Anthropogenic impacts on yellow-spotted river turtle Podocnemis unifilis (Reptilia: Podocnemididae) from the Brazilian Amazon
Los impactos antropogénicos sobre las tortugas cabeza amarilla Podocnemis unifilis (Reptilia: Podocnemididae) en la Amazonia brasileña
DOI:
https://doi.org/10.15446/abc.v21n2.49196Palabras clave:
Amazon, eggs, freshwater turtle, neonates, nests, Amazonia, huevos, nidos, recién nacidos, tortuga acuática. (en)Descargas
The purpuse of this study was to investigated the influence of anthropogenic impacts on Podocnemis unifilis nesting on a stretch from Falsino river, with two forest reserves, and one urban area on the Araguari river, state of Amapá, eastern Amazon (Brazil). A total of 180 nests were found, being 89.4 % in the forest reserves and only 10.6 % in urban areas. On Falsino river, we observed a spawning pattern, because the number of nests was correlated to the length and width of the nesting locations. On Araguari river, the P. unifilis nests were generally found in areas with surrounding vegetation up to 5 meters in height, minimum distance of 120 meters from residences and immediately or after places of higher exploration of pebbles. Females from Falsino river had smaller eggs, but the neonates were bigger and with higher body condition index than the neonates from Araguari river. About 80 % of the nests were prey, mostly because of the large collection of eggs for feeding. Furthermore, it was found that adult turtle hunting has been intense. Although one of the areas is in forest reserves, the human impacts were similar to those caused in urban areas, indicating the need to implement protection programs for the conservation of P. unifilis.
El propósito de este estudio fue investigar la influencia de los impactos antropogénicos en nidos de Podocnemis unifilis en un tramo del Río Falsino, se investigó en dos reservas forestales y tramo urbano del río Araguari, Estado de Amapá, en Amazonia (Brasil). Un total de 180 nidos fueron localizados y 89,4 % estaban en las áreas de reservas forestales, pero sólo el 10,6 % en las zonas urbanas. En el río Falsino, hubo desove patrón, el número de nidos se correlacionó con la longitud y la anchura de los sitios de anidación. En Río Araguari, nidos de P. unifilis se encuentran generalmente en lugares con vegetación circundante de hasta 5 metros de altura, distancia mínima de 120 metros de residencial y de inmediato o después de los lugares de mayor explotación de los guijarros. En el río Falsino, aunque las hembras han demostrado huevos más pequeños, los recién nacidos fueron mayores y la puntuación cuerpo también es mayor que en los recién nacidos del río Araguari. En la región del Río Araguari, aproximadamente el 80 % de los nidos fueron depredados, debido a la gran colección de huevos para la alimentación. Se observó que la presión de caza en las tortugas adultas ha sido intensa. Aunque es una de las áreas a ser bosques protegidos, los impactos humanos fueron similares a los de las zonas urbanas, lo que indica la necesidad de implementar programas de protección para la conservación de P. unifilis.
We investigated the influence of anthropogenic impacts on Podocnemis unifilis nesting on a stretch from Falsino river, with two forest reserves, and one urban area on the Araguari river, state of Amapá, eastern Amazon (Brazil). A total of 180 nests was found, being 89.4% in the forest reserves and only 10.6% in urban areas. On Falsino river, we observed a spawning pattern, because the number of nests was correlated to the length and width of the nesting locations. On Araguari river, the P. unifilis nests were generally found in areas with surrounding vegetation up to 5 m in height, minimum distance of 120 m from residences and immediately or after places of higher exploration of pebbles. Females from Falsino river had smaller eggs, but the neonates were bigger and with higher body condition index than the neonates from Araguari river. About 80% of the nests were prey, mostly because of the large collection of eggs for feeding. Furthermore, it was found that adult turtle hunting have been intense. Although one of the areas is in forest reserves, the human impacts were similar to those caused in urban areas, indicating the need to implement protection programs for the conservation of P. unifilis.
Doi: https://doi.org/10.15446/abc.v21n2.49196
ANTHROPOGENIC IMPACTS ON YELLOW-SPOTTED RIVER TURTLE Podocnemis unifilis (REPTILIA: PODOCNEMIDIDAE) FROM THE BRAZILIAN AMAZON
Los impactos antropogénicos sobre las tortugas cabeza amarilla Podocnemis unifilis (Reptilia: Podocnemididae) en la Amazonia brasileña
Débora Regina Santos ARRAES1, Helenilza Ferreira Albuquerque CUNHA1, Marcos TAVARES-DIAS2.
1 Universidade Federal do Amapá, UNIFAP, Rodoviaria Juscelino Kubitschek, Km 2, Marco Zero. Macapá, Brasil.
2 Empresa Brasileira de Pesquisa Agropecuária, Embrapa Amapá, Rodovia Juscelino Kubitschek, Km 5, 2600 Universidade. Macapá, Brasil.
For correspondence. arraesdebora@hotmail.com
Received: 15th March 2015, Returned for revision: 28th March 2015, Accepted: 23rd November 2015. Associate Editor: John Carr.
Citation / Citar este artículo como: Arraes DRS, Cunha HFA, Tavares-Dias M. Anthropogenic impacts on yellow-spotted river turtle Podocnemis unifilis (Reptilia: Podocnemididae) from the Brazilian Amazon. Acta biol. Colomb. 2016;21(2):413-421. doi: https://doi.org/10.15446/abc.v21n2.49196
ABSTRACT
The purpuse of this study was to investigated the influence of anthropogenic impacts on Podocnemis unifilis nesting on a stretch from Falsino river, with two forest reserves, and one urban area on the Araguari river, state of Amapá, eastern Amazon (Brazil). A total of 180 nests were found, being 89.4 % in the forest reserves and only 10.6 % in urban areas. On Falsino river, we observed a spawning pattern, because the number of nests was correlated to the length and width of the nesting locations. On Araguari river, the P. unifilis nests were generally found in areas with surrounding vegetation up to 5 meters in height, minimum distance of 120 meters from residences and immediately or after places of higher exploration of pebbles. Females from Falsino river had smaller eggs, but the neonates were bigger and with higher body condition index than the neonates from Araguari river. About 80 % of the nests were prey, mostly because of the large collection of eggs for feeding. Furthermore, it was found that adult turtle hunting has been intense. Although one of the areas is in forest reserves, the human impacts were similar to those caused in urban areas, indicating the need to implement protection programs for the conservation of P. unifilis.
