Tumor del estroma gastrointestinal localizado en el duodeno con presentación clínica de síndrome carcinoide. Reporte de caso
Duodenal gastrointestinal stromal tumor and carcinoid syndrome. Case report
DOI:
https://doi.org/10.15446/cr.v10n1.103349Palabras clave:
Tumores del estroma gastrointestinal, Tumores neuroendocrinos, Tumor carcinoide (es)Gastrointestinal Stromal Tumors, Neuroendocrine Tumors, Carcinoid Tumor (en)
Descargas
Resumen
Introducción. Los tumores neuroendocrinos gastrointestinales son neoplasias que se derivan de células neuroendocrinas y pueden formarse en todo el sistema gastrointestinal. El 3% de los pacientes con este tipo de tumores desarrollan síndrome carcinoide, caracterizado por diarrea, enrojecimiento cutáneo, sibilancias o síntomas similares al asma y lesiones cutáneas similares a las de la pelagra con hiperqueratosis y pigmentación. Por su parte, los tumores del estroma gastrointestinal (GIST) son los tumores mesenquimales más frecuentes en el tracto digestivo, siendo el duodeno la ubicación más rara (4-5% de los GIST).
Presentación del caso. Mujer de 58 años que en agosto del 2020 consultó al servicio de urgencias del Hospital Dr. Hernán Henríquez Aravena de Temuco (Chile) por sintomatología sugerente de síndrome carcinoide. Se realizó tomografía computarizada de abdomen y pelvis con contraste que mostró lesión sólida hipervascular, hipodensa y probablemente necrótica ubicada en la segunda porción del duodeno. Dados los hallazgos, se practicó una duodenopancreatectomía con reconstrucción mediante pancreatoyeyunoanastomosis con técnica de Blumgart en la cual no se presentaron complicaciones y se evidenció páncreas blando y conducto pancreático principal fino. Los análisis histopatológico e inmunohistoquímico fueron compatibles con GIST.
Conclusión. Se presenta el caso de una paciente con síntomas clínicos y hallazgos en imágenes diagnósticas sugerentes de tumor neuroendocrino del páncreas, en quien se confirmó GIST localizado en el duodeno mediante biopsia. Este tipo de tumores pueden confundirse con tumores neuroendocrinos debido a la expresión de receptores de somatostatina, por lo cual es indispensable hacer un diagnóstico correcto.
Abstract
Introduction: Gastrointestinal neuroendocrine tumors are neoplasms derived from neuroendocrine cells that can form throughout the gastrointestinal system. Around 3% of patients with this type of tumor develop carcinoid syndrome, which is characterized by diarrhea, flushed skin, wheezing/asthma-like symptoms, and pellagra-like skin lesions with hyperkeratosis and pigmentation. Regarding gastrointestinal stromal tumors (GIST), they are the most common mesenchymal tumors in the digestive tract, with duodenal tumors being the rarest subtype (4-5% of all GIST).
Case presentation: A 58-year-old female patient presented to the emergency department of the Hospital Dr. Hernán Henríquez Aravena of Temuco (Chile) in August 2020 due to symptoms suggestive of carcinoid syndrome. A contrast computed tomography of the abdomen and pelvis showed a hypervascular, hypodense and probably necrotic lesion located in the second portion of the duodenum. Given the findings, a pancreaticoduodenectomy with reconstruction was performed by pancreaticojejunostomy with Blumgart's technique in which there were no complications, highlighting a soft pancreas and a thin pancreatic duct, with favorable follow-up. Histopathological and immunohistochemical analyzes were compatible with a duodenal GIST.
Conclusion: This is the case of a patient with clinical symptoms and diagnostic imaging findings suggestive of neuroendocrine tumor of the pancreas, in whom GIST located in the duodenum was confirmed by biopsy. This type of tumors can be mistaken for neuroendocrine tumors due to the expression of somatostatin receptors, so it is essential to make a correct diagnosis.
https://doi.org/10.15446/cr.v10n1.103349
Duodenal gastrointestinal stromal tumor and carcinoid syndrome. Case report
Keywords: Gastrointestinal Stromal Tumors; Neuroendocrine Tumors; Carcinoid Tumor.
Palabras clave: Tumores del estroma gastrointestinal; Tumores neuroendocrinos; Tumor carcinoide.
Héctor Fabio Losada-Morales
Universidad de la Frontera - Faculty of Medicine - Department of Surgery, Trauma, and Anesthesiology - Temuco - Chile.
Hospital Dr. Hernán Henríquez Aravena - Surgery Service - Temuco - Chile.
