Composition, trophic structure and activity patterns of the understory bats of the Bitaco Forest Reserve
Composición, estructura trófica y patrón de actividad de murciélagos de sotobosque en la Reserva Forestal Bitaco
DOI:
https://doi.org/10.15446/abc.v23n2.64062Palabras clave:
environmental temperature, precipitation, trophic levels. (en)niveles tróficos, precipitación, temperatura ambiental. (es)
Descargas
The present study describes composition and trophic structure, and assesses the effect of environmental temperature and rainfall on the pattern of nocturnal activity of understory bats in an Andean forest, in the first half of the night. Seven sampling events were conducted, lasting six nights each one. Ten mist nets were used to capture bats, which were checked every hour between 18:00 and 00:00 hours. Bat species, sex and capture time were registered. In addition, environmental temperature and rainfall were also recorded during sampling. The bat assemblage of the understory of the study area was represented mostly by frugivore species, within this guild there was a lower richness of nomadic than sedentary species. For the first half of the night, bat activity peaked between 18:00 and 19:00 hours and declined to a minimum level between 22:00 and 23:00 hours. The most accurate model to explain variation in bat captures included only the effect of air temperature, which positively affected the number of captures. In conclusion, for the sampled period the activity of the bat assemblage in the study area was not related to rainfall and exhibited a slight but significant relationship with air temperature.
En el presente trabajo se describe la composición y estructura trófica, y se evalúa el efecto de la temperatura ambiental y la precipitación sobre el patrón de actividad nocturna de los murciélagos de sotobosque que habitan que un bosque Andino, para la primera mitad de la noche. Para esto se realizaron siete campañas de muestreo, con una duración de seis noches cada una, en las que se utilizaron diez redes de niebla que operaron entre las 18:00 y 00:00 horas, siendo revisadas cada hora. Además, se registró la temperatura ambiental y la precipitación durante los eventos de muestreo. El ensamblaje de murciélagos de sotobosque del área de estudio estuvo representado principalmente por especies frugívoras, y dentro de este gremio, se registró una menor riqueza de especies nómadas que sedentarias. La actividad de los murciélagos, para la primera mitad de la noche, fue máxima entre las 18:00 y 19:00 horas, disminuyendo hasta alcanzar una actividad mínima entre las 22:00 y 23:00 horas. El modelo más adecuado para explicar la variación en las capturas de murciélagos incluyó sólo el efecto de la temperatura ambiental, la cual afectó positivamente el número de capturas. En conclusión, para el periodo evaluado, la actividad del ensamble de murciélagos de sotobosque en el área de estudio no se relacionó con la precipitación y exhibió relación leve pero significativa con la temperatura ambiental.
Recibido: 17 de abril de 2017; Revisión recibida: 1 de agosto de 2017; Aceptado: 16 de marzo de 2018
ABSTRACT
The present study describes composition and trophic structure, and assesses the effect of environmental temperature and rainfall on the pattern of nocturnal activity of understory bats in an Andean forest, in the first half of the night. Seven sampling events were conducted, lasting six nights each one. Ten mist nets were used to capture bats, which were checked every hour between 18:00 and 00:00 hours. Bat species, sex and capture time were registered. In addition, environmental temperature and rainfall were also recorded during sampling. The bat assemblage of the understory of the study area was represented mostly by frugivore species, within this guild there was a lower richness of nomadic than sedentary species. For the first half of the night, bat activity peaked between 18:00 and 19:00 hours and declined to a minimum level between 22:00 and 23:00 hours. The most accurate model to explain variation in bat captures included only the effect of air temperature, which positively affected the number of captures. In conclusion, for the sampled period the activity of the bat assemblage in the study area was not related to rainfall and exhibited a slight but significant relationship with air temperature.
Keywords:
environmental temperature, precipitation, trophic levels.RESUMEN
En el presente trabajo se describe la composición y estructura trófica, y se evalúa el efecto de la temperatura ambiental y la precipitación sobre el patrón de actividad nocturna de los murciélagos de sotobosque que habitan que un bosque Andino, para la primera mitad de la noche. Para esto se realizaron siete campañas de muestreo, con una duración de seis noches cada una, en las que se utilizaron diez redes de niebla que operaron entre las 18:00 y 00:00 horas, siendo revisadas cada hora. Además, se registró la temperatura ambiental y la precipitación durante los eventos de muestreo. El ensamblaje de murciélagos de sotobosque del área de estudio estuvo representado principalmente por especies frugívoras, y dentro de este gremio, se registró una menor riqueza de especies nómadas que sedentarias. La actividad de los murciélagos, para la primera mitad de la noche, fue máxima entre las 18:00 y 19:00 horas, disminuyendo hasta alcanzar una actividad mínima entre las 22:00 y 23:00 horas. El modelo más adecuado para explicar la variación en las capturas de murciélagos incluyó sólo el efecto de la temperatura ambiental, la cual afectó positivamente el número de capturas. En conclusión, para el periodo evaluado, la actividad del ensamble de murciélagos de sotobosque en el área de estudio no se relacionó con la precipitación y exhibió relación leve pero significativa con la temperatura ambiental.