Keywords: Amazon, eggs, freshwater turtle, neonates, nests.
RESUMEN
El propósito de este estudio fue investigar la influencia de los impactos antropogénicos en nidos de Podocnemis unifilis en un tramo del Río Falsino, se investigó en dos reservas forestales y tramo urbano del río Araguari, Estado de Amapá, en Amazonia (Brasil). Un total de 180 nidos fueron localizados y 89,4 % estaban en las áreas de reservas forestales, pero sólo el 10,6 % en las zonas urbanas. En el río Falsino, hubo desove patrón, el número de nidos se correlacionó con la longitud y la anchura de los sitios de anidación. En Río Araguari, nidos de P. unifilis se encuentran generalmente en lugares con vegetación circundante de hasta 5 metros de altura, distancia mínima de 120 metros de residencial y de inmediato o después de los lugares de mayor explotación de los guijarros. En el río Falsino, aunque las hembras han demostrado huevos más pequeños, los recién nacidos fueron mayores y la puntuación cuerpo también es mayor que en los recién nacidos del río Araguari. En la región del Río Araguari, aproximadamente el 80 % de los nidos fueron depredados, debido a la gran colección de huevos para la alimentación. Se observó que la presión de caza en las tortugas adultas ha sido intensa. Aunque es una de las áreas a ser bosques protegidos, los impactos humanos fueron similares a los de las zonas urbanas, lo que indica la necesidad de implementar programas de protección para la conservación de P. unifilis.
Palabras claves: Amazonia, huevos, nidos, recién nacidos, tortuga acuática.
INTRODUCTION
Podocnemis unifilis Troschel, 1848 (yellow-spotted river turtle) is a Podocnemididae that inhabits streams, lakes, and wetlands of the Amazon and Orinoco river basins (Pritchard and Trebbau, 1984). It is the greatest distribution for a chelonian species of freshwater of Amazon; and can be found in Colombia, Venezuela, Ecuador, Peru, Bolivia, Guyana, French Guiana, Suriname and Brazil. In Brazil, this turtle species is distributed in all states of the North region, as well as in the states of Goiás and Mato Grosso (Thorbjarnarson et al., 1993), both in the central region of the country.
Currently, this turtle species is considered vulnerable (Iucn, 2012), due to the intense exploitation of its natural stocks. For natural populations of chelonians, the two main threats are the excessive exploitation by man (Pantoja-Lima et al., 2014) and the modification of their habitats (Pantoja-Lima et al., 2009). The first threat is due to the great consumption of meat, offal and eggs of turtles, since these animals are a major source of protein in the diet of indigenous and riverine populations from the Amazon, besides being used for medicinal purposes and manufacture of ornaments and utensils. The second threat is due to the nesting changes in habitats and microhabitats, which may interfere with the success of nests, since in some of the stages of the egg incubation the sex determination is influenced by characteristics of the environment, such as: vegetation, soil gradation of spawning places, ambient temperature, air and soil humidity, factors that can cause serious alterations in the sex ratio and genetic variability of P. unifilis populations (Ferreira-Junior et al., 2007; Ferreira-Junior, 2009a; Ferreira-Junior, 2009b).
Several studies (Fachín-Terán, 1993; Fachín-Terán et al., 1995; Rebelo and Pezzuti, 2000; Fachín-Terán and Von Mulhen, 2003; Fachín-Teran et al., 2004; Caputo et al., 2005; Fachín-Terán and Von Mulhen, 2006; Ceballos et al., 2014) on the influence of egg collection, adult chelonians hunting and effects of habitat destruction reported that the decline of chelonians populations is more strongly associated with increased activities that alter mainly the landscapes of Amazon floodplains. In the Araguari river basin (eastern Amazon), the nesting of P. unifilis occurs from September to November, eggs hatching occurrs in December and the mean incubation period is of 63.5 ± 5.2 days. In this region, the urban population growth and changes of P. unifilis habitat, due to the exploitation of pebbles on the riverbed and constructions of hydroelectric power plant may be causing changes in nesting patterns, not yet evaluated (Arraes and Tavares-Dias, 2014). Thus, the present study has compared the human impact effects on nesting patterns and body characteristics of P. unifilis neonates in areas of forest reserves (region of low impact) and urban area (region of high impact) in this important basin for the northern Brazil.
MATERIALS AND METHODS
Study area
From May to December 2011, for 10 days a month, the monitoring of the P. unifilis potential spawning places was performed (License ICMBio: 28856-1) in the Araguari river basin system, in eastern Amazon, northern Brazil. This study was conducted in a 70 km extension of this river basin, divided in two subsequent transects of 35 km each (Fig. 1).
On the Falsino river stretch, there are two sustainable use conservation units, the Amapá National Forest (Flona-AP) and Amapá State Forest (Flota-AP), in the city of Ferreira Gomes. The region has low anthropogenic influence, due to the existence of only two residences of riverines, inspection and difficult access to the region. This first sample site is located 35 km above the confluence with the Araguari river. After 20 km from the end of the Falsino River stretch, the first point of the Araguari river stretch, which flows towards the mouth and covers the urban of the city of Porto Grande, is located. Its riverbank has severely altered vegetal coverage due to the urban population growth, the presence of several residences with recreational and economic enterprises, like exploration companies and stones for construction. Furthermore, this stretch is near the reservoir of the Hydroelectric Power Plant of (HEP) Coaracy Nunes, operating in the region for over 30 years, and suffers direct influence of the HEP Ferreira Gomes, which is under construction. In addition, in this region there will be installed the third hydroelectric power plant, the HEP Cachoeira-Caldeirão, which is in the licensing phase.