Clínica Alemana Temuco - Surgery Service - Temuco - Chile
Norberto Andrés Portillo-López
Universidad de la Frontera - Faculty of Medicine - Department of Surgery, Trauma, and Anesthesiology - Temuco - Chile.Renato Esteban Becker-Hecker
Hospital Dr. Hernán Henríquez Aravena - Department of Anatomic Pathology - Temuco - Chile.
Universidad de la Frontera - Faculty of Medicine - Department of Anatomic Pathology - Temuco - Chile
Renato Esteban Becker-Hecker.
Hospital Dr. Hernán Henríquez Aravena -
Department of Anatomic Pathology - Temuco - Chile.
Universidad de la Frontera - Faculty of Medicine -
Department of Anatomic Pathology - Temuco - Chile
Marcelo Eduardo Klein-Díaz
Hospital Dr. Hernán Henríquez Aravena - Radiology Service - Temuco - Chile.
Corresponding author
Héctor Losada-Morales. Departamento de Cirugía, Traumatología y Anestesiología, Facultad de Medicina, Universidad de la Frontera. Temuco. Chile. E-mail: hector.losada@ufrontera.cl
Received: 25/06/2022 Accepted: 12/12/2022
Resumen
Introducción. Los tumores neuroendocrinos gastrointestinales son neoplasias que se derivan de células neuroendocrinas y pueden formarse en todo el sistema gastrointestinal. El 3% de los pacientes con este tipo de tumores desarrollan síndrome carcinoide, caracterizado por diarrea, enrojecimiento cutáneo, sibilancias o síntomas similares al asma y lesiones cutáneas similares a las de la pelagra con hiperqueratosis y pigmentación. Por su parte, los tumores del estroma gastrointestinal (GIST) son los tumores mesenquimales más frecuentes en el tracto digestivo, siendo el duodeno la ubicación más rara (4-5% de los GIST).
Presentación del caso. Mujer de 58 años que en agosto del 2020 consultó al servicio de urgencias del Hospital Dr. Hernán Henríquez Aravena de Temuco (Chile) por sintomatología sugerente de síndrome carcinoide. Se realizó tomografía computarizada de abdomen y pelvis con contraste que mostró lesión sólida hipervascular, hipodensa y probablemente necrótica ubicada en la segunda porción del duodeno. Dados los hallazgos, se practicó una duodenopancreatectomía con reconstrucción mediante pancreatoyeyunoanastomosis con técnica de Blumgart en la cual no se presentaron complicaciones y se evidenció páncreas blando y conducto pancreático principal fino. Los análisis histopatológico e inmunohistoquímico fueron compatibles con GIST.
Conclusión. Se presenta el caso de una paciente con síntomas clínicos y hallazgos en imágenes diagnósticas sugerentes de tumor neuroendocrino del páncreas, en quien se confirmó GIST localizado en el duodeno mediante biopsia. Este tipo de tumores pueden confundirse con tumores neuroendocrinos debido a la expresión de receptores de somatostatina, por lo cual es indispensable hacer un diagnóstico correcto.
Abstract
Introduction: Gastrointestinal neuroendocrine tumors are neoplasms derived from neuroendocrine cells that can form throughout the gastrointestinal system. Around 3% of patients with this type of tumor develop carcinoid syndrome, which is characterized by diarrhea, flushed skin, wheezing/asthma-like symptoms, and pellagra-like skin lesions with hyperkeratosis and pigmentation. Regarding gastrointestinal stromal tumors (GIST), they are the most common mesenchymal tumors in the digestive tract, with duodenal tumors being the rarest subtype (4-5% of all GIST).
Case presentation: A 58-year-old female patient presented to the emergency department of the Hospital Dr. Hernán Henríquez Aravena of Temuco (Chile) in August 2020 due to symptoms suggestive of carcinoid syndrome. A contrast computed tomography of the abdomen and pelvis showed a hypervascular, hypodense and probably necrotic lesion located in the second portion of the duodenum. Given the findings, a pancreaticoduodenectomy with reconstruction was performed by pancreaticojejunostomy with Blumgart’s technique in which there were no complications, highlighting a soft pancreas and a thin pancreatic duct, with favorable follow-up. Histopathological and immunohistochemical analyzes were compatible with a duodenal GIST.
Conclusion: This is the case of a patient with clinical symptoms and diagnostic imaging findings suggestive of neuroendocrine tumor of the pancreas, in whom GIST located in the duodenum was confirmed by biopsy. This type of tumors can be mistaken for neuroendocrine tumors due to the expression of somatostatin receptors, so it is essential to make a correct diagnosis.
