-related issues and the second to assist manufacturers and technical training, the first was the one who answered questions on issues related to eligibility criteria for blood and tissues donor, approval of products, and tips for finding clinical trials in a specific search. The human resource CBER DIC is comprised of individuals who had received a course of at least 30 hours of education in science, public affairs specialists and education. Each branch had about 6 people with a wide range of professions, such as nursing, social work, animal science, journalism and molecular genetics. About 600 questions were answered per month and the response time of most queries was approximately 1 to 3 days. On the CDER, Division of Drug Information (DDI) was responsible for answering questions from the public about products for human use. The topics of the queries were related to clinical aspects of particular molecule or a group of drugs and drug regulation either new, as generic or OTC. In the DDI 12 pharmacists and one nurse worked and they received about 1,000 phone calls and 900 emails per week. The answers were usually given by phone the next day and the mails were responded within two days [7]. The main difference of these DICs of the FDA with those DICs of hospitals and universities was that the former does not provide medical advice to abstain from prescribing information approved by the FDA; therefore, these DICs did not necessarily perform an extensive literature search that took into account other uses other than those approved by the FDA. The DDI had great confidence in the information posted on the FDA Web site but also used traditional information resources of a DIC. An additional reference used was the internal databases that allow to review previously resolved questions. The two DICs answered questions that might require the use of multiple sources in their respective divisions, including offices of pro- duct review, quality, medical policies, etc. [7].
Reviewing the situation in other parts of the world, a study was found in 2006 in Bangalore, India, where the World Health Organization (WHO) conducted a training workshop which included an introduction to information activities of drugs and rational use thereof. The course was part of a program to spread the influence of drug information centers and training programs in clinical pharmacy that had been developed in southern India during the previous 10 years. This program was coordinated by the Board of Pharmacy of the state of Karnataka (KSPC) and sponsored by the WHO office in India [8]. The KSPC founded a DIC in 1997 but the project included the creation of five independent DICs across the country [9]. The projected centers might provide information to health personnel and the general public, and also meet the reporting of suspected adverse reactions [8]. These centers would act as state DICs able to respond to the level of detail requested [9]. The WHO planned to grant limited financial resources to purchase information resources but in the long term would require financial support from the state [8]. As part of the financing it would receive an initial amount to purchase reference books and extra money monthly for 6 months for recurrent expenditure [9].
Very little was known about the DICs of eastern Asia. In 1996 a drug information pharmacist handed a formal setting and it was a relatively new concept in this part of the world. It was not until 1980 that the first organized DIC was created in Singapore. Then in 1996, Lim and Chui conducted a study conducted by pharmaceutical DICs there. At the time of the study there were 3 DICs located in hospitals and 1 in the Pharmaceutical Department of the Ministry of Health. Hospitals were in charge for the funding for the first 3, while the latter were funded by the government and no fees were charged in cases for services or external applicants. All centers were well equipped with cabinets, telephone, fax, photocopier, computers, and Internet access (with the exception of one DIC). The most used bibliographic resource was Micromedex, followed by Martindale, AHFS Drug and compendia as Index of Malaysia & Singapore and British National Formulary. Less than 4% of the consultations needed secondary sources of literature, but Medline was the resource of choice in such cases. All DICs had access to a medical library.
Of the four DICs of Singapore surveyed, three had one on staff full-time pharmacist. None had any formal training in drug information as graduate training; one of them had a master's degree in Clinical Pharmacy, while another was studying for the same title. There were no quality assurance programs except for a center that was sending feedback forms to users once a year, but all DICs had developed operational policies and procedures manuals.
The four centers of Singapore recorded manually each question received; these records contained the date, the applicant's name, a contact number, the question asked, the answer given, the response time, references used and the signature of the pharmacist who gave the information. The average monthly consultation was between 92 and 259. Most queries were answered in five minutes, only less than 10% required more than 1 hour to be resolved. In approximately 80% of the consultations was used only one reference. The most frequent consultants were physicians (51%), pharmacists (32%) and nurses (10%).
In hospital's DICs, the most common questions were dosing, availability of a drug and identification; others were choosing a therapy, administration, adverse effects and drug safety. In the DIC located in the Ministry of Health request printed literature consultation drug was the most common, followed by questions about adverse reactions, product availability, formulation and identification [10].