Palabras clave:
niveles tróficos, precipitación, temperatura ambiental.INTRODUCTION
Bats are an important component of ecosystems since they participate in several ecological processes such as pest control, seed dispersal, and plant pollination. Bats are found at every trophic level feeding on fruits, insects, nectar, pollen, fish, blood, vertebrates, and leaves (Medellín et al., 2000; Clarke et al., 2005; Vargas Espinoza et al., 2008). Bat activity is influenced by a series of intrinsic and extrinsic factors such as metabolic water balance (Adams and Thibault, 2006), energetic demands (Bozinovic et al., 1985; Voigt and Lewanzik, 2011), weight (Audet and Thomas, 1997), reproductive state, moonlight intensity (Fenton et al., 1977; Reith, 1982; Lang et al., 2006), environmental temperature (O'Farrell et al., 1967; O'Farrell and Bradley, 1970; Erickson and West, 2002), rainfall (Fenton et al., 1977; Erickson and West, 2002; Geluso and Geluso, 2012), and wind speed (Santos-Moreno et al., 2010). As activity increases, the probability of bats becoming captured in mist nets increases (Geluso and Geluso, 2012). Consequently, activity is one of the most relevant factors for capture success, and bat activity levels can be quantified through capture frequency by using mist nets.
Air temperature is the factor that affects the most nocturnal and seasonal activity in bats, with higher captures in mist nets when environmental temperature is higher (O'Farrell and Bradley, 1970). Erickson and West (2002) reported similar results using ultrasonic detectors. Environmental temperature can even modulate activity patterns in a different manner for males and females of the same species. It was reported that males of Pipistrellus hesperus were captured over the entire range of temperature, whereas females were not captured in nets when temperatures were low; which suggests a decrease in female activity at low temperatures (O'Farrell et al., 1967). McGuire and Boyle (2013) found, in insectivorous bats, a temporary decrease in female activity associated with a reduction in food supply and lower reproductive success (fewer reproductive females, late delivery dates, females in poor body condition), which occurs in colder weather.
For frugivorous bats, Sánchez and Giannini (2014) found that Sturnira lilium and Sturnira erythromos exhibited particular and contrasting responses to changes in temperature across and elevational range. Sturnira lilium captures increased at sites with high-temperature, whereas S. erythromos captures increased at sites with low mean annual temperature and narrow mean monthly temperature range, which characterize montane environments.
Rainfall is another environmental factor that has been recurrently linked to bat nocturnal activity since variation in the capture rate of insectivorous bats can be significantly associated with occurrence of rain before sampling (Geluso and Geluso, 2012). However, the effect on bat activity seems to depend on rain intensity. Thies et al. (2006) reported that activity levels of Carollia castanea did not decrease with light to moderate rain, but ceased with heavy rain. In general, it seems that precipitation increases the energetic cost of flight by creating thermoregulation problems associated with wet fur, and interferes with echolocation and the ability to detect food (Fenton et al., 1977; Voigt et al., 2011; Snell-Rood, 2012). Nevertheless, Sánchez and Giannini (2014) did not find a significant relation between the activity of frugivorous bats and rainfall.
The current knowledge of bat assemblages in lowland forests of Colombia (Estrada-Villegas et al., 2010; Murillo-García and Bedoya-Duran, 2014; Murillo García et al., 2014) is higher than in montane forests (Pérez-Torres and Ahumada, 2004; Bejarano-Bonilla et al., 2007; Rodríguez-Posada, 2010). Studies have evaluated variations in diversity and diet through altitudinal transects in the central Cordillera, they have found that richness and abundance of species is significantly reduced with altitude; possibly due to decreases in temperature, habitat complexity, or availability of food resources. Environmental temperature and rainfall can act independently or in conjunction to determine bat species diversity (Graham, 1983) and activity levels of Andean assemblages.
Due to the lack of information on bat assemblages of Colombian Andes, the overall objective of this research was to describe composition, trophic structure and nocturnal activity patterns of understory bats in an Andean forest, in the first half of the night. Activity was measured as a function of the capture rate using mist nets and the effect of air temperature and precipitation on bat activity levels was evaluated.
MATERIALS AND METHODS
Study area
The Bitaco Forest Reserve (BFR) is located in the villages of Chicoral and Zaragoza, 26 km northwest of Santiago de Cali, in the municipality of La Cumbre. It covers an altitudinal range from 1850 to 2100 masl on the eastern slope of the Western Andean Range in the upper part of the Bitaco River sub-basin. The reserve covers 195 ha ofvery wet pre-montane forest, according to Holdridge's classification (CVC, 2006). The flora is represented mainly for plants of the families Rubiaceae, Orchidaceae, Melastomataceae, Solanaceae, Lauraceae, and Asteraceae. The most abundant genera are Miconia, Psychotria, Solanum, Anthurium, Monstera, and Philodendron. Due to the dense humidity layer that ascends from Pacific lowlands to the mountains, this area is subject to a persistent light rain inside the forest. The air temperature oscillates between 12 and 21 °C, whereas relative humidity fluctuates between 80 and 87 %. The reserve has the highest precipitation levels in the Bitaco River sub-basin, with values ranging from 1400 to 4000 mm per year. January, February, July, and August are the months with lowest rainfall, and October is the rainiest (CVC, 2006).