Introduction
Gastrointestinal neuroendocrine tumors (GI-NETs) are rare neoplasms that can form throughout the gastrointestinal system and are derived from neuroendocrine cells (1). These tumors can be classified either as non-functioning, because they do not generate symptoms related to the secretion of a certain hormone, or as functioning, because they cause symptoms associated with the release of peptides and hormones, which, also, can cause carcinoid syndrome (2).
Carcinoid syndrome develops in 3% of patients with neuroendocrine tumors in the duodenum (3), which, according to Ito et al. (4), is characterized by diarrhea (78%), flushed skin (78%), wheezing or asthma-like symptoms (12%), and pellagra-like skin lesions with hyperkeratosis and pigmentation (1%). A potential treatment to treat these symptoms is the administration of somatostatin analogues, and subcutaneous administration of octreotide prior to the procedure is recommended for patients who are candidates for surgery to reduce the incidence of carcinoid crisis (2).
Somatostatin receptors (SSTR) are present in 90% of carcinoid tumors, which can be of five different subtypes: SSTR1, SSTR2, SSTR3, SSTR4, and SSTR5. SSTR2 is predominant in neuroendocrine tumors and somatostatin analogs used in therapy predominantly bind to this subtype (5).
Regarding gastrointestinal stromal tumors (GIST), it has been described that they are the most frequent mesenchymal tumors in the digestive tract and that duodenal GIST is the rarest subtype, representing only 4-5% of all GIST, but up to 21% of all tumors resected (6). Although most GIST do not cause symptoms, when symptoms occur they can be highly variable, the most frequent being gastrointestinal bleeding and abdominal pain (7,8).
Case presentation
A 58-year-old female with a history of arterial hypertension (AHT), hypothyroidism and untreated bronchial asthma was admitted to the emergency room of the Hospital Dr. Hernán Henríquez Aravena in Temuco (Chile) in August 2020 due to abdominal pain, persistent diarrhea lasting one month, and vomiting. The patient indicated that she had undergone a cesarean section in 1991 and an open cholecystectomy in 2006.
Upon physical examination on admission the patient was hypertensive (166/96 mmHg), tachycardic (112 bpm), with signs of mild dehydration, diffuse abdominal pain on palpation, and no signs of peritoneal irritation. On the same day of admission, a contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis showed a round, hypodense mass of irregular edges measuring 34x35x32mm on the anterior aspect of the second portion of the duodenum, which had features suggestive of GI-NET. No secondary lymphatic implants or signs of distant metastasis were observed in this CT scan (Figure 1). The patient was discharged with a diagnosis of duodenal tumor and with a suspicion of neuroendocrine tumor, for which complementary studies were requested. Outpatient follow-up was performed by the internal medicine and surgery services.
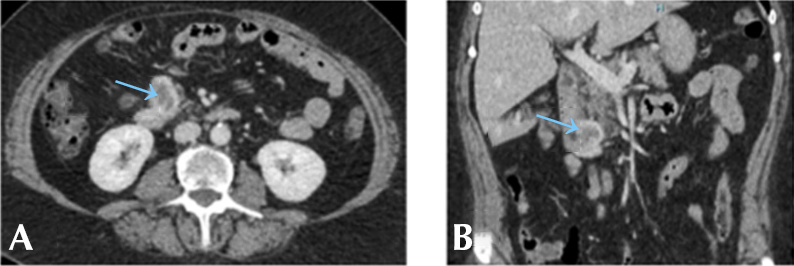
Figure 1. Computed tomography of the abdomen and pelvis. A) axial plane); B) coronal plane). The blue arrows show a parietal heterogeneous hypervascular nodule attached to the second and third portion of the duodenum, which is non-obstructive and causes no vascular involvement.
Source: Images obtained while conducting the study.
The patient was monitored on an outpatient basis by the endocrinology and gastroenterology services and 30 days after discharge an upper gastrointestinal endoscopy was performed, which showed a subepithelial lesion adjacent to the ampulla of Vater. During this procedure, a biopsy was performed, revealing duodenal mucosa with reactive inflammatory changes without villous flattening or evidence of malignancy (Figure 2). Simultaneously, a colonoscopy was performed and the result was normal.
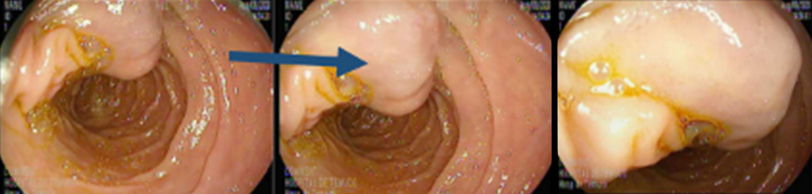
Figure 2. Upper endoscopy showing a 15mm submucosal lesion in the second portion of the duodenum adjacent to the ampulla of Vater.
Source: Image obtained while conducting the study.