Latin American Situation
In 2003, Costa Rica performed a study diagnosis of DICs belonging to national public institutions, in order to determine the degree of fit to the requirements of the PAHO. The results showed that there were 7 public units drug information: 4 DICs and 3 Drug Information Services (DIS), of which 6 were located in a hospital and one at a university. The primary sources of information suggested by PAHO were not available in 5 of the centers. Of the 36 recommended tertiary sources, 15 were not available at any of the centers. The most frequent activities were resolving queries of the community hospital or outpatient users, implementation of education programs for patients and risk groups and programs for student training rotation. The authors conclude that the activities of different DIC in Costa Rica were similar to each other, not just respond to the guidelines of the PAHO, but have similarities with the activities and operations of other DIC worldwide, also showed that the bibliographical support must be strengthened [11].
Moreover, Brazil had established a nationwide network of DIC, which was organized on a decentralized, non-hierarchical manner and operates on cooperation protocols. The network was called The Brazilian Drug Information System (SISMED) and was the result of inter-agency effort to support multidisciplinary health team, optimizing the resources available in this area and promoting rational drug use. Also it could support the development of the pharmaco-epidemiological studies in the country, with emphasis on pharmacovigilance as one of the activities the DIC could do. Among the strategies to implement SISMED were specialized professionals and frequent meetings of the coordinators of the DICs to share experiences. Training courses had been given, and national meetings of professional from the DICs had been made, activities that have helped the development of the national network and the strengthening Brazilian DIC of drug information system in Brazil [12].
In 1992 the Federal Council of Pharmacy of Brazil (CFF), together with the Pan American Health Organization, created the Brazilian Center for Drug Information (CEBRIM-Centro Brasileiro de Informação sobre Medicamentos). Silva et al. conducted a cross-sectional descriptive study between November 2000 and October 2001 in order to analyze the results of CEBRIM and the opinion of its users [13].
The results were that CEBRIM answered 970 questions in the study period, with a monthly average of 81 questions, showing that this DIC answered more questions than many others Brazilian DICs at a rate of 30%. The percentage of patients who consulted the center was very low (34.5%). In 44% of cases the answer was delivered in 24 hours. Of the users surveyed, 89.5% reported receiving response time, 88% thought it was clear, objective and 85% that the information was complete. The 99.2% of patients declared its intention to use the DIC service. The authors conclude that the CEBRIM served its purpose of providing drug information in an objective, timely and updated manner. It could be considered that the center plays an important role in promoting rational drug use and is a useful tool for health professionals in the care of patients [13].
Since 2011 the Latin America and the Caribbean DIC Network (REDCIMLAC for its acronym in Spanish) has been formed, as an initiative of the Drug Utilization Research Group-LA and with support from PAHO/WHO to link the DIC in Latin America and the Caribbean, respecting their autonomy (web page: http://web2.redcimlac.org/) [14]. This network consists of 19 countries and 29 information centers. These centers were created and in some cases supported by PAHO / WHO so they all retain the basic guidelines of this organization white paper [14].
In figure 2 the creation of the DICs included in this review is summarized by year.
Although it was not possible to compare the same variables for all DIC it could be observed that they did not limit solely to answer consultations but they also participate in different activities, in some cases in education-related activities, in other cases in the productions of bulletins, participation in pharmacy and therapeutics committees, and other activities. It was also observed that most belong to hospitals or pharmacy schools.
With respect to financing, it was found that the economic resources come from independent sources like hospitals, government, universities, and the charging of fees for consultation; only one DIC study in the US reported that 4% of DIC receives economic resources from the pharmaceutical industry.
It is concluded that the concept and functions of the DIC are constant over time and across countries. As expected, an increase in the number of countries with an established CIM is evident worldwide.
Acknowledgements
The authors thank the Universidad Nacional de Colombia and the Network of Latin America and Caribbean Drug Information Centers (REDCIMLAC).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
1. R. D'Alessio, U. Busto, N. Girón, "Guía para el desarrollo de servicios farmacéuticos hospitalarios: información de medicamentos", Serie Medicamentos Esenciales y Tecnología N.° 54. Organización Panamericana de la Salud, 1997.
2. Grupo de trabajo regional sobre centros de información de medicamentos de la Organización Panamericana de la Salud, "Centros de Información de Medicamentos: una estrategia de apoyo al uso racional de medicamentos", PAHO HQ Library, Santo Domingo, 1995.