Field work
Seven sampling events were undertaken between May and November 2014, each event last a total of six nights. In order to capture bats, ten 12m-long mist nets were used and set in two different areas of the reserve to cover the different landscape units present in the reserve. The location of the nets was changed every three nights, and nets were checked every hour from 18:00 to 00:00. Taxonomic identification was carried out based on Díaz et al. (2016), the species list was made following the proposal of Solari et al. (2013), with the changes proposed by Ramírez-Chaves and Suárez Castro (2014), and Ramírez-Chaves et al. (2016). Environmental temperature was measured using continuous recorders (Tidbit® v2) placed in each net, and precipitation was measured using a pluviometer; both measurements were registered during the sampling periods. Individuals captured were classified in trophic categories following Soriano (2000) to study the functional structure of the community.
Statistical analyses
Representativeness of the sampling was evaluated by comparing the observed richness against the expected richness based on the non-parametric richness estimators Jackknife 1 and Jackknife 2. The activity pattern of the understory bat assemblage was described graphically based on the frequency of capture records per hour. A Poisson regression was performed, based on a generalized linear model, to evaluate the effect of environmental temperature and rainfall on bat activity (number of captures). This distribution model must comply with the equi-dispersion assumption, that is, the variance must be equal to the mean. However, the data show dependent variable's variance of 6.33 which is greater than the mean 2.95, which leads to over-dispersion in the data. For this, the standard errors were therefore corrected using a quasi-generalized model with variance determined by φ x μ, where μ is the mean and φ is the dispersion parameter.
RESULTS
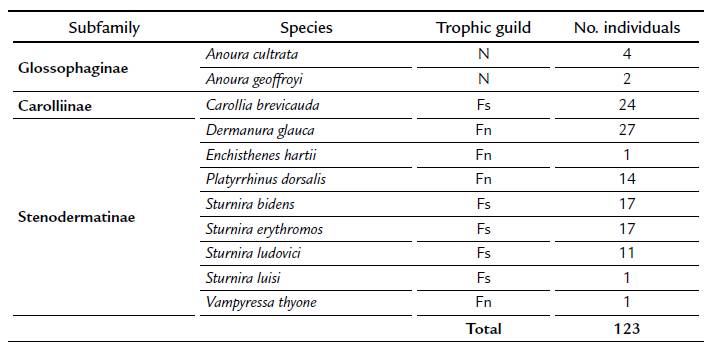
Sample representativeness ranged from 69 to 79 % of the expected species richness. A total of 123 individuals belonging to 11 species of the Phyllostomidae family were captured during 42 nights of sampling. Three trophic guilds were represented: 4.88 % of captured individuals were nectarivores, 34.96 % were nomadic frugivores, and 60.16 % were sedentary frugivores (Table 1).The most captured species were Dermanura glauca (21.95 % of total captures), Carollia brevicauda (19.51 %), Sturnira erythromos (13.82 %), Sturnira bidens (13.82 %), Sturnira ludovici (12.20 %), and Platyrrhinus dorsalis (11.38 %). The species Sturnira luisi, Anoura cultrata, Anoura geoffroyi, Enchisthenes hartii, and Vampyressa thyone accounted for the remaining 7.32 % of captures.
Table 1: Understory bat species and number of individuals caught in the BFR. N: nectarivore, Fn: nomadic frugivore, Fs: sedentary frugivore.
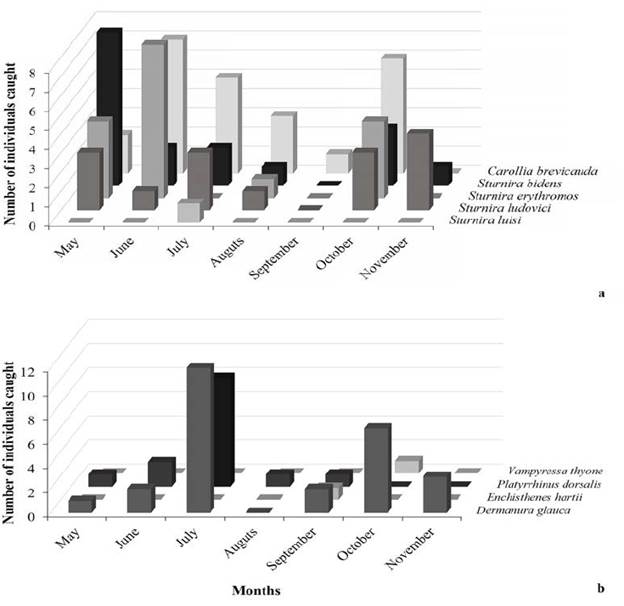
According with Soriano (2000), we found that four out of five sedentary frugivores were obtained during almost every sampling month at relatively high abundance; except September, when only one C. brevicauda individual was recorded. Moreover, only one individual of the sedentary species S. luisi was captured during sampling, this record is in the maximum altitudinal limit of distribution for this species (Solari et al., 2013). The nomadic frugivore species were not recorded equally during all sampling months. Only two out of the four species were caught at relatively high abundances. Dermanura glauca was abundant in July and October, and P. dorsalis was abundant in July; however, during the remaining sampling months few individuals of these species were caught. Three out of the four nomadic frugivore species were captured in September, when the lowest abundance of sedentary frugivore was recorded (Fig. 1).
