Production of sucroesters using solvent-free reactive systems containing emulsifiers
Producción de ésteres de sacarosa utilizando sistemas reactivos libres de solvente con emulsionantes
DOI:
https://doi.org/10.15446/ing.investig.v38n1.61432Keywords:
Sucroester, solvent-free, transesterification, emulsifiers, Heterogeneous (en)Éster de sacarosa, libre de solventes, transesterificación, emulsionante (es)
The transesterification reaction of sucrose and fatty acid methyl esters to produce sucroesters was experimentally evaluated using commercial emulsifiers as compatibility agents. Reactions were carried out at temperatures between 100 and 140°C, using emulsifier concentrations in the range of 5 to 15 %wt, and potassium carbonate as catalyst. Fatty acid methyl esters consumption and sucroesters production was monitored by HPLC analysis of samples. Methyl esters conversions around 40 % were obtained with 68 %wt monoester content in sucroesters mixture. Despite the reaction times were reduced by operating at high temperatures and high emulsifier’s concentration, multiple substitution and color degradation were observed. Higher productivities of sucroester and higher selectivity to monoesters were obtained when potassium palmitate was used as contacting agent. The lower monoester content in the final product was obtained when using a commercial sucroester emulsifier. Results of this study can be used for preliminary process design in a solvent-free production of biobased sucroesters.
Production of sucroesters using solvent-free
reactive systems containing emulsifiers
Producción de ésteres de sacarosa utilizando sistemas reactivos
libres de solvente con emulsionantes
Ma. Fernanda Gutierrérrez 1, Álvaro Orjuela 2, J. Luis Rivera 3, and Andrea Suaza 4
1 >B. S.c., Universidad Nacional de Colombia, Colombia. M.Sc. Technische Universität Berlin, Germany. Ph.D. candidate, Universidad Nacional de Colombia, Colombia. E-mail: mfgutierrezs@unal.edu.co
2 B. S.c., Universidad Nacional de Colombia, Colombia. M.S.c., Universidad Nacional de Colombia, Colombia. Ph.D., Michigan State University, USA. Affiliation: Associate Professor, Universidad Nacional de Colombia, Colombia. E-mail: aorjuelal@unal.edu.co
3 B. S.c., Universidad Nacional de Colombia, Colombia. Universidad Nacional de Colombia, Colombia. E-mail: jlriverag@unal.edu.co
4 B. S.c., Universidad Nacional de Colombia, Colombia. Specialist in Cosmetics, Universidad Nacional de Colombia, Colombia. M.Sc. Student, Universidad Nacional de Colombia, Colombia. E-mail: asuazam@unal.edu.co
How to cite: Gutierrez M. F., Orjuela A., Rivera J. L., Suaza A. (2018). Production of sucroesters using solvent-free reactive systems containing emulsifiers. Ingeniería e Investigación, 38(1), 16-23.
DOI:10.15446/ing.investig.v38n1.61432.
ABSTRACT
The transesterification reaction of sucrose and fatty acid methyl esters to produce sucroesters was experimentally evaluated using commercial emulsifiers as compatibility agents. Reactions were carried out at temperatures between 100 and 140°C, using emulsifier concentrations in the range of 5 to 15 %wt, and potassium carbonate as catalyst. Fatty acid methyl esters consumption and sucroesters production was monitored by HPLC analysis of samples. Methyl esters conversions around 40 % were obtained with 68 %wt monoester content in sucroesters mixture. Despite the reaction times were reduced by operating at high temperatures and high emulsifier’s concentration, multiple substitution and color degradation were observed. Higher productivities of sucroester and higher selectivity to monoesters were obtained when potassium palmitate was used as contacting agent. The lower monoester content in the final product was obtained when using a commercial sucroester emulsifier. Results of this study can be used for preliminary process design in a solvent-free production of biobased sucroesters.
Keywords: Sucroester, solvent-free, transesterification, emulsifiers, Heterogeneous.