The region has a humid tropical climate with mean temperatures ranging from 27.3 to 29.9 °C, precipitation exceeding 2000 mm/year, relief mainly flat with some undulating areas. The soil is considerably diverse: yellow latosol; red-yellow latosol; clayish red-yellow latosol and clayish red-yellow, the dominant vegetation on the river banks is upland forest, wet forest and regeneration (Bernard, 2006).
Turtle sampling procedures and data analysis
From May to December 2011, during the monthly visits lasting ten days each, all possible P. unifilis spawning places were monitored on both stretches to nest identification. All spawning sites were then georeferenced and plotted on a map with the aid of the software ArcGIS 10. Using a tape measure, the length, width and maximum height of the nesting sites in relation to the river were measured. The spawning substrates were also classified into four types: sand, pebble, black soil and dry leaves. The upland surrounding vegetation height was classified and then it was defined into three scores: i) grassy vegetation, regeneration, scattered trees up to 5 m; ii) predominant tree species over 5 m up to 20 m and iii) predominant tree species above 20 m heigh.
The P.unifilis nests location was visual, from tracks left in the sand by the female the night before and also with the aid of a 15 cm wooden stick inserted into the soil of the beach in lightly stirred regions. After checking the eggs near the soil surface, the nests were again covered (Fachín-Terán, 1993). All nests were georeferenced and marked with the following information: nest number, date and time the nest was found, geographic coordinates and probable date of hatching. This nest identification was done discreetly and carefully, avoiding the population to easily identify them. After closing the nest, a small stone was placed just above the eggs, tied to a string at its end with a small plastic bottle with wood identification plate (stick), buried about one meter away.
The distribution and abundance analysis of nests was performed using the Kernel estimator, following the recommendations of Câmara and Carvalho (2002), which indicated the intensity of nests in both areas investigated. A total of 23 randomly selected nests were opened shortly after laying, to quantify the number of eggs, total depth and diameter of the nesting chamber opening, egg mass, maximum length and maximum width of the eggs. The eggs of these nests were carefully handled, avoiding rotations or direct exposure to the sun. After measuring, the eggs were returned to their nests, and each one was also measured in relation to the distance to the vegetation, distance to the river and height of these in relation to the river.
The number of nests in each area was evaluated considering that each female has only one annual laying. During the eggs hatching, nests were successfully opened for checking the laying size (number of eggs per nest). The success and apparent predation of nests were calculated from the number of nests with success or the number of nests with failure divided by the total number of nests multiplied by 100. When leaving the nests towards the river, neonates were collected for determination of the carapace maximum length and maximum width, plastron maximum length and maximum width, carapace-plastron maximum width, carapace-plastron distance, head width and maximum length, interocular distance, carapace maximum height, using a manual caliper. The body mass of neonates was obtained with the aid of an appropriate digital scale. The Body Condition Index (BCI) was determined from the values of carapace maximum length (cm) and body mass (g), following the recommendations of Le-Cren (1951).
The estimation of nest predation was performed considering as successfully nests those in which at least one baby was released into the wild. Mayfield method (1961, 1975) helped to account the number of days that the nests remained in their spawning site, the initial day was the day they were found (0), and the hatching day, predation, collection or hatching was the last day the nests were found (X). When it was not possible to track days 0 and X, they were estimated by calculating the mean between the last day observed (or not observed for day 0) and the first day on which the egg was no longer found (first day it was found, for day 0). We calculated the difference between the initial and final day and, then, the mean for each region.
Assessment of anthropogenic impacts
The stretch on the Falsino river, area of the two forest reserves, was considered a control region in relation to the amount of potentially impactful activities, because the presence of activities is very low (there are only two residences), in the case of activities with exploitation of minerals and rocks for civil construction. On the stretch of Araguari river, activities that affect the environment of the banks were checked by recording the geographical coordinates, with the aid of a Global Positioning System (GPS) for preparation of maps supported by the software ArcGIS 10 and ArcMap. Thus, comparisons between the two areas of study were performed.
On Araguari river, the distance of the nests in relation to the residences was evaluated from the determination of circular areas with radius of 50, 100, 150, 200 and 250 m, with reference to the points of nests that were generated by using the Multiple Ring Buffer. In addition, there was the exploitation intensity ratio of pebble from the Kernel estimator, which, according to Câmara and Carvalho (2002), evaluates the point's intensity. This analysis includes the location points of found nests.
The impact on the collection of adult's eggs was investigated by using the technique of formal interviews, informal interviews, and participant observation to obtain information about the species of chelonians in the region, the exploitation of P. unifilis adult nests, such as hunting frequency, hunting purpose, travel distance for hunting and the main capture instruments used by the hunter. Due to the great diversity of popular names that a same species of chelonian has in the Amazon, identification photography of the species was shown to the interviewee, so that he/she could define exactly which species was being reported. To answer these questions, we interviewed 30 residents of the Araguari river banks who agreed to speak openly about it, lived in the urban area of the city of Porto (state of Amapá) and developed fishing and hunting turtles or their eggs, activities for subsistence purposes or commercialization. The interviews were analyzed through descriptive statistics, with reference to the sample size of all interviewees.
Statistical analyzes
Data were tested for homoscedasticity using Bartlett test, and for normality using the Shapiro-Wilk test. The t-test was used to compare data of the animals between the Araguari river and Falsino river, but data without non-Gaussian distribution were compared by Mann-Whitney test (U). For correlation analysis, the Spearman rank coefficient (rs) was used.
RESULTS
The distribution and abundance of P. unifilis nests indicate differences between the two studied stretches. On the Falsino river, along 35 km, in 22 spawning areas, 89.5 % (N=161) of the nests were found. On the 35 km stretch of the Araguari river, in 12 spawning places, there was only 10.5 % (N=19) of nests (8.5 lower) and the distribution was restricted to the last 16 km, towards the mouth of this river (Fig. 1).
The distance from nests to vegetation, from nests to the river and height of nests in relation to, did not show statistical differences between the two studied stretches (Table 1). The P. unifilis spawning places showed similar measurements in length and width. However, there are differences regarding the mean height of the spawning beaches, which was higher on the Araguari river (Table 2).