Figure 1: Number of individuals (of bats) caught between May and November 2014 in the BFR. a) Sedentary frugivores, b) Nomadic frugivores.
For the first half of the night, the activity of understory bats in BFR reached a maximum between 18:00 and 19:00 hours and decreased slowly until a minimum activity level between 22:00 and 23:00 hours (Fig. 2). The peaks of activity for the most abundant species of the assemblage occurred at different times. Of the 27 D. glauca individuals captured, nine were caught between 19:00 and 21:00 hours, and the remaining 18 were caught between 21:00 and 00:00 hours. Of the 24 C. brevicauda individuals captured, 19 were caught between 19:00 and 21:00 hours, and five were caught between 21:00 and 00:00 hours.
Figure 2: Number of individuals (of bats) captured by hour of the night and air temperature in the BFR.
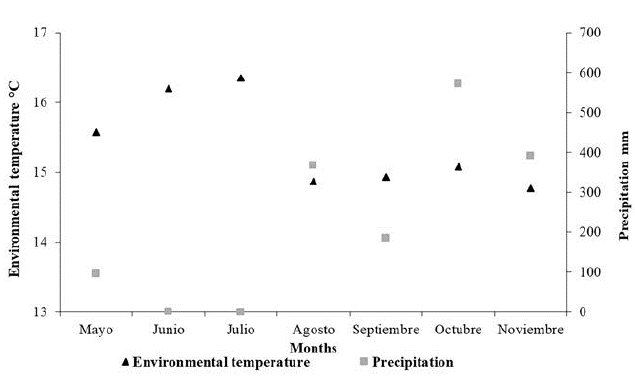
In contrast to rainfall, values for temperature were higher from May to June and lower between August and November (Fig. 3). In example, during June and July were recorded the highest average values of the temperature, 16.20 and 16.35 °C respectively. However, there was no precipitation at the sampling periods corresponding to these months. On the other hand, October and November presented the highest records of precipitation with 572 and 392 mm respectively, and low temperature, being November the coldest month in the sampling period with 14.77 °C. During the study, daily temperature fluctuates between 13.58 °C and 17.13 °C, exhibiting a low range of variation (3.55 °C) compared to historical records (12.00-21.00 °C, with a range of 9.00 °C).
Figure 3: Environmental temperature and precipitation during sampling period in BFR.
During the warmest and driest month of sampling (July), the captures were higher than the other months, 33 individuals in total. In contrast, during the months with lowest temperatures and highest rainfall recorded (August, September and November), we obtained the lowest captures (six to eight individuals).
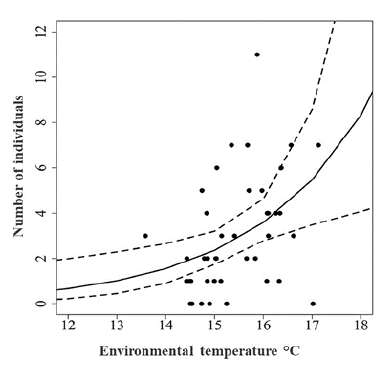
The analysis of the relationship between bat activity (capture frequency per hour) and temperature and rainfall showed that the generalized linear model was not affected by the exclusion of the precipitation variable (X(1)2= 0.24, p= 0.62), whereas it was affected when air temperature was removed (X(1)2= 6.62, p= 0.01). Therefore, the most adequate model to explain the variation in bat captures was the air temperature. According to this model, air temperature affected slightly and positively the number of captures (β= 0.42, gl=40, p= 0.007) (Fig. 4), in such a way that more individuals were captured at higher air temperatures.
Figure 4: Dispersion graph of the effect of air temperature on the number of individuals captured and adjusted generalized linear model.
