Síndrome de dolor regional complejo secundario a infección por SARS-CoV-2. Reporte de caso
Complex regional pain syndrome secondary to SARS-CoV-2 infection: Case report
DOI:
https://doi.org/10.15446/cr.v10n1.99564Palabras clave:
Síndrome de dolor regional complejo, Causalgia, Infecciones por coronavirus (es)Complex Regional Pain Syndromes, Coronavirus Infections, Causalgia (en)
Descargas
Resumen
Introducción. El síndrome de dolor regional complejo (SDRC) es una afección rara cuyo diagnóstico se realiza con base en un examen físico y los síntomas reportados por el paciente ya que no existe una prueba diagnóstica definitiva. El tratamiento de esta condición, que también es limitado y a menudo no produce alivio completo de los síntomas, se centra en aumentar la movilidad y el uso del miembro afectado. Se presenta el caso de una paciente que desarrolló SDRC como consecuencia de una infección por SARS-CoV-2. Dado que hasta el momento no se ha documentado una relación causal entre estas dos entidades, este se considera un caso atípico.
Presentación del caso. Mujer de 28 años quien consultó a una institución de cuarto nivel de atención de Bogotá (Colombia) por dolor de características neuropáticas y movilidad limitada del miembro superior izquierdo. Tres semanas antes la paciente había presentado infección por SARS-CoV-2. Dada la sintomatología, se consideró que cursaba con SDRC secundario a infección por SARS-CoV-2. Debido a que los síntomas persistían a pesar del manejo analgésico administrado, se decidió realizar una intervención analgésica invasiva con la cual se logró una mejoría parcial.
Conclusiones. Se reporta el caso de una paciente que posterior a una infección por SARS-CoV-2 presentó sintomatología correspondiente al SDRC con una secuencia temporal que permite vincular ambas entidades, configurando así una rara asociación. La información aquí descrita permite establecer un punto de partida para el estudio y el mejor entendimiento de ambas enfermedades teniendo en cuenta sus características y puntos comunes.
Abstract
Introduction: Complex regional pain syndrome (CRPS) is a rare condition that is diagnosed based on physical examination and patient-reported symptoms since there is no definitive diagnostic test. The treatment for this condition, which is also limited and does not often result in complete relief of symptoms, focuses on increasing mobility and use of the affected limb. This is a case report of a patient who developed CRPS as a consequence of SARS-CoV-2 infection. Since no causal relationship between these two conditions has been reported to date, this is considered an atypical case.
Case Report: A 28-year-old female patient visited a quaternary care clinic in the city of Bogotá, Colombia, due to neuropathic pain and limited mobility of the left upper limb. Three weeks prior, the patient had presented with SARS-CoV-2 infection. In view of the symptoms reported, it was considered that she had CRPS secondary to SARS-CoV-2 infection. Since symptoms persisted despite the analgesic management administered, it was decided to perform an invasive analgesic intervention that led to partial improvement.
Conclusions: This is a case report of a patient who, following a SARS-CoV-2 infection, developed symptoms related to CRPS with a timeline that allows connecting both conditions, thus constituting a rare association. The information described here allows establishing a starting point for the study and better understanding of both diseases taking into account their characteristics and common points.
https://doi.org/10.15446/cr.v10n1.99564
Complex regional pain syndrome secondary to SARS-CoV-2 infection: Case report
Keywords: Complex Regional Pain Syndromes; Causalgia; Coronavirus Infections.
Palabras clave: Síndrome de dolor regional complejo; Causalgia; Infecciones por coronavirus.
Juan Esteban González-Camargo
Universidad Nacional de Colombia
- Bogotá Campus - Faculty of Medicine -
Department of Physical Medicine and Rehabilitation
- Bogotá D.C. - Colombia
Angela Viviana Navas-Granados
Clínica Universitaria Colombia - Neurology Service - Bogotá D.C. - Colombia
Christian Vladimir Guauque-Marcelo
Clínica Universitaria Colombia - Pain and Palliative Care Service - Bogotá D.C. - Colombia
Jorge Arturo Diaz-Ruiz
Clínica Universitaria Colombia - Physical Medicine and Rehabilitation Service - Bogotá D.C. - Colombia
Corresponding author
Juan Esteban González-Camargo.