On the Falsino river, there was a highly significant positive correlation (rs=0.788, p=0.0001) between the number of nests and the length of the beaches, but this did not occur on the Araguari river (rs=0.107, p=0.739). Highly significant positive correlation was found between the number of nests and the width of the beach on the Falsino river (rs=0.8462, p=0.0001), but it was not significant on the Araguari river (rs=-0.095; p=0.828). The lack of correlation was also observed between the number of nests and the height of the beach on the stretches on the Falsino river (rs=0.125, p=0.056) and Araguari river (rs=-0.004, p=0.647).
To P. unifilis nesting, sand was the predominant substrate, used both on the stretch on the Falsino river (80.7 %) and the stretch of the Araguari river (57.9 %), but the deposition on folic substrate occurred only on Falsino river, while on land substrate occurred only on Araguari river. On the Falsino river, nests were surrounded by vegetation, height between 5 and 20 meters. However, on the Araguari river, the nests were placed, mostly, in places that had predominant vegetation with height up to 5 meters. Furthermore, on both stretches, the nests were placed in locations with direct incidence of sunlight.
On the Falsino river, the mean number of P. unifilis eggs was 12.07 ± 4.37 (five to 20 eggs/nest) and on the Araguari river was 15.88 ± 7.45 (four-26 eggs/nest), so, there was a difference between these two studied areas (U=28.00, p=0.0389). The size of P. unifilis eggs from Araguari river was higher when compared to the eggs found on the Falsino river (Table 3). However, it was not possible to evaluate the different incubation periods in both areas, as the number of eggs that have had success on the Araguari river was very low, due to man collections.
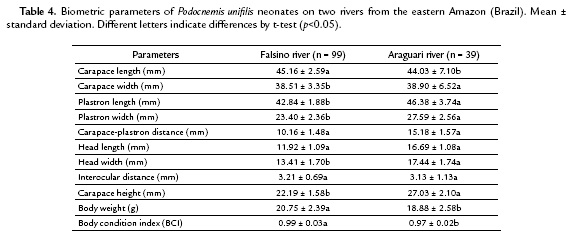
The size of neonates from both investigated sites showed differences between the two areas for carapace maximum length, plastron length, head maximum length and body mass. The body condition index of the neonates also showed differences among animals of both stretches (Table 4).
On Araguari river, 78.9 % of the nests were unsuccessful in hatching due to egg collection by man, exclusively. Similarly, on the Falsino river 80.1 % of the nests were unsuccessful and 93.8 % of the failure was due to egg collection by man, 3.8 % due to animals' predation and 2.5 % due to flooding of nests because of the sudden rise of the water level. The period nests remained in the laying local on Falsino river was 26.8 days and on Araguari river it was 40.13 days, showing a statistically significant difference between these two areas (t=-2.2751, p=0.0170).
On Araguari river, among the 19 found nests, none was closer than 120 m from residences; 15.78 % were located between 121 and 150 m from residences; 5.3 % between 151 and 200 m from residences, 26.3 % between 201 and 250 m from residences. The majority (52.6 %) of the nests was located at a distance greater than 250 m from residences (Fig. 2).
On Araguari river, an assessment of the intensity of extraction of pebbles showed that 78.9 % of the P. unifilis nests were located in regions of greater exploitation of this economic activity or below that (Fig. 3). Sand movements deep in the river to the surface during the removal of pebbles, cause changes in the accumulation and flow of sand, what led to the formation of sandy environments on the banks of this river, preferred locations for P. unifilis nesting.
In the urban area of the city of Porto Grande, there were 30 interviewees from different homes. The major questions of the interview were related to egg collection, P. unifilis hunting and other species of chelonians in the region. From these, 80.0 % of interviewees indicated they have collected both eggs and animals in the last two years, 13.3 % had these practices about two to four years ago and 6.6 % over four years ago. To accomplish these collections, 93.3 % go alone or in groups of up to five people. The main means of transport (73.4 %) are wooden boat with low power motor or small boats made of iron with medium power motor (23.3 %). The distance shown to collect was 66 km. The time required for egg collection and turtle hunting was five hours, occurring mainly during the night, from 6 pm to six am (93.3 %) and during the dry season, between September and December 2011 (66.7 %).
The Amazonian turtle hunting was carried on rivers (50.0 %) and main streams (13.33 %), and had as main collect tool/technique gillnets (36.6 %), hooks (26.6 %), firearms (13.3 %), manual collection on beaches (13.3 %) and diving (10.0 %). When it is practiced in deep parts of the rivers, small boats made of iron with medium power engine are used to force the P. unifilis to emerge. According to the information, for each collection, the amount of eggs was 17 to 300 units; the number of captured animals was 1 to 50 with body mass ranging from 1 to 6 kg. In addition, 90.0 % indicated that P. unifilis is the most collected species of chelonian in the region, with preference for females (67.8 %).
The eggs and animals (56.6 %) are more easily found around the reserves (State and National Forests of Amapá), due to the higher presence of P. unifilis in these places and the little inspection. Podocnemis unifilis (76.5 %) and box turtle Chelonoidis sp. (20.5 %) were identified as most preferred the species by the community of the region. Thus, 70.0 % indicated that, in the region, the number of P. unifilis has declined due to hunting (53.3 %) and modification of spawning beaches, due to the exploitation of pebbles (16.6 %) and mining (23.3 %) in the region.
The majority (86.6 %) of the interviewees also reported that these collected eggs and chelonians were, usually, for family consumption; 10.0 % for consumption and trade and 13.3 % only for trade in Porto Grande and the city of Macapá. The chelonians captured alive are sold at values ranging from U$ 14.0 to 28.0 per unity. This variation is due to the size and gender, as pregnant females achieve higher price. The eggs are sold at prices ranging from U$ 1.86.0 to 2.80 by dozen.