Furthermore, 60 days after the initial visit, a positron emission tomography/computed tomography with Ga 68 DOTATATE (68Ga-DOTATOC PET/CT) was performed in which the following findings were reported: hypervascular, hypodense, exophytic and probably necrotic solid lesion measuring 3x7x3.7cm in the second portion of the duodenum with a maximum standardized uptake value (SUVmax) of 8.66 and no signs of distant metastasis (Figure 3).
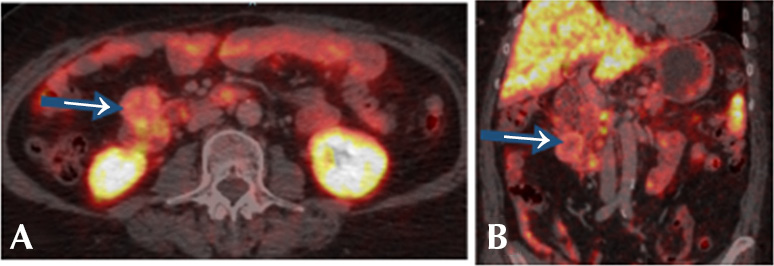
Figure 3. Positron emission tomography/computed tomography with Ga 68 DOTATATE. A) axial plane; B) coronal plane. Blue arrows show a heterogeneous, hypervascular, hypodense and probably necrotic solid nodule attached to the second and third portion of the duodenum with maximum standardized uptake value of 8.66.
Source: Image obtained while conducting the study.
Subsequently, 5 months after the initial visit, the patient was readmitted to the emergency department of the same institution due to suspicion of carcinoid syndrome after presenting recurrent episodes of sweating, palpitations, hypertension, tachycardia, chest pain, flushing (+), and persistent diarrhea of up to 5 episodes per day. A glycosylated hemoglobin (HbA1C) test was performed and the result was found to be within normal parameters (5.6%). The woman was admitted to the hospital and treated by the endocrinology service, which indicated the initiation of empirical treatment with somatostatin analogues (sandostatin LAR 20mg orally every 28 days). Concurrently, her case was evaluated during a medical board by the biliopancreatic surgery team, which decided to perform a cephalic pancreaticoduodenectomy.
In August 2021, a pancreaticoduodenectomy with reconstruction by means of pancreatojejunostomy with Blumgart’s technique was performed, with no complications and in which a soft pancreas and a thin pancreatic duct were evidenced. The patient developed a pancreatic fistula type B, for which she required percutaneous drainage, total parenteral nutrition, and management with octreotide (0.1mg subcutaneous every 8 hours) for 10 days. She was discharged from hospital 28 days after surgery.
The final biopsy report of the surgical specimen (Figure 4) reported the following findings: morphological and immunohistochemical alterations compatible with spindle cell GIST located in the duodenum (Figure 5), grade 1, with negative margins and a mitotic index <1/50 high power fields (HPF), which presented a low risk of malignancy according to the modified National Institutes of Health (NIH) classification (Table 1).
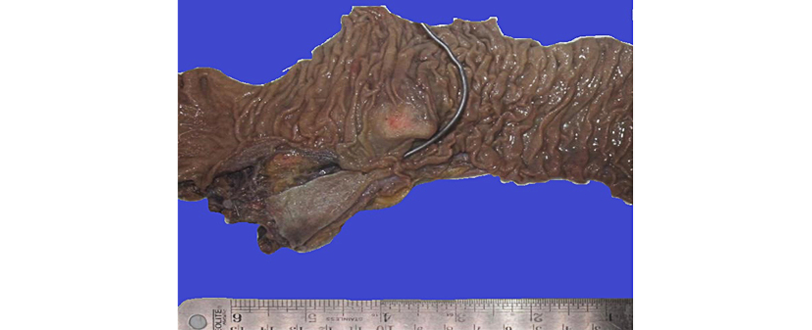
Figure 4. Macroscopy of surgical specimen obtained during pancreaticoduodenectomy. A nodular lesion is observed under the duodenal mucosa located 2cm from the major axis, 6cm from the proximal edge, 15.5cm from the distal edge, and 0.7cm from the ampulla of Vater.
Source: Image obtained while conducting the study.