3. D. Scala, A. Bracco, S. Cozzolino, A. Cristinziano, C. De Marino, A. Di Martino, E. Gonzalez, A. Mancini, F. Romagnuolo, L. Zeuli, Italian drug information centres: benchmark report, Pharm. World Sci., 23, 217 (2001).
4. H. Müllerová, J. Vlcek, European drug information centres-survey of activities, Pharm. World Sci., 20, 131 (1998).
5. J.M. Rosenberg, T. Koumis, J.P. Nathan, L.A. Cicero, H. McGuire, Current status of pharmacist-operated drug information centers in the United States, Am. J. Health-Syst. Pharm., 61, 2023 (2004).
6. J.M. Rosenberg, S. Schilit, J.P. Nathan, T. Zerilli, H. McGuire, Update on the status of 89 drug information centers in the United States, Am. J. Health-Syst. Pharm., 66, 1718 (2009).
7. K.C. Dada, M.E. Kremzner, S.K. Bhanot, R. Lal, Role of FDA's drug information centers, Am. J. Health-Syst. Pharm., 65, 803 (2008).
8. International Pharmaceutical Federation (FIP), Developing drug information centres in India, WHO Drug Information, 21, 130 (2007).
9. BioBoard, KSPC to Set up Drug Information Centers with WHO Funding, Asia Pacific Biotech News, 11, 1 (2007).
10. L.Y. Lim, W.K. Chui, Pharmacist-operated drug information centres in Singapore, J. Clin. Pharm. Ther., 24, 33 (1999).
11. V. Hall, C. Gómez, F. Fernandez-Llimos, Situation of drug information centers and services in Costa Rica, Pharm. Pract. (Granada), 4, 83 (2006).
12. C. Vidotti, R. Hoefler, E.V. Silva, G. Bergsten-Mendes, Sistema Brasileiro de Informação sobre Medicamentos (SISMED), Cad. Saude Publica, 16, 1121 (2000).
13. E. Silva, L. Castro, L. Bevilaqua, C. Vidotti, R. Hoefler, CEBRIM: the Brazilian Drug Information Center: characterization of services provided and user's opinions, Rev. O.F.I.L., 13, 55 (2003).
14. Red de Centros de Información de Medicamentos de Latinoaméricana y el Caribe. URL: http://web2.redcimlac.org/, consultado en abril de 2013.
How to cite this article
M.A. Nova-Manosalva, J.J. López-Gutiérrez, M. Cañas, Drug information centers: An overview to the concept, Rev. Colomb. Cienc. Quím. Farm., 45(2), 243-255 (2016).
Referencias
(1) R. D’Alessio, U. Busto, N. Girón, “Guía para el desarrollo de servicios farmacéuticos hospitalarios: información de medicamentos”, Serie Medicamentos Esenciales y Tecnología N. ° 54. Organización Panamericana de la Salud, 1997.
(2) Grupo de trabajo regional sobre centros de información de medicamentos de la Organización Panamericana de la Salud, “Centros de Información de Medicamentos: una estrategia de apoyo al uso racional de medicamentos”, PAHO HQ Library, Santo Domingo, 1995.
(3) D. Scala, A. Bracco, S. Cozzolino, A. Cristinziano, C. De Marino, A. Di Martino, E. Gonzalez, A. Mancini, F. Romagnuolo, L. Zeuli, Italian drug information centres: benchmark report, Pharm. World Sci., 23, 217 (2001).
(4) H. Müllerová, J. Vlcek, European drug information centres-survey of activities, Pharm. World Sci., 20, 131 (1998).
(5) J.M. Rosenberg, T. Koumis, J.P. Nathan, L.A. Cicero, H. McGuire, Current status of pharmacist-operated drug information centers in the United States, Am. J. Health-Syst. Pharm., 61, 2023 (2004).
(6) J.M. Rosenberg, S. Schilit, J.P. Nathan, T. Zerilli, H. McGuire, Update on the status of 89 drug information centers in the United States, Am. J. Health-Syst. Pharm., 66, 1718 (2009).
(7) K.C. Dada, M.E. Kremzner, S.K. Bhanot, R. Lal, Role of FDA’s drug information centers, Am. J. Health-Syst. Pharm., 65, 803 (2008).
(8) International Pharmaceutical Federation (FIP), Developing drug information centres in India, WHO Drug Information, 21, 130 (2007).
(9) BioBoard, KSPC to Set up Drug Information Centers with WHO Funding, Asia Pacific Biotech News, 11, 1 (2007).