DISCUSSION
In conservation units Flona-AP and Flota-AP, Falsino river (AP), the number of P. unifilis nests was 8.5 times higher than that found on Araguari river, the urban area of the city of Porto Grande, State of Amapá (Brazil), which has lower number of females. Similarly, in Bolivia, along the rivers Itenéz and Paraguá, the abundance of P. unifilis was higher in areas distant from urban sites (Coway-Gomez, 2007). In the Amazon, meat, viscera and turtle eggs are important sources of protein for riverine and indigenous populations, but are appreciated by urban human communities due to the cultural habit maintained by generations of riverine origin (Duarte et al. 2008). Thus, P. unifilis population has a long history of decline due to the use of its meat and eggs by riverine communities, as well as by pressure on its nesting habitats (Arraes and Tavares-Dias, 2014). Pantoja-Lima et al. (2014) estimated that about 34 tons of turtles are consumed annually only along the margins of a major fishing river in the state of Amazonas, central Amazon (Brazil).
In the Araguari river basin, the number of P. unifilis nests showed strong positive correlation with the length and width of spawning places only on Falsino river. However, on Araguari river, local close to the urban area and of excessive egg collection, most nests were found in areas with vegetation up to 5 meters in height. This nesting behavior in areas with low-growing vegetation seems to indicate the search for locations with greater sunlight intensity when compared to areas with not much vegetation. Possibly, it is an attempt to shorten the incubation period, to reduce, then, the predation and collection of eggs. Most of the nests were also found in a region with less altered riparian vegetation and there was an animal preference for nesting places with a minimum distance of 250 meters from residences, indicating that P. unifilis prefers places with fewer disturbances caused by man. On this river, movements at the bottom of the riverbed for pebbles extracting leads the sandy sediment to its banks and accumulates it, increasing and/or forming new nesting sites for this turtle. This led us to observe a greater or lower number of nests in these locations of exploitation of this activity in the Araguari river region.
In addition, the height of the beaches was greater than on Falsino river, due to the existence of beaches with higher levels. However, when comparing the distances of nests in relation to the vegetation or to the river, there was no difference between the two investigated stretches, indicating that these patterns of choice for nesting were not influenced by this anthropic factor. For turtle nesting, the choice of habitat has great ecological and evolutionary effect for the species, since the microhabitat of egg incubation influences the phenotype and development of neonates. Higher temperatures increase the chances of female births, changing the structure of the population. Wetter soils prolong the incubation time, increasing, thus, the absorption of vitelline reserves (Ferreira-Junior et al., 2007; Escalona et al., 2009; Ferreira-Junior, 2009a; Ferreira-Junior, 2009b). Moreover, P. unifilis avoids risks associated with its nesting, such as increased predation of eggs and neonates, as well as the vulnerability of nests to flooding (Thorbjarnarson et al., 1993; Escalona and Fa, 1998; Pantoja-Lima et al., 2009).
On Araguari river, the P. unifilis eggs were smaller when compared to animals of the Falsino river, although a larger number of nests have been found there. The difference between the numbers of eggs per nest may be related to the energetic effort that females are employing for their reproduction, because in affected areas the females may be depositing a larger amount of eggs per nest, to reduce the chances of predation during this process. On Falsino river, multiple laying may be occurring, that is, each female is depositing eggs in more than one nest during the same annual cycle. The size of the eggs appears to be associated with the population standard, because on Falsino river, the group of females, in addition to being larger, considering that 161 matrices were found nesting, may be constituted by animals with predominantly young age, due to a better annual recruiting, thus causing the production of eggs with smaller size. On the Araguari river, where only 19 females were found nesting, there is a low recruitment of neonates due to the excessive egg collection, which has been happening for a long time. Furthermore, it is possible that on Araguari river females are larger and are performing greater maternal investment as a way to compensate for the low recruitment of neonates, because of the frequent collection of eggs and environmental factors influencing negatively the successful egg hatching. However, additional studies are needed to understand the factors influencing the P. unifilis population structure in the Araguari river basin system. For the turtle species, egg size can be determined by biological patterns of females, mainly related to their age, among other factors. Because these animals do not have parental care, the apparent energetic effort employed for reproduction can ensure better development of the offspring; thus, larger eggs may have greater hatching success and generate larger neonates (Valenzuela, 2001; Loehr et al., 2004, Warner et al., 2010; Ceballos et al., 2014). Conditions of soil moisture and temperature that cause water loss in the egg may lead to a lower vitelline absorption and, then, generate smaller neonates (Ferreira-Junior, 2009a; Ferreira-Junior, 2009b). In P. unifilis, changes in the age structure of the population of adult females are long-term indicators, because when a population consists primarily of larger females, it indicates a low recruitment of young females, due to the high and constant loss of eggs and/or neonates over the years (Hernandez et al., 2010).
On Falsino river, vegetal coverage region has changed little and, consequently, with lower ambient temperatures and greater humidity, the mean incubation period was 64.5 days. In this area, in contrast to egg size, the measures of body mass and length of P. unifilis neonates were higher, resulting in neonates with best BCI due to the longer incubation and absorption of vitelline reserves. Although it was not possible to assess the period of incubation of the eggs on Araguari river, urban area with little vegetal coverage, it can be inferred that in this location the hatching period was shorter than on Falsino river. On the other hand, the eggs of females of Araguari river were bigger, but the neonates presented smaller body sizes and smaller BCI values, quantitative index of body condition that can be influenced by environmental conditions in which animals are located and also by the use of food resources (Congdon et al., 1999; Loehr et al., 2004; Lambrada-Martagón et al., 2010) and available energy.
CONCLUSIONS
Although the number of nests and neonates of P. unifilis found was greater in conservation units, the percentage of withdrawal was the same in both areas, demonstrating that there is the need for higher inspection in the area, since the conservation units are intended to contribute to the greater preservation of these species and to maintain their feeding and reproduction environments unchanged. In the area of the Araguari river, exploitation of eggs and females, over the decades, might be influencing the size of P. unifilis eggs, due to changes in its population structure. Furthermore, it appears that spawning habitat and microhabitat changes are negatively influencing the size of these turtle neonates. Thus, there is an imminent need for action to contain the negative environmental impacts in the Araguari river basin, through management programs, protection of nests and encouraging the P. unifilis culture, which should ease the pressure on its natural stocks, not yet evaluated in the region.