DISCUSSION
Assemblage characterization
All captured individuals were Phyllostomid bats caught in the understory of the BFR. This family encompasses almost the entire spectrum of trophic guilds found in chiropterans, and is also the best-represented family in Neotropical bat assemblages (Muñoz-Arango, 1986; Ospina-Ante and Gómez, 1999; Bejarano-Bonilla et al., 2007); with approximately half of all bat species recorded in this biogeographic region (Soriano, 2000). Even though Vespertillionidae and Molossidae are bat families that have been reported in high altitude forests of Colombia, they were not recorded during the present study (Estrada-Villegas et al., 2001; Pérez-Torres and Ahumada 2004; Bejarano-Bonilla et al., 2007). This can be associated with the sampling technique since individuals of these families are insectivorous, which are not easily captured using nets due to their flying and foraging habits (Kalko, 1998; Bejarano-Bonilla et al., 2007;). Besides, several insectivorous bats are also associated with open areas and perturbed environments (Pérez-Torres and Ahumada, 2004), which were habitats not covered during the sampling.
The high representativeness of frugivores in the studied assemblage is consistent with those reported for other Andean areas (Soriano, 2000; Estrada-Villegas et al., 2001). There was a low abundance of nomadic frugivores, which has been reported in other studies (Soriano, 2000; Estrada-Villegas et al., 2001). This could be due to the strategy used by this trophic guild, they feed on plant species with a massive production of fruits over a short period of time (seasonal). Consequently, nomadic bats must move across different areas following food resources, so their refuges or perching sites are also frequently changed. In addition, captures of sedentary bats were uniformly distributed during the sampling months, whereas captures of frugivorous fluctuates across the study period.
The higher abundance of frugivore than nectarivore species, reported for the study area, is in agreement with those reported for the Phyllostomidae family (Fleming, 1986). Following the proposal of Baker et al. (2016) subfamilies of Phyllosthomidae such as Stenodermatinae (of Sturnirini, Stenodermatini and Artibeina tribes) and Carolliinae are composed mostly by frugivore species, which are common at high altitude areas according to the works of Pérez-Torres and Ahumada (2004) and Estrada-Villegas et al. (2010). There was low representativeness of nectarivores bats in BFR. The low richness of nectarivores bats can be related to limitations in availability of food since nectar production is limited at low temperatures and high elevations (Stiles, 1980). For example, Inga vera has a peak in nectar production at the beginning of the night at low elevations, but nectar production occurs in mid-afternoon above 1000 masl and is therefore inaccessible to bats. In general, total fruit biomass is higher than nectar and insects, so higher densities of frugivore bats are expected compared with other trophic guilds (Hunter, 1992). The high richness recorded for species of Sturnira has also been reported for high altitude forests (Estrada-Villegas et al., 2001; Perez-Torres and Ahumada, 2004; Bejarano-Bonilla et al., 2007), as this genus comprises generalist species with a wide distribution range (Humphrey and Bonaccorso, 1979; Medellín et al., 2000; Bejarano-Bonilla et al., 2007).
Bats activity and environmental factors
The nocturnal activity of chiropterans is influenced by the interaction of several intrinsic and extrinsic factors, which results in variation of nocturnal activity patterns across species of bat assemblages. In the BFR the highest activity level, for the first half of the night, occurred at the first hour after the sunset (18:00-19:00) and decreased gradually until 23:00 hours. A similar pattern has been reported across studies that describe how bats emerge from their shelters, during the first hours of the night, to forage and then return to their resting sites (Davis and Dixon, 1976; O'Shea and Vaughan, 1977; Adams and Thibault, 2006).
Variations in air temperature are tightly linked to altitude (Graham, 1983; Soriano, 2000), as has been reported in several studies, is one of the environmental factors that strongly influences bat activity levels (O'Farrell et al., 1967; O'Farrell and Bradley, 1970; O'Shea and Vaughan, 1977; Soriano, 2000; Sánchez and Giannini, 2014). The variation in the temperature range registered in BFR was narrow, compared to historical records, which is characteristic of montane environments; however, our results showed that a greater number of individuals were captured at higher air temperatures, which suggests that air temperature had a significant and positive relationship on the activity of understory bats in cloud forests. During the fieldwork, night temperatures between 13.58 and 17.13 °C were recorded, so the lower and upper limits reported for the study area (12 and 21°C, respectively) were not represented throughout sampling period, it is expected that the highest temperatures occur only during the day so bats were not exposed to the extreme temperatures reported for the study area.
Environmental temperature is an important factor in the regulation of activity because it directly affects heat production by organisms. According to McNab (1974) and McNab (1980) this relationship could be expressed in simplified form as M=C*(Tb-Ta), where M is heat production, C is thermal conductance, Tb is body temperature, and Ta is air temperature. This relationship is acceptable for organisms in areas with low-temperature. In the case of a chiropteran, heat production should be equal to heat loss to maintain a constant body temperature, but the surface-volume relationship of these small-sized mammals favors heat loss (Dunbar and Brigham, 2010). Therefore, energy expenditure can be reduced either when conductance is low or when the difference between environmental and body temperature decrease. Consequently, bats can be more active as environmental temperature increases, as we found, with a less investment in heat production.