(10) L.Y. Lim, W.K. Chui, Pharmacist-operated drug information centres in Singapore, J. Clin. Pharm. Ther., 24, 33 (1999).
(11) V. Hall, C. Gómez, F. Fernandez-Llimos, Situation of drug information centers and services in Costa Rica, Pharm. Pract. (Granada), 4, 83 (2006).
(12) C. Vidotti, R. Hoefler, E.V. Silva, G. Bergsten-Mendes, Sistema Brasileiro de Informação
Sobre Medicamentos (SISMED), Cad. Saude Publica, 16, 1121 (2000).
(13) E. Silva, L. Castro, L. Bevilaqua, C. Vidotti, R. Hoefler, CEBRIM: the Brazilian Drug Information Center: characterization of services provided and user’s opinions, Rev. O.F.I.L., 13, 55 (2003).
(14) Red de Centros de Información de Medicamentos de Latinoaméricana y el Caribe.
URL:
Consultado en abril de 2013.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Mansour Almuqbil, Lamees Alrojaie, Haya Alturki, Abdullah Alhammad, Yasmin Alsharawy, Aljawharah Alkoraishi, Abdulaziz Almuqbil, Sara Alrouwaijeh, Syed Wajid, Mohamed N. Al-Arifi. (2022). The role of drug information centers to improve medication safety in Saudi Arabia - a study from healthcare professionals' perspective. Saudi Pharmaceutical Journal, 30(4), p.377. https://doi.org/10.1016/j.jsps.2022.01.024.
2. Giovanni Caivano, Fabio Massimo Sciarra, Pietro Messina, Enzo Maria Cumbo, Luigi Caradonna, Emanuele Di Vita, Salvatore Nigliaccio, Davide Alessio Fontana, Antonio Scardina, Giuseppe Alessandro Scardina. (2025). Antimicrobial Resistance and Causal Relationship: A Complex Approach Between Medicine and Dentistry. Medicina, 61(10), p.1870. https://doi.org/10.3390/medicina61101870.
3. Matthew P. Van Cuyk, Jason C. Cooper, James P. New. (2019). Clinical Pharmacy Education, Practice and Research. , p.123. https://doi.org/10.1016/B978-0-12-814276-9.00009-X.
4. G K Sadagoban, Aiswarya Baiju, Samantha Sanjeev, M Ayilya, Swathi Swaroopa Borra. (2021). A cumulative review on the utilisation of drug information services provided in India. Journal of Pharmaceutical Health Services Research, 12(3), p.452. https://doi.org/10.1093/jphsr/rmab029.
5. Johannes Heck, Dirk O. Stichtenoth, Ruxandra Sabau, Christoph Schröder, Stefan Engeli, Thorben Pape, Nina O’Connell, Carsten Schumacher, Olaf Krause, Felix Koop. (2022). Clinical-pharmacological drug information center of Hannover Medical School: experiences and analysis from a tertiary care university hospital. Scientific Reports, 12(1) https://doi.org/10.1038/s41598-022-24005-y.
6. Benjamin Krichevsky, Stefan Engeli, Stefanie M. Bode‐Böger, Felix Koop, Martin Schulze Westhoff, Sebastian Schröder, Carsten Schumacher, Thorben Pape, Dirk O. Stichtenoth, Johannes Heck. (2025). Human vs. artificial intelligence: Physicians outperform ChatGPT in real‐world pharmacotherapy counselling. British Journal of Clinical Pharmacology, https://doi.org/10.1002/bcp.70321.
7. Santhosh Shivabasappa, Akila Srinivasan. (2025). Introduction to Basics of Pharmacology and Toxicology. , p.51. https://doi.org/10.1007/978-981-95-1920-0_3.
8. Nishanthi Anandabaskar. (2019). Introduction to Basics of Pharmacology and Toxicology. , p.223. https://doi.org/10.1007/978-981-32-9779-1_14.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2016 Revista Colombiana de Ciencias Químico-Farmacéuticas

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
El Departamento de Farmacia de la Facultad de Ciencias de la Universidad Nacional de Colombia autoriza la fotocopia de artículos y textos para fines de uso académico o interno de las instituciones citando la fuente. Las ideas emitidas por los autores son responsabilidad expresa de estos y no de la revista.
Todo el contenido de esta revista, excepto dónde está identificado, está bajo una Licencia Creative Commons de Atribución 4.0 aprobada en Colombia. Consulte la normativa en: http://co.creativecommons.org/?page_id=13