ACKNOWLEDGMENTS
This study was developed according to the principles adopted by the Brazilian College of Animal Experiments (COBEA) and human ethic. We would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Program for Biodiversity Research (PPBIO) for the Masters scholarship to the first author. M. Tavares-Dias was supported by a Research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
REFERENCES
Arraes DRS, Tavares-Dias M. Nesting and neonates of the yellow-spotted river turtle (Podocnemis unifilis, Podocnemididae) in the Araguari river basin, eastern Amazon, Brazil. Acta Amaz. 2014;44:387-392. Doi:10.1590/1809-4392201302864.
Bernard E. Inventários biológicos na Floresta Nacional do Amapá-Expedições I e II. IEPA, Conservação Internacional, Ibama, Sema; 2006. p.87.
Câmara G, Carvalho MS. Análise espacial de eventos. São José dos Campos: INPE; 2002. p.14.
Caputo FP, Canestrelli D, Boitani L. Conserving the terecay (Podocnemis unifilis, Testudines: Pelomedusidae) through a community-based sustainable harvest of its eggs. Biol Cons. 2005;126:84-92. Doi:10.1016/j.biocon.2005.05.004.
Ceballos CP, Romero I, Gómez-Saldarriaga C, Miranda K. Reproduction and conservation of the Magdalena river turtle (Podocnemis lewyana) in the Claro Cocorná Sur River, Colombia. Acta biol Colomb. 2014;19(3):393-400.
Congdon JD, Nagle RD, Dunham AE, Beck CW, Kinney OM, Yeomans SR. The relationship of body size to survivorship of hatchling snapping turtles (Chelydra serpentina): an evaluation of the "bigger is better" hypothesis. Oecologia. 1999;121(2):224-235. Doi:10.1007/s04420050924.
Coway-Gomez K. Effects of human settlements on abundance of Podocnemis unifilis and P. expansa turtles in northeastern Bolivia Chelonian. Conserv Biol. 2007;6(2):199-205. Doi:10.2744/1071-8443(2007)6[199:EOHSOA]2.0.CO;2.
Duarte JAM, Costa FS, Andrade PCM. Revisão sobre as características das principais espécies de quelônios aquáticos amazônicos. In: Andrade, P.C. Criação e manejo de quelônios no Amazonas. Manaus: ProVárzea/Ibama;2008. p. 24-54.
Escalona T, Fa JE. Survival of nests of the terecay turtle (Podocnemis unifilis) in the Nichare-Tawadu rivers, Venezuela. J Zoology. 1998;244(2):303-312. Doi:10.1111/j.1469-7998.1998.tb00034.x.
Escalona T, Engstrom TN, Hernandez OE, Bock BC, Vogt RC, Valenzuela N. Population genetics of the endangered South American freshwater turtle, Podocnemis unifilis, inferred from microsatellite DNA data. Conserv Genet. 2009;10(6):1683-1696. Doi:10.1007/s10592-008-9746-3.
Fachín-Terán A. Caracteristicas de Podocnemis unifilis (Repetitia, Testudines) en el Rio Samiria Loreto. Boletim de Lima. 1993;87:69-74.
Fachin-Teran A, Von Mulhen EM. Reproducción de la taricaya Podocnemis unifilis Troschel 1848 (Testudines: Podocnemididae) en la várzea del medio Solimões, Amazonas, Brasil. Ecologia Aplicada. 2003;2(1):125-132.
Fachin-Teran A, Vogt RC, Gomez, MFS. Food habits of an assemblage of five species of turtles in the Rio Guapore, Rondônia, Brazil. J Herpetol. 1995;29(4):536-547. Doi:10.2307/1564736.
Fachín-Teran A, Von-Mulhen EM. Período de desova e sucesso reprodutivo do tracajá Podocnemis unifilis, Troschel 1848 (Testudines: Podocnemididae) na várzea da RDSM - médio Solimões, Brasil. Uakari. 2006;2(1):63-75.
Fachín-Teran A, Vogt, RC, Thorbjarnarson JB. Patterns of use and hunting of turtles in the Mamirauá sustainable development reserve, Amazonas, Brazil. In: Silvius KM, Bodmer RE, Fragoso JMV, editors. People in nature: Wildlife Conservation in South and Central America. New York: Columbia University Press; 2004. p. 362-377.
Ferreira-Júnior PD, Castro AZ, Castro PTA. The importance of nidification environment in the Podocnemis expansa and Podocnemis unifilis phenotypes (Testudines: Podocnemididae) South American. J Herpetol. 2007;2(1):39-46. Doi:10.2994/1808-9798(2007)2[39:TIONEI]2.0.CO;2.
Ferreira-Júnior PD. Efeitos de fatores ambientais na reprodução de tartarugas. Acta Amaz. 2009a;39(2):319-334.
Ferreira-Júnior PD. Aspectos Ecológicos da determinação sexual em tartarugas. Acta Amaz. 2009b;39(1):139-154.
Hernandez O, Espinosa-Blano AS, May Lugo C, Jiménez-Oraa M, Seijas AE. Artificial incubation of yellow-headed sideneck turtle Podocnemis unifilis eggs to reduce losses to flooding and predation, Cojedes and Manapire rivers, southern Venezuela. Conserv Evidence. 2010;7:100-105.
IUCN. IUCN Red List of Threatened Species. Version 3.1; cited 25 de Junho 2012. Available from: http://www.iucnredlist.org.
Lambrada-Martagón V, Rodriguez LCM, Gardner SC, Escalona VHC, Savin TZ. Health indices of the green turtle (Chelonia mydas) along the pacific coast of Baja California Sur, Mexico. II. body condition index. Chelonian Conserv Biol. 2010;9(2):173-183. Doi:10.2744/CCB-0807.1.
Le-Cren ED. The lenght-weight relationship and seasonal cycle in gonad and conditions in the perch Perca fluviatilis. J Anim Ecol. 1951;20(2):201-219.
Loehr VJT, Henen BT, Hofmeyr MD. Reproduction of the smallest tortoise, the namaqualand speckled padloper, Homopus signatus signatus. Herpetologica. 2004;60(4):444-454. Doi: 10.1655/03-59.
Mayfield H. Nesting success calculated from exposure. Wilson Bull. 1961;73:255-261.