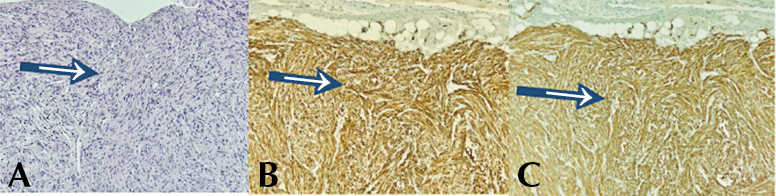
Figure 5. Microscopy and immunohistochemistry of surgical specimen obtained during pancreaticoduodenectomy. A) spindle cell stromal neoplasm consisting of cells with elongated nuclei and eosinophilic cytoplasm (hematoxylin-eosin stain, 100x); B) DOG1: diffuse and intense immunoreactivity (cytoplasmic staining pattern, 100x); C. CD117: diffuse and intense immunoreactivity (100x cytoplasmic staining pattern).
Source: Image obtained while conducting the study.
Table 1. Modified National Institutes of Health classification for gastrointestinal stromal tumors.
|
Risk |
Tumor size (cm) |
Mitotic count (by high magnification fields) |
Tumor location |
|
Very low |
2 |
>5/50 |
Any location |
|
Low |
2.1-5 |
>5/50 |
Any location |
|
Intermediate |
>5 |
6-10/50 |
Gastric |
|
5.1-10 |
>5/50 |
Gastric |
|
|
High |
Any size |
Any mitotic rate |
Perforated tumor |
|
>5 |
>5/50 |
Any location |
|
|
>10 |
Any mitotic rate |
Any location |
|
|
Any size |
>10/50 |
Any location |
|
|
2.1-5 |
>5/50 |
Non-gastric |
|
|
5.1-10 |
<5/50 |
Non-gastric |
Source: Elaborated based on Agaimy (9).
The patient, who had no symptoms or complications during the postoperative period, was followed up for 1 year by the biliopancreatic surgery and endocrinology services and was found to be in good general condition during follow-up examinations.
Discussion
Duodenal GIST is a rare neoplasm with a highly variable clinical presentation related to its origin, growth rate, and size (7). This tumor may be asymptomatic or involve symptoms such as upper gastrointestinal bleeding and abdominal pain (6-8). Its diagnosis is difficult since the imaging studies currently available only allow for suspicion, while confirmation is achieved histologically, with endosonography and biopsy being the gold standard tests (10).
In this report, the patient initially sought medical assistance for abdominal pain, persistent diarrhea and vomiting, symptoms that are nonspecific. On her second admission, she presented symptoms consistent with carcinoid syndrome (sweating, palpitations, hypertension, tachycardia, precordial pain, flushed skin, and persistent diarrhea). These symptoms were added to the Ga 68-DOTATATE PET/CT scan findings and, as a result, the initial diagnostic hypothesis of GI-NET was confirmed. At this point, it should be noted that, among GI-NETs, 90% of carcinoid tumors involve the presence of SSTRs.
SSTRs belong to the G protein-coupled receptor (GPCR) superfamily. Somatostatin is expressed in two biologically active forms: somatostatin 14, which is the molecular form originally identified in the hypothalamus, and somatostatin 28, which was subsequently isolated and characterized. Both somatostatin 14 and 28 are widely distributed throughout the body; however, the presence and abundance of their expression depend on the tissue where they are located. Thus, the 14-amino acid peptide is produced up to 4 times more in the hypothalamus, whereas somatostatin 28 is produced in the intestine; somatostatin 14 is the only one found in other tissues such as the stomach, the retina, or the pancreatic islets (11).
As mentioned above, there are 5 SSTR subtypes (SSTR1, SSTR2, SSTR3, SSTR4, and SSTR5), which have been identified via autoradiographic studies in a large number of organs, including the brain, pituitary gland, pancreas, gastrointestinal tract, and placenta. It has also been established that SSTR1, SSTR2 and SSTR5 receptors, and probably SSTR3, are found in the pituitary gland of a healthy subject. The SSTR2 receptor is the main mediator in growth hormone secretion inhibition.
It is worth mentioning that somatostatin is distributed in most, but not all organs (nervous system, intestine, pancreas, salivary glands, urinary excretory system, etc.) (5).
In a study performed on 478 GIST samples to evaluate the expression levels and prognostic values of SSTRs in GIST patients, Zhao et al. (12) demonstrated a high positive expression of SSTR1 (81.9%) and SSTR2 (87.6%) and a low expression of SSTR3 (56.1%) and SSTR5 (47.2%) in this type of tumors. This opens up a new line of research and a possible therapeutic target that offers new possibilities for the management of these tumors.
On the other hand, the Ga 68-DOTATATE PET/CT test has emerged as a highly sensitive hybrid SSTR imaging modality for neuroendocrine tumors, as well as for imaging and characterizing other tumors with high expression of SSTR, such as paragangliomas and specific mesenchymal tumors (13). This test has a high affinity for the SSTR2 receptor, but no affinity for the SSTR3 and SSTR5 receptors. There are several studies that have shown that the SSTR2 receptor is the subtype most frequently expressed in primary and metastatic GI-NETs (14-16).