Facultad de Medicina, Universidad Nacional
de Colombia. Bogotá. Colombia.
E-mail: juegonzalezca@unal.edu.co
Received: 18/11/2021 Accepted: 22/06/2022
Resumen
Introducción. El síndrome de dolor regional complejo (SDRC) es una afección rara cuyo diagnóstico se realiza con base en un examen físico y los síntomas reportados por el paciente ya que no existe una prueba diagnóstica definitiva. El tratamiento de esta condición, que también es limitado y a menudo no produce alivio completo de los síntomas, se centra en aumentar la movilidad y el uso del miembro afectado. Se presenta el caso de una paciente que desarrolló SDRC como consecuencia de una infección por SARS-CoV-2. Dado que hasta el momento no se ha documentado una relación causal entre estas dos entidades, este se considera un caso atípico.
Presentación del caso. Mujer de 28 años quien consultó a una institución de cuarto nivel de atención de Bogotá (Colombia) por dolor de características neuropáticas y movilidad limitada del miembro superior izquierdo. Tres semanas antes la paciente había presentado infección por SARS-CoV-2. Dada la sintomatología, se consideró que cursaba con SDRC secundario a infección por SARS-CoV-2. Debido a que los síntomas persistían a pesar del manejo analgésico administrado, se decidió realizar una intervención analgésica invasiva con la cual se logró una mejoría parcial.
Conclusiones. Se reporta el caso de una paciente que, posterior a una infección por SARS-CoV-2, presentó sintomatología correspondiente al SDRC con una secuencia temporal que permite vincular ambas entidades, configurando así una rara asociación. La información aquí descrita permite establecer un punto de partida para el estudio y el mejor entendimiento de ambas enfermedades, teniendo en cuenta sus características y puntos comunes.
Abstract
Introduction: Complex regional pain syndrome (CRPS) is a rare condition that is diagnosed based on physical examination and patient-reported symptoms since there is no definitive diagnostic test. The treatment for this condition, which is also limited and does not often result in complete relief of symptoms, focuses on increasing mobility and use of the affected limb. This is a case report of a patient who developed CRPS as a consequence of SARS-CoV-2 infection. Since no causal relationship between these two conditions has been reported to date, this is considered an atypical case.
Case Report: A 28-year-old female patient visited a quaternary care clinic in the city of Bogotá, Colombia, due to neuropathic pain and limited mobility of the left upper limb. Three weeks prior, the patient had presented with SARS-CoV-2 infection. In view of the symptoms reported, it was considered that she had CRPS secondary to SARS-CoV-2 infection. Since symptoms persisted despite the analgesic management administered, it was decided to perform an invasive analgesic intervention that led to partial improvement.
Conclusions: This is a case report of a patient who, following a SARS-CoV-2 infection, developed symptoms related to CRPS with a timeline that allows connecting both conditions, thus constituting a rare association. The information described here allows establishing a starting point for the study and better understanding of both diseases taking into account their characteristics and common points.
Introduction
Complex regional pain syndrome (CRPS) is a rare, progressive condition characterized by severe pain, usually in the extremities. This condition usually occurs after sustaining a direct injury (i.e. fractures, sprains, dislocations, and nerve injuries), but can also occur after surgery, stroke or acute myocardial infarction, but it may not even have a clear cause (1,2). The prevalence of CRPS may vary depending on social and ethnic factors, and middle-aged women seem to suffer more frequently from this condition (female-to-male ratio of 4:1), with the upper limbs being the most typically affected (3).