Bats show several mechanisms to reduce or regulate the heat loss in cold environments, which varies from external characteristics as fur length and density that permit modify the thermal conductance (McNab, 2002), to behavioral as regulation of nocturnal activity to reduce the amount of departures from the roosting sites (Bozinovic et al., 1985). This behavioral trait can explain the low activity (number of captures) when the environmental temperature decreases, because bat captures are positively related to activity (Geluso and Geluso, 2012).
It is probably that the range of night environmental temperature during the sampling period doesn't include low critical temperatures for captured bats, which included frugivorous and nectarivorous species. Soriano et al. (2002) found that frugivorous bat Sturnira erythromos, was able of maintaining high and constant body temperatures (normothermia) in temperatures between 14.0 and 25.5 °C. Nevertheless, below 14 °C they were unable of keeping body temperature constant and suffered hypothermia. Which result in a decrease in activity for these bats and can be a mechanism for saving energy or represent a state to facultative torpor. In BFR we did not register many nights with critical environmental temperatures for frugivorous species like S. erythromos, so most of the captures were at night temperatures above 14 °C. However, contrary to expectations, on many sampling nights there were any captures. Soriano et al. (2002) reported that some individuals of S. erythromos presented hypothermia even at environmental temperatures above 14 °C, due to a combined response to temperature and low supply of water and food; which can explain why some nights with temperatures above 14 °C we did not capture any bats and suggest that critical temperatures can be similar for the bat species in the study area. Alternatively, it could be that for several species captured in the study area, the critical values of temperature were higher than 14 °C.
Precipitation has also been identified as a modulating factor of bat activity levels due to thermoregulation problems associated with moist fur and the interference of rain with echolocation (Fenton et al., 1977; Weinbeer et al., 2006). However, this relationship doesn't always exist or isn't significant (Sánchez and Giannini, 2014). Based on results from the present study, there was no evidence that would suggest that there is an effect of rainfall on the activity level of bats from the understory in the BFR. Due to the temporal scale of the study, it is probably that the level of variation in rainfall during the study period was probably not sufficient to show a significant effect of precipitation on bat activity.
CONCLUSIONS
The bat assemblage of the understory of the BFR was represented mostly by frugivore species, which is consistent with that reported for other Andean areas of the country. Within this guild there was a lower richness of nomadic than sedentary species.
According to the results for the first half of the night, the activity levels of the bat assemblage that inhabits the understory of the BFR was maximal between 18:00 and 19:00 hours, soon after they emerge from their refuges to forage. This activity decreased slowly and was minimal between 22:00 and 23:00 hours, when they probably return to their roosting sites. It was found that activity levels increase as air temperature rise, but precipitation did not appear to have an effect on the activity level of bats in the reserve. It can be inferred that bats determine their activity with the temperature in this particular case.
ACKNOWLEDGMENT
We thank Sebastián Orjuela, Martín Llanos, and Cristian Armando Hernández for their help during fieldwork; and to Betty Cadena for her hospitality and sharing her knowledge of the study area. This study was carried out within the Joven Investigador scholarship program, which was funded by Colciencias and Universidad del Valle.
REFERENCES
Referencias
Adams RA, Thibault KM. Temporal resource partitioning by bats at water holes. J Zool. 2006;270(3):466-472. Doi:10.1111/j.1469-7998.2006.00152.x
Audet D, Thomas DW. Facultative hypothermia as a thermoregulatory strategy in the phyllostomid bats, Carollia perspicillata and Sturnira lilium. J Comp Physiol B. 1997;167(2):146-152. Doi:10.1007/s003600050058
Bejarano-Bonilla DA, Yate-Rivas A, Bernal-Bautista MH. Diversidad and distribución de la fauna quiróptera en un transecto altitudinal en el departamento del Tolima, Colombia. Caldasia. 2007;29(29):297-308.
Baker RJ, Solari S, Cirranello A, Simmons NB. Higher level classification of phyllostomid bats with a summary of DNA synapomorphies. Acta Chiropt. 2016;18(1):1-38. Doi:10.3161/15081109ACC2016.18.1.001
Bozinovic F, Contreras LC, Rosenmann M, Torres-Mura JC. Bioenergetica de Myotis chiloensis (Quiróptera: Vespertilionidae). Rev Chil Hist Nat. 1985;58:39-45.
Clarke FM, Pio DV, Racey PA. A comparison of logging systems and bat diversity in the Neotropics. Conserv Biol. 2005;19(4):1194-1204. Doi:10.1111/j.1523-1739.2005.00086.x-i1
CVC-Corporación Autónoma Regional del Valle del Cauca. Plan de manejo participativo Reserva Forestal de Bitaco. Santiago de Cali: Corporación Autónoma Regional del Valle del Cauca, Sistema Departamental de Áreas Protegidas del Valle; 2006. p. 19-26.
Davis WB, Dixon JR. Activity of bats in a small village clearing near Iquitos, Peru. J Mammal. 1976:57(4):747-749.