Imaging studies with somatostatin analogs are extremely useful for identifying primary tumors and for staging disease, especially GI-NETs of the small bowel. In addition, according to Olivia-Gonzáles et al. (17) there are reports of sensitivity ranging from 80% to almost 100%. These tests also facilitate decision making regarding the course of medical treatment (18).
In the case described, the anatomic pathology report of the biopsy specimen obtained during the pancreatoduodenectomy showed morphologic and immunohistochemical alterations compatible with a GIST located in the duodenum, which ruled out the initial diagnosis of GI-NET.
In general, GISTs affect patients over 50 years of age and are discovered incidentally (19); however, in most cases the diagnosis is made histologically, being, therefore, endosonography and post-surgical biopsy the main methods of diagnostic confirmation, as was the case of the patient reported. In her case, there was no preoperative suspicion of GIST, but it was thought to be a functioning neuroendocrine tumor, and for this reason endosonography was not performed prior to surgery.
In the reviewed literature, no reports were found of an atypical clinical presentation such as that of the patient in the present case. However, one publication supported the use of Ga 68-DOTATATE PET/CT scan in cases of GIST for diagnostic, therapeutic, and follow-up purposes (20).
Due to the lack of early clinical manifestations, duodenal GISTs are usually diagnosed when they have reached a size that is large enough to cause symptoms related to the effect of the mass or gastrointestinal bleeding. Most publications on duodenal GISTs are case reports or series, and there are no studies that relate GISTs expressing SSTR to the symptomatology of the patients. Therefore, the clinical manifestations, radiological diagnosis, surgical treatment and prognostic factors are still not clearly established and generate controversy (21).
As for histology, the modified NIH classification (9) categorizes GISTs into four categories depending on the risk of malignancy (very low risk, low risk, intermediate risk, and high risk) taking into account their location or rupture, mitotic count, and size (Table 1). In the present case, the biopsy revealed a 4cm duodenal tumor with a mitotic count <1/50 HPF, which was classified as low risk (22).
Conclusion
This is a case report of a patient with clinical symptoms and diagnostic imaging findings suggestive of neuroendocrine tumor of the pancreas, but in whom the result of the biopsy performed on the surgical specimen collected confirmed a GIST tumor located in the duodenum. This type of tumors can be mistaken for neuroendocrine tumors due to the expression of SSTR, so it is essential to make a correct diagnosis.
Ethical considerations
The authors state that no experiments involving humans or animals were performed for the preparation of this case report and that the patient’s details are not included in this article.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
None stated by the authors.
References
1.Zúñiga-Monge D. Tumores neuroendocrinos gastrointestinales. Medicina Legal de Costa Rica. 2013;30(1):89-98.
2.Miranda G, Luna L. Tumor neuroendocrino ileal con síndrome carcinoide, patología de difícil diagnóstico y pronóstico variable. An. Fac. med. 2015;76(2):193-8. https://doi.org/mvft.
3.Sato Y, Hashimoto S, Mizuno KI, Takeuchi M, Terai S. Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol. 2016;22(30):6817-28. https://doi.org/f8xzvf.
4.Ito T, Lee L, Jensen RT. Carcinoid-syndrome: recent advances, current status and controversies. Curr Opin Endocrinol Diabetes Obes. 2018;25(1):22-35. https://doi.org/mvfs.
5.Lumbreras-Gavilanes A. Somatostatina: bioquímica, fisiología y uso farmacológico [tesis]. Madrid: Universidad Complutense de Madrid; 2017.
6.Popivanov G, Tabakov M, Mantese G, Cirocchi R, Piccinini I, D’Andrea V, et al. Surgical tratment of gastrointestinal stromal tumors of the duodenum: a literature review. Transl Gastroenterol Hepatol. 2018;3:71. https://doi.org/mvfv.
7.Cavallaro G, Polistena A, D’Ermo G, Pedullà G, De Toma G. Duodenal gastrointestinal stromal tumors: review on clinical and surgical aspects. Int. J Surg. 2012;10(9):463-5. https://doi.org/mvfw.
8.Melo C, Canhoto C, Manata F, Bernardes A. Surgical treatment of giant gist with acute gastrointestinal bleeding: Case report. Int J Surg Case Rep. 2018;53:354-7. https://doi.org/mvfx.
9.Agaimy A. Gastrointestinal stromal tumors (GIST) from risk stratification systems to the new TNM proposal: more questions than answers? A review emphasizing the need for a standardized GIST reporting. Int J Clin Exp Pathol. 2010;3(5):461-71.