Although its pathophysiology is not yet fully established, it is known that patients with this condition exhibit classical inflammation mediated mainly by tumor necrosis factor (TNF), interleukin-1 (IL1) and interleukin-6 (IL6); dysregulation in the production of proinflammatory cytokines in the peripheral nerve (which can even lead to degeneration of nerve fibers); and alterations of the autonomic nervous system that give rise to the symptoms that come with pain (increased skin temperature, erythema, and changes in skin appendages) (1).
Similarly, an increase in neuropeptides such as substance P and calcitonin gene-related peptide (CGRP) have been shown to be associated with the release of proinflammatory mediators, such as TNF-a, IL-1b, IL-6, and nerve growth factor (NGF), some of which potentiate peripheral sensitization to noxious stimuli (1). Moreover, within its etiopathogenesis, it has been described that the central sensory and motor circuits are reorganized, leading to an increase in nociception and movement dysfunction of the affected limb (4).
This is the case of a female patient with CRPS associated with SARS-CoV-2 infection, whose causal relationship has not been documented so far, so it can be considered as an atypical case.
Case presentation
A 28-year-old female patient from Bogotá (Colombia), a secretary at a health care institution with no significant medical history except for SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR) test 25 days prior to admission, visited the emergency room of a quaternary care institution in Bogotá due to persistent pain and limitation of movement in her left elbow.
SARS-CoV-2 infection had initially presented with mild respiratory symptoms and joint pain in knees, elbows, wrists, and interphalangeal joints of both hands. These symptoms, with the exception of left elbow pain, had resolved 8 days prior to her admission to the emergency room.
Pain in the left elbow was subsequently associated with allodynia, limitation of forearm mobility, edema, and increased thermal sensation in the area. Another symptom was a decrease in the palpebral fissure of the left eye.
Physical examination on admission revealed vital signs within normal parameters; left forearm with redness, grade 2/3 edema, and increased temperature; pain on passive or active movement of the left arm with reduced range of motion in the elbow and hand; synovitis in the metacarpophalangeal joints; and lividity in the left hand (Figure 1).
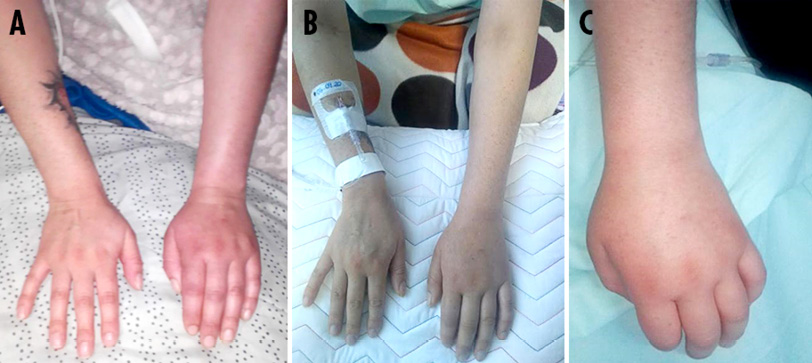
Figure 1. A) edema and redness of soft tissues on left forearm; b) synovitis in metacarpophalangeal joints; C) lividity in left hand.
Source: Images obtained while conducting the study.
On neurological examination, there was evidence of decreased strength in the left forearm (MRC scale: 3/5) (5), which was related to pain and hypoesthesia and was predominant in the area of innervation of the ulnar and median nerves; in addition, the patient had allodynia in the forearm and hand. Cranial nerve analysis revealed hypoesthesia in the V1 (ophthalmic nerve) and V2 (maxillary nerve) regions of the left trigeminal nerve.
During admission and the following 24 hours, blood tests were performed to rule out autoimmune disease (rheumatoid factor, complement blood test, anti-neutrophil cytoplasmic antibodies [ANCA], anti-myeloperoxidase antibodies [anti-MPO], and antinuclear antibodies [ANA: anti-LA, anti-RO, anti-SM, and anti-DNA]), which were negative. Measurements of muscle enzyme levels and acute phase reactants (performed 5 days after admission) were also taken and were found to be within normal parameters.