Díaz MM, Solari S, Aguirre LF, Aguiar LM, Barquez, RM. Clave de Identificación de los murciélagos de Sudamérica–Chave de identificação dos morcegos da America do Sul. Publicación Especial No. 2 PCMA; 2016. 160 p.
Dunbar MB, Brigham RM. Thermoregulatory variation among populations of bats along a latitudinal gradient. J Comp Physiol B. 2010;180(6):885-893. Doi:10.1007/s00360-010-0457-y
Erickson JL, West SD. The influence of regional climate and nightly weather conditions on activity patterns of insectivorous bats. Acta Chiropt. 2002;4(1):17-24. Doi:10.3161/001.004.0103
Estrada-Villegas S, Pérez-Torres J, Stevenson PR. Ensamble de murciélagos en un bosque sub-andino colombiano and análisis sobre la dieta de algunas especies. Mastozool Neotrop. 2010;17(1):31-41.
Fenton MB, Boyle, NGH, Harrison TM, Oxley DJ. Activity patterns, habitat use, and prey selection by some African insectivorous bats. Biotropica. 1977;9(2):73-85. Doi:10.2307/2387662
Fleming TH. Opportunism versus specialization: the evolution of feeding strategies in frugivorous bats. In: Estrada A, Fleming TH, editors. Frugivores and seed dispersal. Dordrecht: Dr W. Junk Publishers; 1986. p. 105-118.
Geluso KN, Geluso K. Effects of environmental factors on capture rates of insectivorous bats, 1971-2005. J Mammal. 2012;93(1):161-169. Doi:10.1644/11-MAMM-A-107.1
Graham GL. Changes in bat species diversity along an elevational gradient up the Peruvian Andes. J Mammal. 1983;64:559-571. Doi:10.2307/1380511
Humphrey SR, Bonaccorso FJ. Population and community ecology. In: Baker RJ, Jones JK Jr., Carter DC, editor(s). Biology of bats of the New World family Phyllostomidae. Parte III. Lubbock: Special publication the Museum Texas Tech University; 1979. p. 409-441.
Hunter MD. Interactions within herbivore communities mediated by the host plants: The keystone herbivore concept. In: Hunter MD, Ohgushi T, Price PW, editors. Effects of resource distribution on animal-plant interactions. San Diego: Academic Press; 1992. p. 287-325.
Kalko EKV. Organization and diversity of tropical bat communities through space and time. Zoology. 1998;101:281-297.
Lang AB, Kalko EK, Romer H, Bockholdt C, Dechmann D K. Activity levels of bats and katydids in relation to the lunar cycle. Oecologia. 2006;146(4):659-666. Doi:10.1007/s00442-005-0131-3
McGuire LP, Boyle WA. Altitudinal migration in bats: evidence, patterns, and drivers. Biol Rev Camb Philos Soc. 2013;88(4):767-786.
McNab BK. The energetics of endotherms. Ohio J Sci. 1974;74(6):370-380.
McNab BK. On estimating thermal conductance in endotherms. Physiol Zool. 1980;53(2):145-156.
McNab BK. Short-term energy conservation in endotherms in relation to body mass, habits, and environment. J Therm Biol. 2002;27(6):459-466.
Medellín RA, Equihua M, Amin MA. Bat diversity and abundance as indicators of disturbance in Neotropical rainforests. Conserv Biol. 2000;14(6):1666-1675. Doi:10.1111/j.1523-1739.2000.99068.x
Murillo-García OE, Bedoya-Duran MJ. Distribución y abundancia de murciélagos en bosques con diferente grado de intervención en el Parque Nacional Natural Gorgona (Colombia). Rev Biol Trop. 2014;62(1):419-434.
Murillo OE, Bedoya MJ, Velandia-Perilla JH, Yusti-Muñoz AP. Riqueza de especies, nuevos registros y actualización del listado taxonómico de la comunidad de murciélagos del Parque Nacional Natural Gorgona, Colombia. Rev Biol Trop. 2014;62(1):407-417.
Muñoz-Arango J. Murciélagos del Parque Natural El Refugio (Antioquia, Colombia). Actualidades Biológicas. 1986;12:66-76.
O’Farrell MJ, Bradley WG. Activity patterns of bats over a desert spring. J Mammal. 1970;51(1):18-26. Doi:10.2307/1378527
O’Farrell MJ, Bradley WG, Jones GW. Fall and winter bat activity at a desert spring in southern Nevada. Southwest Nat. 1967;12(2):163-171. Doi:10.2307/3669270
O’Shea TJ, Vaughan TA. Nocturnal and seasonal activities of the pallid bat, Antrozous pallidus. J Mammal. 1977;58(3):269-284. Doi:10.2307/1379326
Ospina-Ante O, Gómez LG. Riqueza, abundancia relativa and patrones de actividad temporal de la comunidad de murciélagos de la Reserva Natural La Planada, Nariño, Colombia. Rev Acad Colomb Cienc Exactas Fis Nat. 1999;23:659-669.