Mayfield H. Suggestions for calculating nest success. Wilson Bull. 1975;87:456-466.
Pantoja-Lima J, Pezzuti JCB, Teixeira AS, Félix-Silva D, Rebêlo GH, Monjeló LAS, Kemenes A. Seleção de locais de desova e sobrevivência de ninhos de quelonios Podocnemis no baixo Rio Purus, Amazonas, Brasil. Rev Colom Ciên Anim. 2009;1(1):37-59.
Pantoja-Lima J, Aride PHR, Oliveira AT, Félix-Silva D, Pezzuti JCB, Rebêlo GH. Chain of commercialization of Podocnemis spp. turtles (Testudines: Podocnemididae) in the Purus River, Amazon basin, Brazil: current status and perspectives. J Ethnobiol Ethnomed. 2014;10(1):8. Doi:10.1186/1746-4269-10-8.
Pritchard P, Trebbau P. The turtles of Venezuela. Society for the Study of Amphibians and Reptiles. Ohio: Oxford; 1984. p. 440.
Rebêlo G, Pezzuti J. Percepções sobre o consumo de quelônios na Amazônia. sustentabilidade e alternativas ao manejo atual. Ambiente & Sociedade. 2000;6(7):85-104. Doi:10.1590/S1414-753X2000000100005.
Thorbjarnarson JB, Perez N, Escalona T. Nesting of Podocnemis unifilis in the Capanaparo river, Venezuela. J Herpetol. 1993;27(3):344-347.
Valenzuela N. Constant, shift, and natural temperature effects on sex determination in Podocnemis expansa turtles. Ecology. 2001;82(11):3010-3024. Doi:10.1890/0012-9658(2001)082[3010:CSANTE]2.0.CO;2.
Warner DA, Jorgensen CF, Janzen FJ. Maternal and abiotic effects on egg mortality and hatchling size of turtles: temporal variation in selection over seven years. Funct Ecol. 2010;24(4):857-866. Doi:10.1111/j.1365-2435.2010.01714.x.
Referencias
Arraes DRS, Tavares-Dias M. Nesting and neonates of the yellow-spotted river turtle (Podocnemis unifilis, Podocnemididae) in the Araguari river basin, eastern Amazon, Brazil. Acta Amaz. 2014;44:387-392. Doi:10.1590/1809-4392201302864.
Bernard E. Inventários biológicos na Floresta Nacional do Amapá-Expedições I e II. IEPA, Conservação Internacional, Ibama, Sema; 2006. p.87.
Câmara G, Carvalho MS. Análise espacial de eventos. São José dos Campos: INPE; 2002. p.14.
Caputo FP, Canestrelli D, Boitani L. Conserving the terecay (Podocnemis unifilis, Testudines: Pelomedusidae) through a community-based sustainable harvest of its eggs. Biol Cons. 2005;126:84-92. Doi:10.1016/j.biocon.2005.05.004.
Ceballos CP, Romero I, Gómez-Saldarriaga C, Miranda K. Reproduction and conservation of the Magdalena river turtle (Podocnemis lewyana) in the Claro Cocorná Sur River, Colombia. Acta biol Colomb. 2014;19(3):393-400.
Congdon JD, Nagle RD, Dunham AE, Beck CW, Kinney OM, Yeomans SR. The relationship of body size to survivorship of hatchling snapping turtles (Chelydra serpentina): an evaluation of the "bigger is better" hypothesis. Oecologia. 1999;121(2):224-235. Doi:10.1007/s04420050924.
Coway-Gomez K. Effects of human settlements on abundance of Podocnemis unifilis and P. expansa turtles in northeastern Bolivia Chelonian. Conserv Biol. 2007;6(2):199-205. Doi:10.2744/1071-8443(2007)6[199:EOHSOA]2.0.CO;2.
Duarte JAM, Costa FS, Andrade PCM. Revisão sobre as características das principais espécies de quelônios aquáticos amazônicos. In: Andrade, P.C. Criação e manejo de quelônios no Amazonas. Manaus: ProVárzea/Ibama;2008. p. 24-54.
Escalona T, Fa JE. Survival of nests of the terecay turtle (Podocnemis unifilis) in the Nichare-Tawadu rivers, Venezuela. J Zoology. 1998;244(2):303-312. Doi:10.1111/j.1469-7998.1998.tb00034.x.
Escalona T, Engstrom TN, Hernandez OE, Bock BC, Vogt RC, Valenzuela N. Population genetics of the endangered South American freshwater turtle, Podocnemis unifilis, inferred from microsatellite DNA data. Conserv Genet. 2009;10(6):1683-1696. Doi:10.1007/s10592-008-9746-3.
Fachín-Terán A. Caracteristicas de Podocnemis unifilis (Repetitia, Testudines) en el Rio Samiria Loreto. Boletim de Lima. 1993;87:69-74.
Fachin-Teran A, Von Mulhen EM. Reproducción de la taricaya Podocnemis unifilis Troschel 1848 (Testudines: Podocnemididae) en la várzea del medio Solimões, Amazonas, Brasil. Ecologia Aplicada. 2003;2(1):125-132.
Fachin-Teran A, Vogt RC, Gomez, MFS. Food habits of an assemblage of five species of turtles in the Rio Guapore, Rondônia, Brazil. J Herpetol. 1995;29(4):536-547. Doi:10.2307/1564736.
Fachín-Teran A, Von-Mulhen EM. Período de desova e sucesso reprodutivo do tracajá Podocnemis unifilis, Troschel 1848 (Testudines: Podocnemididae) na várzea da RDSM - médio Solimões, Brasil. Uakari. 2006;2(1):63-75.
Fachín-Teran A, Vogt, RC, Thorbjarnarson JB. Patterns of use and hunting of turtles in the Mamirauá sustainable development reserve, Amazonas, Brazil. In: Silvius KM, Bodmer RE, Fragoso JMV, editors. People in nature: Wildlife Conservation in South and Central America. New York: Columbia University Press; 2004. p. 362-377.