Furthermore, 7 days after being admitted, the following imaging and electrodiagnostic tests were performed: Doppler ultrasound, electromyography and nerve conduction velocity test of the left upper limb; magnetic resonance imaging (MRI)
to characterize the brachial plexus, left hand, forearm, and shoulder; MRI of the brain; MRI of the orbits; and computed tomography angiography of the chest, abdomen, and neck. All of them yielded normal results, as well as a three-phase bone scan performed on the tenth day of admission.
From admission until discharge, the patient was treated with intravenous diclofenac (75mg intramuscular every 12 hours) and oral acetaminophen
(1g every 12 hours). On the second day of hospitalization, it was decided to initiate symptomatic management with intravenous hydromorphone with patient-controlled
analgesia (PCA) infusion pump (doses up to 0.3mg with 10-minute blocks, maximum 7.2mg in 4 hours); this medication was administered for 9 days. Then, 7 days after admission, oral methadone was added (dose up to 10mg oral every 12 hours, equivalent dose of daily oral morphine: 100mg) and administered for
3 days, i.e., until the tenth day of hospitalization.
During her hospital stay, the patient was also given oral pregabalin (75mg every 12 hours), which was started on the third day of admission and used for 4 days. Despite the implementation of this therapeutic approach, no complete response was obtained and the patient reported the persistence of moderate to high levels of pain according to the visual analog scale (VAS) score: 3/10 - 5/10.
On the tenth day of admission, since the patient met the Budapest and Kozin CRPS diagnostic criteria and her clinical condition did not improve, a stellate ganglion block was performed with anesthetic and non-particulate steroid together with the pain clinic service as follows: first, the thyroid gland and the cricoid cartilage were located at the level of the C6 vertebrae; then, 50mm echogenic needle advancement technique was performed in field vision and the tip of the needle was brought to the posterior space of the left carotid artery in the fascia between the longus colli and longus capiti muscles, where, through the administration of saline solution, it was verified that there was an adequate distribution; finally, 5mL of mixed analgesics (30mg of 1% lidocaine without epinephrine and 8mg of non-particulated dexamethasone) were instilled, thus achieving an 80% reduction in pain in the immediate postoperative period according to the VAS score.
The day after the intervention, a clinical follow-up was performed, showing continued analgesic effect, reduction of the cutaneous sympathetic response in the left upper limb, and reduction of allodynia in the affected area. Consequently, the patient was discharged from the hospital and outpatient follow-up was indicated. It should be noted that no adverse effects were reported in any of the interventions performed during the hospital stay.
In a virtual follow-up that took place four and a half months after the intervention, the patient reported persistence of the pain and functional limitation, thus she continued to be followed up by a pain specialist for symptomatic management. The timeline with all the events of the case is presented in Figure 2.
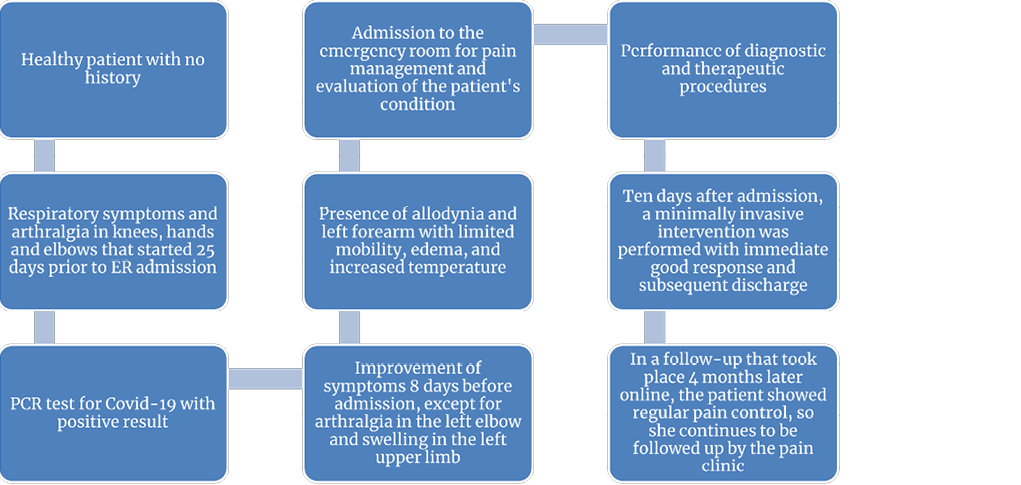
Figure 2. Timeline of the case.