Pérez-Torres J, Ahumada JA. Murciélagos en bosques alto-andinos, fragmentados and continuos, en el sector occidental de la sabana de Bogotá (Colombia). Univ Sci. 2004;9:33-46.
Ramírez-Chaves HE, Suárez-Castro AF. Adiciones y cambios a la lista de mamíferos de Colombia: 500 especies registradas para el territorio nacional. Mammalogy Notes. 2014;1(2):223-235.
Ramírez-Chaves HE, Suárez-Castro AF, González-Maya JF. Cambios recientes a la lista de los mamíferos de Colombia. Mammalogy Notes. 2016;3(1):1-9.
Reith CC. Insectivorous bats fly in shadows to avoid moonlight. J Mammal. 1982;63(4): 685-688. Doi:10.2307/1380284
Rodríguez-Posada ME. Murciélagos de un bosque en los Andes Centrales de Colombia con notas sobre su taxonomía and distribución. Caldasia. 2010;32(1):205-220.
Sánchez MS, Giannini NP. Altitudinal patterns in two syntopic species of Sturnira (Mammalia: Chiroptera: Phyllostomidae) in the montane rain forests of Argentina. Biotropica. 2014;46(1):1-5.
Santos-Moreno A, Ruiz-Velásquez E, Sánchez-Martínez A. Efecto de la intensidad de la luz lunar and de la velocidad del viento en la actividad de murciélagos filostomidos de Mena Nizanda, Oaxaca, México. Rev Mex Biodivers. 2010;81(3):839-845. Doi:10.7550/rmb.21531
Snell-Rood EC. The effect of climate on acoustic signals: does atmospheric sound absorption matter for bird song and bat echolocation? J Acoust Soc Am. 2012;131(2):1650-1658. Doi:10.1121/1.3672695
Solari S, Muñoz-Saba Y, Rodríguez-Mahecha JV, Defler TR, Ramírez-Chaves HE, Trujillo F. Riqueza, endemismo y conservación de los mamíferos de Colombia. Mastozool Neotrop. 2013;20(2):301-365.
Soriano PJ. Functional structure of bat communities in tropical rainforests and Andean cloud forests. Ecotropicos. 2000;13(1):1-20.
Soriano PJ, Ruiz A, Arends A. Physiological responses to ambient temperature manipulation by three species of bats from Andean cloud forests. J Mammal. 2002;83(2):445-457. Doi:10.1644/1545-1542(2002)083<0445:PRTATM>2.0.CO;2
Stiles FG. Ecological and evolutionary aspects of bird-flower coadaptations. In Noring R. editor. Proceedings of the XVII International Ornithological Congress. Berlin: International Ornithological Congress; 1980. p. 1173-1178.
Thies W, Kalko EK, Schnitzler HU. Influence of environment and resource availability on activity patterns of Carollia castanea (Phyllostomidae) in Panama. J Mammal. 2006;87(2):331-338. Doi:10.1644/05-MAMM-A-161R1.1
Vargas Espinoza A, Aguirre LF, Galarza MI, Gareca E. Ensamble de murciélagos en sitios con diferente grado de perturbación en un bosque montano del Parque Nacional Carrasco, Bolivia. Mastozool Neotrop. 2008;15(2):297-308.
Voigt CC, Lewanzik D. Trapped in the darkness of the night: thermal and energetic constraints of daylight flight in bats. Proc Biol Sci. 2011;278(1716):2311-2317. Doi:10.1098/rspb.2010.2290
Weinbeer M, Meyer C F, Kalko EK. Activity pattern of the trawling Phyllostomid bat, Macrophyllum macrophyllum, in Panamá. Biotropica. 2006;38(1):69-76.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. J. Perez-Guerra, J. Gonzalez-Velez, J. Murillo-Escobar, J. C. Jaramillo-Fayad. (2024). Prediction of areas with high risk of roadkill wildlife applying maximum entropy approach and environmental features: East Antioquia, Colombia. Landscape and Ecological Engineering, 20(1), p.75. https://doi.org/10.1007/s11355-023-00581-7.
2. Janeira Liseth Rosero-Taramuel, Ingrith Yuliany Mejía-Fontecha, Alexandra Marín-Ramírez, Valentina Marín-Giraldo, Héctor E. Ramírez-Chaves. (2023). Urban and peri-urban bats (Mammalia: Chiroptera) in Manizales, Colombia: exploring a conservation area in sub-Andean and Andean ecosystems. Mammalia, 87(6), p.545. https://doi.org/10.1515/mammalia-2022-0138.
3. Daisy Alejandra Gómez-Ruiz, Jesús Antonio Cogollo, Daniela Trujillo, Andrés Oliveros, Ana Cristina Cadavid R. (2023). Dípteros ectoparásitos asociados a murciélagos en un intervalo urbano-rural del norte de los Andes, Colombia. Caldasia, 45(3) https://doi.org/10.15446/caldasia.v45n3.102948.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2018 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).