10.Losada-Morales H, Sanhueza-Vega F, Klein-Diaz M, Becker-Hecker R. GIST duodenal asociado a hemorragia digestiva alta. Reporte de caso. Rev. Cir. 2021;73(2):212-6. https://doi.org/mvh8.
11.La Salvia A, Espinosa-Olarte P, Riesco-Martinez MDC, Anton-Pascual B, Garcia-Carbonero R. Targeted Cancer Therapy: What’s New in the Field of Neuroendocrine Neoplasms? Cancers. 2021;13(7):1701. https://doi.org/gmh56t.
12.Zhao WY, Zhuang C, Xu J, Wang M, Zhang ZZ, Tu L, et al. Somatostatin receptors in gastrointestinal stromal tumors: new prognostic biomarker and potential therapeutic strategy. Am J Transl Res. 2014;6(6):831-40.
13.Aloj L, Giger O, Mendichovszky IA, Challis BG, Ronel M, Harper I, et al. The role of [68 Ga]Ga-DOTATATE PET/CT in wild-type KIT/PDGFRA gastrointestinal stromal tumours (GIST). EJNMMI Res. 2021;11(1):5. https://doi.org/mvh9.
14.Kaemmerer D, Peter L, Lupp A, Schulz S, Sänger J, Prasad V, et al. Molecular imaging with 68Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38(9):1659-68. https://doi.org/fbxmjp.
15.Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17(1):R53-73. https://doi.org/bd92cs.
16.Kulaksiz H, Eissele R, Rössler D, Schulz S, Höllt V, Cetin Y, et al. Identification of somatostatin receptor subtypes 1, 2A, 3, and 5 in neuroendocrine tumours with subtype specific antibodies. Gut. 2002;50(1):52-60. https://doi.org/dr42d3.
17.Oliva-González JP, Martínez-Ramírez Aldo, González-González JJ, Almeida-Arias DA, Calderón-Marín CF, Quesada-Cepero Waldo, et al. Gammagrafía de receptores de somatostatina con 68Ga-DOTATATE PET/CT: experiencia inicial en Cuba. Nucleus. 2017:(62):14-23.
18.Arne G, Nilsson B, Dalmo J, Kristiansson E, Arvidsson Y, Forssell-Aronsson E, et al. Gastrointestinal stromal tumors (GISTs) express somatostatin receptors and bind radiolabeled somatostatin analogs. Acta Oncol. 2013;52(4):783-92. https://doi.org/f24knj.
19.Oyanedel R, O’Brien A, Pizarro A, Zamora E, Menias C. Tumor estromal gastrointestinal (GIST): formas de presentación. Rev. Chil. Radiol. 2005;11(1):13-8. https://doi.org/dx9htw.
20.Loaiza-Bonilla A, Bonilla-Reyes PA. Somatostatin Receptor Avidity in Gastrointestinal Stromal Tumors: Theranostic Implications of Gallium-68 Scan and Eligibility for Peptide Receptor Radionuclide Therapy. Cureus. 2017;9(9):e1710. https://doi.org/mvkv.
21.Beltrán MA. Tumores del estroma gastrointestinal (GIST) del duodeno: presentación clínica, estudio diagnóstico y tratamiento actual. Rev Chil Cir. 2014;66(4):381-93. https://doi.org/mvkw.
22.Parab TM, De Rogatis MJ, Boaz AM, Grasso SA, Issack PS, Duarte DA, et al. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol. 2019;10(1):144-54. https://doi.org/gjqt8t.
Referencias
References
Zúñiga-Monge D. Tumores neuroendocrinos gastrointestinales. Medicina Legal de Costa Rica. 2013;30(1):89-98.
Miranda G, Luna L. Tumor neuroendocrino ileal con síndrome carcinoide, patología de difícil diagnóstico y pronóstico variable. An. Fac. med. 2015;76(2):193-8. https://doi.org/mvft.
Sato Y, Hashimoto S, Mizuno KI, Takeuchi M, Terai S. Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol. 2016;22(30):6817-28. https://doi.org/f8xzvf.
Ito T, Lee L, Jensen RT. Carcinoid-syndrome: recent advances, current status and controversies. Curr Opin Endocrinol Diabetes Obes. 2018;25(1):22-35. https://doi.org/mvfs.
Lumbreras-Gavilanes A. Somatostatina: bioquímica, fisiología y uso farmacológico [tesis]. Madrid: Universidad Complutense de Madrid; 2017.
Popivanov G, Tabakov M, Mantese G, Cirocchi R, Piccinini I, D’Andrea V, et al. Surgical tratment of gastrointestinal stromal tumors of the duodenum: a literature review. Transl Gastroenterol Hepatol. 2018;3:71. https://doi.org/mvfv.