PCR: polymerase chain reaction.
Source: Own elaboration.
Discussion
CRPS is a rare condition that was first described in the sixteenth century and renamed as we know it today by the International Association of Study of Pain (IASP) in 1993 (6). It is classified into two main groups: type 1 CRPS, in which there is no physical injury to the nerve involved, and type 2 CRPS, in which there is evident damage to the nerve structure (6-8). The case of the patient reported here was classified as type 1 CRPS since she met the Budapest diagnostic criteria (7) and there was no specific neuropathic damage according to clinical, paraclinical, and imaging information.
In 2003, Sandroni et al. (9) published a study carried out in Olmsted County (United States) with data recorded between 1989 and 1999, in which they established that the incidence of CRPS was 5.46 cases per 100 000 inhabitants.
CRPS is a condition that occurs more frequently in women, with a female-to-male
ratio of 4:1, and in patients between the fifth and sixth decade of life (3,9), affecting the upper limbs more than the lower limbs with a ratio of 3:2 (10). The risk factors for the development of this syndrome include smoking, menopause, consumption of angiotensin-converting enzyme inhibitors, and migraine (11). In the present case, the patient’s only predisposing factor was the female sex.
In cases of CRPS, elevated levels of neuropeptides such as CGRP, bradykinin, and substance P are observed since they are released at peripheral nerve endings as a result of tissue injury, triggering neurogenic inflammation (12,13). It has also been established that there are systemic and central nervous system mechanisms related to pain, such as elevated levels of proinflammatory cytokines in cerebrospinal fluid (14-16) and reorganization of cortical neurons, which contribute to the persistence of this symptom (17,18).
Even though the CRPS diagnosis is made based on clinical evaluation, some diagnostic tests such as X-rays, MRI and bone scans can be useful. However, it should be kept in mind that they are not effective in all cases (2).
Clinical manifestations of CRPS include edema, allodynia, cutaneous vasomotor changes, and alterations in sudomotor function that are usually distal to the lesion. There are different diagnostic criteria such as the Kozin and Budapest scales; the latter has a high sensitivity (99%) but a moderate specificity (68%), assesses the presence of symptoms, and rules out other differential diagnoses (Tables 1 and 2) (19).
Table 1. Budapest criteria for the diagnosis of complex regional pain syndrome.
|
Criterion 1. Pain |
Continuing pain, disproportionate to any inciting event |
|
Criterion 2. Symptoms |
The patient must report at least one symptom in three of the following four categories: • Sensory: hyperesthesia and/or allodynia • Vasomotor: temperature asymmetry, changes in skin color, and/or skin color asymmetry • Sudomotor: edema, sweating changes, and/or sweating asymmetry • Motor: decreased range of motion, motor dysfunction, and/or trophic changes (hair, nails, skin) |
|
Criterion 3. |
The patient must report at least one sign in two or more of the following categories: • Sensory: hyperalgesia (pinprick) and/or allodynia (light touch or temperature) • Vasomotor: skin temperature asymmetry >1°C, changes in skin color, and/or skin color asymmetry • Sudomotor: edema, sweating changes, and/or sweating asymmetry • Motor: decreased range of motion, motor dysfunction (weakness, tremor, dystonia), and/or trophic changes (hair, nails, sin) |
|
Criterion 4 |
Other diseases that may explain the previous signs and symptoms should be ruled out |
Source: Own elaboration based on Pergolizzi et al. (19)
Table 2. Kozin criteria for the diagnosis of complex regional pain syndrome.