Ferreira-Júnior PD, Castro AZ, Castro PTA. The importance of nidification environment in the Podocnemis expansa and Podocnemis unifilis phenotypes (Testudines: Podocnemididae) South American. J Herpetol. 2007;2(1):39-46. Doi:10.2994/1808-9798(2007)2[39:TIONEI]2.0.CO;2.
Ferreira-Júnior PD. Efeitos de fatores ambientais na reprodução de tartarugas. Acta Amaz. 2009a;39(2):319-334.
Ferreira-Júnior PD. Aspectos Ecológicos da determinação sexual em tartarugas. Acta Amaz. 2009b;39(1):139-154.
Hernandez O, Espinosa-Blano AS, May Lugo C, Jiménez-Oraa M, Seijas AE. Artificial incubation of yellow-headed sideneck turtle Podocnemis unifilis eggs to reduce losses to flooding and predation, Cojedes and Manapire rivers, southern Venezuela. Conserv Evidence. 2010;7:100-105.
IUCN. IUCN Red List of Threatened Species. Version 3.1; cited 25 de Junho 2012. Available from: http://www.iucnredlist.org.
Lambrada-Martagón V, Rodriguez LCM, Gardner SC, Escalona VHC, Savin TZ. Health indices of the green turtle (Chelonia mydas) along the pacific coast of Baja California Sur, Mexico. II. body condition index. Chelonian Conserv Biol. 2010;9(2):173-183. Doi:10.2744/CCB-0807.1.
Le-Cren ED. The lenght-weight relationship and seasonal cycle in gonad and conditions in the perch Perca fluviatilis. J Anim Ecol. 1951;20(2):201-219.
Loehr VJT, Henen BT, Hofmeyr MD. Reproduction of the smallest tortoise, the namaqualand speckled padloper, Homopus signatus signatus. Herpetologica. 2004;60(4):444-454. Doi: 10.1655/03-59.
Mayfield H. Nesting success calculated from exposure. Wilson Bull. 1961;73:255-261.
Mayfield H. Suggestions for calculating nest success. Wilson Bull. 1975;87:456-466.
Pantoja-Lima J, Pezzuti JCB, Teixeira AS, Félix-Silva D, Rebêlo GH, Monjeló LAS, Kemenes A. Seleção de locais de desova e sobrevivência de ninhos de quelonios Podocnemis no baixo Rio Purus, Amazonas, Brasil. Rev Colom Ciên Anim. 2009;1(1):37-59.
Pantoja-Lima J, Aride PHR, Oliveira AT, Félix-Silva D, Pezzuti JCB, Rebêlo GH. Chain of commercialization of Podocnemis spp. turtles (Testudines: Podocnemididae) in the Purus River, Amazon basin, Brazil: current status and perspectives. J Ethnobiol Ethnomed. 2014;10(1):8. Doi:10.1186/1746-4269-10-8.
Pritchard P, Trebbau P. The turtles of Venezuela. Society for the Study of Amphibians and Reptiles. Ohio: Oxford; 1984. p. 440.
Rebêlo G, Pezzuti J. Percepções sobre o consumo de quelônios na Amazônia. sustentabilidade e alternativas ao manejo atual. Ambiente & Sociedade. 2000;6(7):85-104. Doi:10.1590/S1414-753X2000000100005.
Thorbjarnarson JB, Perez N, Escalona T. Nesting of Podocnemis unifilis in the Capanaparo river, Venezuela. J Herpetol. 1993;27(3):344-347.
Valenzuela N. Constant, shift, and natural temperature effects on sex determination in Podocnemis expansa turtles. Ecology. 2001;82(11):3010-3024. Doi:10.1890/0012-9658(2001)082[3010:CSANTE]2.0.CO;2.
Warner DA, Jorgensen CF, Janzen FJ. Maternal and abiotic effects on egg mortality and hatchling size of turtles: temporal variation in selection over seven years. Funct Ecol. 2010;24(4):857-866. Doi:10.1111/j.1365-2435.2010.01714.x.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Alexandre Fernandes Veloso, Odair Diogo da Silva, Tatiane Dias Santana, Márcia Karine de Souza Santos, Letícia Carina Pinheiro Camilo, Dionei José da Silva. (2024). Entre o sucesso e o risco: padrões de ninhos e biometria de Podocnemis unifilis, uma espécie introduzida no Rio Paraguai. Cuadernos de Educación y Desarrollo, 16(9), p.e5684. https://doi.org/10.55905/cuadv16n9-117.
2. Vivian P. Páez, Brian C. Bock, Felipe A. Toro-Cardona, Viviana M. Cartagena-Otálvaro. (2024). Lessons learned during a 12-year monitoring project with the endangered Magdalena River turtle (Podocnemis lewyana): hunting pressure, habitat degradation, and methodological considerations. Environmental Monitoring and Assessment, 196(9) https://doi.org/10.1007/s10661-024-12944-0.
3. Sandra Bibiana Correa, Peter van der Sleen, Sharmin F Siddiqui, Juan David Bogotá-Gregory, Caroline C Arantes, Adrian A Barnett, Thiago B A Couto, Michael Goulding, Elizabeth P Anderson. (2022). Biotic Indicators for Ecological State Change in Amazonian Floodplains. BioScience, 72(8), p.753. https://doi.org/10.1093/biosci/biac038.
4. Thong Pham Van, Olivier Le Duc, Benjamin Leprince, Cedric Bordes, Vinh Quang Luu, Luca Luiselli. (2020). Hunters'structured questionnaires enhance ecological knowledge and provide circumstantial survival evidence for the world's rarest turtle. Aquatic Conservation: Marine and Freshwater Ecosystems, 30(1), p.183. https://doi.org/10.1002/aqc.3225.
5. Iago Barroso da Silva, Camila Kurzmann Fagundes, Geovana Linhares de Oliveira, Gabriela Silva Ribeiro Gonçalves, Daniel de Paiva Silva, Gleomar Fabiano Maschio. (2025). Drifting survival: Impacts of climate change on the distribution of continental chelonians in the Amazon. Journal for Nature Conservation, 84, p.126850. https://doi.org/10.1016/j.jnc.2025.126850.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2016 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).