Cavallaro G, Polistena A, D’Ermo G, Pedullà G, De Toma G. Duodenal gastrointestinal stromal tumors: review on clinical and surgical aspects. Int. J Surg. 2012;10(9):463-5. https://doi.org/mvfw.
Melo C, Canhoto C, Manata F, Bernardes A. Surgical treatment of giant gist with acute gastrointestinal bleeding: Case report. Int J Surg Case Rep. 2018;53:354-7. https://doi.org/mvfx.
Agaimy A. Gastrointestinal stromal tumors (GIST) from risk stratification systems to the new TNM proposal: more questions than answers? A review emphasizing the need for a standardized GIST reporting. Int J Clin Exp Pathol. 2010;3(5):461-71..
Losada-Morales H, Sanhueza-Vega F, Klein-Diaz M, Becker-Hecker R. GIST duodenal asociado a hemorragia digestiva alta. Reporte de caso. Rev. Cir. 2021;73(2):212-6. https://doi.org/mvh8.
La Salvia A, Espinosa-Olarte P, Riesco-Martinez MDC, Anton-Pascual B, Garcia-Carbonero R. Targeted Cancer Therapy: What's New in the Field of Neuroendocrine Neoplasms? Cancers. 2021;13(7):1701. https://doi.org/gmh56t.
Zhao WY, Zhuang C, Xu J, Wang M, Zhang ZZ, Tu L, et al. Somatostatin receptors in gastrointestinal stromal tumors: new prognostic biomarker and potential therapeutic strategy. Am J Transl Res. 2014;6(6):831-40.
Aloj L, Giger O, Mendichovszky IA, Challis BG, Ronel M, Harper I, et al. The role of [68 Ga]Ga-DOTATATE PET/CT in wild-type KIT/PDGFRA gastrointestinal stromal tumours (GIST). EJNMMI Res. 2021;11(1):5. https://doi.org/mvh9.
Kaemmerer D, Peter L, Lupp A, Schulz S, Sänger J, Prasad V, et al. Molecular imaging with 68Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38(9):1659-68. https://doi.org/fbxmjp.
Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17(1):R53-73. https://doi.org/bd92cs.
Kulaksiz H, Eissele R, Rössler D, Schulz S, Höllt V, Cetin Y, et al. Identification of somatostatin receptor subtypes 1, 2A, 3, and 5 in neuroendocrine tumours with subtype specific antibodies. Gut. 2002;50(1):52-60. https://doi.org/dr42d3.
Oliva-González JP, Martínez-Ramírez Aldo, González-González JJ, Almeida-Arias DA, Calderón-Marín CF, Quesada-Cepero Waldo, et al. Gammagrafía de receptores de somatostatina con 68Ga-DOTATATE PET/CT: experiencia inicial en Cuba. Nucleus. 2017:(62):14-23.
Arne G, Nilsson B, Dalmo J, Kristiansson E, Arvidsson Y, Forssell-Aronsson E, et al. Gastrointestinal stromal tumors (GISTs) express somatostatin receptors and bind radiolabeled somatostatin analogs. Acta Oncol. 2013;52(4):783-92. https://doi.org/f24knj.
Oyanedel R, O'Brien A, Pizarro A, Zamora E, Menias C. Tumor estromal gastrointestinal (GIST): formas de presentación. Rev. Chil. Radiol. 2005;11(1):13-8. https://doi.org/dx9htw.
Loaiza-Bonilla A, Bonilla-Reyes PA. Somatostatin Receptor Avidity in Gastrointestinal Stromal Tumors: Theranostic Implications of Gallium-68 Scan and Eligibility for Peptide Receptor Radionuclide Therapy. Cureus. 2017;9(9):e1710. https://doi.org/mvkv.
Beltrán MA. Tumores del estroma gastrointestinal (GIST) del duodeno: presentación clínica, estudio diagnóstico y tratamiento actual. Rev Chil Cir. 2014;66(4):381-93. https://doi.org/mvkw.
Parab TM, De Rogatis MJ, Boaz AM, Grasso SA, Issack PS, Duarte DA, et al. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol. 2019;10(1):144-54. https://doi.org/gjqt8t.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2024 Case reports

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Los autores al someter sus manuscritos conservarán sus derechos de autor. La revista tiene el derecho del uso, reproducción, transmisión, distribución y publicación en cualquier forma o medio. Los autores no podrán permitir o autorizar el uso de la contribución sin el consentimiento escrito de la revista.
El Formulario de Divulgación Uniforme para posibles Conflictos de Interés y los oficios de cesión de derechos y de responsabilidad deben ser entregados junto con el original.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons 4.0 que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).


