|
Criterion 1 |
Pain and tenderness of a limb |
|
Criterion 2 |
Symptoms or signs of unsteadiness, Raynaud’s phenomenon, cold and pale skin, hot or erythematous skin, hyperhidrosis |
|
Criterion 3 |
Swelling of limb, edema with or without fovea |
|
Criterion 4 |
Trophic skin changes, atrophy, desquamation, hypertrichosis, hair loss, nail changes, thickening of the palmar aponeurosis |
|
Diagnosis |
Defined: meets 4 criteria Probable: meets criteria 1, 2, and 3 Possible: meets criteria 1 and 2 |
Source: Own elaboration based on Castillo et al. (4)
Treatment for CRPS is symptomatic and the drugs on the World Health Organization analgesic ladder can be used, with the exception of opioids (20). Some anti-free radical drugs and bisphosphonates have shown moderate efficacy in reducing inflammation; likewise, vasodilators such as nifedipine, despite their side effects, have been shown to be partially effective. In cases where pharmacological treatments are not sufficient, invasive measures such as sympathetic blocks and spinal cord stimulation can be considered (21). Continued physical therapy is recommended as a fundamental part of the rehabilitation process because it has been shown to have a possible beneficial effect on patients with this condition (22).
It is important to keep in mind that, although various therapeutic options have been proposed, none have shown sufficient effectiveness to be considered as standard treatment. Furthermore, in some cases, due to the failure to achieve improvement in patients, amputation of the affected limb has been considered to improve their quality of life (21).
In the present case, it is noteworthy that the patient developed CRPS following a SARS-CoV-2 infection that initially manifested with respiratory and osteoarticular symptoms. Although the pathophysiology of neurological damage due to SARS-CoV-2 is not fully elucidated and there are no previous reports of CRPS due to this infection, there are multiple studies in the literature describing the presentation of peripheral neuropathy due to SARS-CoV-2 (23). The latter, added to the inflammation of the peripheral nerve that has been implicated in the pathophysiology of CRPS (12) and the timeline of the events that occurred in this patient, allows establishing a relationship of association between both conditions.
Conclusions
Type 1 CRPS is a rare and difficult condition in which, after ruling out multiple differential diseases, the clinical features are the main diagnostic tool. In the case reported here, the occurrence of CRPS was associated with SARS-CoV-2 infection, and this is noteworthy because CRPS can be considered as another neurological presentation related to SARS-CoV-2 infection, which has not been described in the literature. However, there is a lack of theoretical support for the etiopathogenesis of this association since the physiopathological mechanisms by which SARS-Cov-2 acts have not been fully established to date. In this sense, further research should aim to clarify the mechanism of action of the virus in the peripheral nervous system and to establish possible treatments.
Ethical considerations
The patient’s informed consent was obtained for the preparation of this case report.
Conflict of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
To the case patient for allowing us to use her clinical records to improve our understanding of her particular case.
References
1.Shim H, Rose J, Halle S, Shekane P. Complex regional pain syndrome: a narrative review for the practising clinician. Br J Anaesth. 2019;123(2):e424-33. https://doi.org/gk7r9g.
2.Harden RN, McCabe CS, Goebel A, Massey M, Suvar T, Grieve S, et al. Complex Regional Pain Syndrome: Practical Diagnostic and Treatment Guidelines, 5th Edition. Pain Med. 2022;23(Suppl 1):S1-S53. https://doi.org/kv3s.
3.Misidou C, Papagoras C. Complex Regional Pain Syndrome: An update. Mediterr J Rheumatol. 2019;30(1):16-25. https://doi.org/ggtj5s.
4.Castillo-Guzmán, Nava-Obregón TA, Palacios-Ríos D, Estrada-Cortinas JA, González-García MC, Mendez-Guerra JF, et al. Complex regional pain syndrome (CRPS), a review. Medicina Universitaria. 2015;17(67):114-21. https://doi.org/f3hh9v.
5.Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. 2008;40(8):665-71. https://doi.org/cdxt95.
6.Cutts S, Gangoo S, Srinivasan SH, Modi N, Pasapula C, Power D. Complex regional pain syndrome: an evolving perspective. Postgrad Med J. 2021;97(1146):250-5. https://doi.org/mm58.
7.Dutton K, Littlejohn G. Terminology, criteria, and definitions in complex regional pain syndrome: Challenges and solutions. J Pain Res. 2015;8:871-7. https://doi.org/gkbzdx.
8.Dey S, Guthmiller KB, Varacallo M. Complex Regional Pain Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
9.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103(1-2):199-207. https://doi.org/cprcks.
10. de Mos M, de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BHC, Sturkenboom MCJM. The incidence of complex regional pain syndrome: A population-based study. Pain. 2007;129(1-2):12-20. https://doi.org/dhjdh9.
11. Farzad M, MacDermid JC, Packham T, Khodabandeh B, Vahedi M, Shafiee E. Factors associated with disability and pain intensity in patients with complex regional pain syndrome. Disabil Rehabil. 2022;44(26):8243-51. https://doi.org/gqmwv7.
12.Taylor SS, Noor N, Urits I, Paladini A, Sadhu MS, Gibb C, et al. Complex Regional Pain Syndrome: A Comprehensive Review. Pain Ther. 2021;10(2):875-92. https://doi.org/gqjzh2.
13. Lloyd ECO, Dempsey B, Romero L. Complex Regional Pain Syndrome. Am Fam Physician. 2021;104(1):49-55.
14. Mills M, Howell CM. Complex regional pain syndrome. JAAPA. 2023;36(8):1-5. https://doi.org/mm6g.
15.Alexander GM, Van Rijn MA, Van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116(3):213-9. https://doi.org/cb9t7w.
16.Alexander GM, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman RJ. Changes in plasma cytokines and their soluble receptors in complex regional pain syndrome. J Pain. 2012;13(1):10-20. https://doi.org/d8fm28.
17. Maihöfner C, Handwerker HO, Neundörfer B, Birklein F. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63(4):693-701. https://doi.org/mm6h.
18. Maihöfner C, Handwerker HO, Neundörfer B, Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61(12):1707-15. https://doi.org/gh6ks8.
19. Pergolizzi JV, LeQuang JA, Nalamachu S, Taylor R, Bigelsen RW. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2(1):1-10. https://doi.org/mm6p.
20. Iolascon G, Snichelotto F, Moretti A. An update on the pharmacotherapeutic options for complex regional pain syndrome. Expert Rev Neurother. 2024;24(2):177-90. https://doi.org/mm6s.
21.Ayyaswamy B, Saeed B, Anand A, Chan L, Shetty V. Quality of life after amputation in patients with advanced complex regional pain syndrome: a systematic review. EFORT Open Rev. 2019;4(9):533-40. https://doi.org/mm6t.
22.Smart KM, Ferraro MC, Wand BM, O’Connell NE. Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Database Syst Rev. 2022;5(5):CD010853. https://doi.org/kv3r.
23.Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. https://doi.org/ggxftr.
Referencias
References
Shim H, Rose J, Halle S, Shekane P. Complex regional pain syndrome: a narrative review for the practising clinician. Br J Anaesth. 2019;123(2):e424-33. https://doi.org/gk7r9g.
Harden RN, McCabe CS, Goebel A, Massey M, Suvar T, Grieve S, et al. Complex Regional Pain Syndrome: Practical Diagnostic and Treatment Guidelines, 5th Edition. Pain Med. 2022;23(Suppl 1):S1-S53. https://doi.org/kv3s.
Misidou C, Papagoras C. Complex Regional Pain Syndrome: An update. Mediterr J Rheumatol. 2019;30(1):16-25. https://doi.org/ggtj5s.
Castillo-Guzmán, Nava-Obregón TA, Palacios-Ríos D, Estrada-Cortinas JA, González-García MC, Mendez-Guerra JF, et al. Complex regional pain syndrome (CRPS), a review. Medicina Universitaria. 2015;17(67):114-21. https://doi.org/f3hh9v.
Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. 2008;40(8):665-71. https://doi.org/cdxt95.
Cutts S, Gangoo S, Srinivasan SH, Modi N, Pasapula C, Power D. Complex regional pain syndrome: an evolving perspective. Postgrad Med J. 2021;97(1146):250-5. https://doi.org/mm58.
Dutton K, Littlejohn G. Terminology, criteria, and definitions in complex regional pain syndrome: Challenges and solutions. J Pain Res. 2015;8:871-7. https://doi.org/gkbzdx.
Dey S, Guthmiller KB, Varacallo M. Complex Regional Pain Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103(1-2):199-207. https://doi.org/cprcks.
de Mos M, de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BHC, Sturkenboom MCJM. The incidence of complex regional pain syndrome: A population-based study. Pain. 2007;129(1-2):12-20. https://doi.org/dhjdh9.
Farzad M, MacDermid JC, Packham T, Khodabandeh B, Vahedi M, Shafiee E. Factors associated with disability and pain intensity in patients with complex regional pain syndrome. Disabil Rehabil. 2022;44(26):8243-51. https://doi.org/gqmwv7.
Taylor SS, Noor N, Urits I, Paladini A, Sadhu MS, Gibb C, et al. Complex Regional Pain Syndrome: A Comprehensive Review. Pain Ther. 2021;10(2):875-92. https://doi.org/gqjzh2.
Lloyd ECO, Dempsey B, Romero L. Complex Regional Pain Syndrome. Am Fam Physician. 2021;104(1):49-55.
Mills M, Howell CM. Complex regional pain syndrome. JAAPA. 2023;36(8):1-5. https://doi.org/mm6g.
Alexander GM, Van Rijn MA, Van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116(3):213-9. https://doi.org/cb9t7w.
Alexander GM, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman RJ. Changes in plasma cytokines and their soluble receptors in complex regional pain syndrome. J Pain. 2012;13(1):10-20. https://doi.org/d8fm28.
Maihöfner C, Handwerker HO, Neundörfer B, Birklein F. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63(4):693-701. https://doi.org/mm6h.
Maihöfner C, Handwerker HO, Neundörfer B, Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61(12):1707-15. https://doi.org/gh6ks8.
Pergolizzi JV, LeQuang JA, Nalamachu S, Taylor R, Bigelsen RW. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2(1):1-10. https://doi.org/mm6p.
Iolascon G, Snichelotto F, Moretti A. An update on the pharmacotherapeutic options for complex regional pain syndrome. Expert Rev Neurother. 2024;24(2):177-90. https://doi.org/mm6s.
Ayyaswamy B, Saeed B, Anand A, Chan L, Shetty V. Quality of life after amputation in patients with advanced complex regional pain syndrome: a systematic review. EFORT Open Rev. 2019;4(9):533-40. https://doi.org/mm6t.
Smart KM, Ferraro MC, Wand BM, O’Connell NE. Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Database Syst Rev. 2022;5(5): CD010853. https://doi.org/kv3r.
Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. https://doi.org/ggxftr.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2024 Case reports

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Los autores al someter sus manuscritos conservarán sus derechos de autor. La revista tiene el derecho del uso, reproducción, transmisión, distribución y publicación en cualquier forma o medio. Los autores no podrán permitir o autorizar el uso de la contribución sin el consentimiento escrito de la revista.
El Formulario de Divulgación Uniforme para posibles Conflictos de Interés y los oficios de cesión de derechos y de responsabilidad deben ser entregados junto con el original.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons 4.0 que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).


