RESUMEN
En este trabajo se evaluó experimentalmente la reacción de transesterificación entre sacarosa y ésteres metílicos de ácidos grasos para producir ésteres de sacarosa, usando emulsificantes comerciales como agentes de compatibilidad. Las reacciones se llevaron a cabo a temperaturas entre 100 y 140°C, usando concentraciones de emulsionantes en un rango entre 5 y 15%p, y carbonato de potasio como catalizador. El consumo de ésteres metílicos de ácidos grasos y la producción de ésteres de sacarosa fueron seguidos por HPLC. Se obtuvieron conversiones de palmitato de metilo alrededor del 40% y ésteres de sacarosa de 68%p de monoéster. A pesar de que los tiempos de reacción se reducen operando a altas temperaturas y altas concentraciones de emulsionante, se observó que el producto final sufría degradación de color. Las mayores productividades de ésteres de sacarosa y la mayor selectividad hacia monoésteres fueron obtenidas cuando se usó palmitato de potasio como agente de contacto. El menor contenido de monoéster en el producto final fue obtenido usando éster de sacarosa comercial. Los resultados de este estudio pueden ser usados por diseño conceptual preliminar en la producción libre de solvente de ésteres de sacarosa biobasados.
Palabras clave: Éster de sacarosa, libre de solventes, transesterificación, emulsionante.
Received: December 6th 2016
Accepted: September 11th 2017
Introduction
Sucroesters of fatty acids are biobased non-ionic surfactants. They exhibit interesting properties as green chemicals such as rapid biodegradability, biocompatibility, and biocide potential for certain microorganisms (Baker et al., 2000; Ferrer et al., 2005; Otomo, 2009; Shi, Li, & Chu, 2011). They are used as emulsifiers in the manufacture of food, cosmetics, pharmaceuticals, and clean and care products. Biobased sucroesters can be produced by esterification of sucrose with fatty acids, or by transesterification with methyl esters (FAMEs) or fatty acid vinyl (FAVEs). Generally, transesterification is preferred because it avoids water formation (and its further separation), limits saponification of fatty acids by basic catalysts, and operates with less corrosive media. The base-catalyzed transesterification reaction (Figure 1) is carried out between two immiscible phases (solid-liquid or liquid-liquid) at temperatures limited by sucrose caramelization (ca. 160°C). Produced methanol is continuously removed in order to shift equilibrium reaction to the desired products. Due to the multiple functional hydroxyl groups in sucrose, the transesterification product is a complex mixture of monoesters, diesters and even octaesters. As a result, tuning of the reaction selectivity to a specific product is a major challenge.
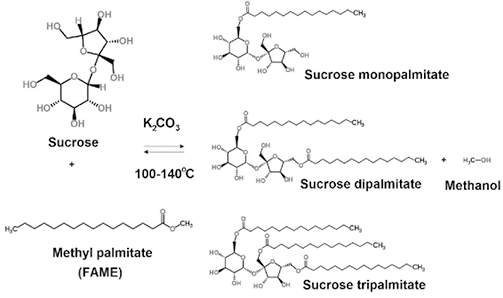
Figure 1. Sucrose fatty acid esters production by transesterification of fatty acid methyl ester (FAME) and sucrose.
Source: Authors
The properties of the sucroester surfactants and their applications are heavily determined by substitution degree (Otomo, 2009). The Hidrofilic-Lipofilic Balance (HLB) of sucrose monoesters is high and they tend to form oil-in-water (o/w) emulsions. In the other extreme of the scale, sucrose octaesters exhibit low HLB values and they are good agents for water-in-oil (w/o) emulsions. In general, the degree of substitution of sucroesters depends on different process conditions such as the reactants molar ratio, temperature, the use of additives in reaction (e.g. solvents, emulsifiers), catalyst’s nature and concentration, etc.
In addition to the control of the esterification degree, a major challenge of sucroesters production is the lack of compatibility of reactants. The presence of solid-solid-liquid interfaces among the catalyst (K2CO3), sucrose and FAMEs results in low reaction rates and conversions. With the aim of improving compatibility, transesterification has been carried out in solvents such as dimethylsulphoxide (DMSO) or dimethylformamide (DMF), in which both reactants are soluble (Deshpande et al., 2013; Hass et al., 1959). Despite the improved solubility, the required solvent loading is nearly 60%wt of the reaction mixture reducing reaction productivity. However, the solvent process involves an exhaustive solvent separation processes in order to avoid trace residues in the final product (Farone et al., 1996; Nakamura et al., 1995; Osipow et al., 1969).
Another alternative to improve compatibility between reactants is the use of contacting agents that help dispersing the solid sucrose into the liquid FAMEs. Alkaline and multivalent fatty acid soaps have demonstrated emulsifying action in this reaction (Fitremann et al., 2007; Galleymore et al., 1981; James & Smith, 2009, 2012; Le-Coent et al., 2003). Also, sucroesters have been used to improve reactants compatibility (Le-Coent et al., 2003; Zhao et al., 2014). Mono- and diacylglicerol have been also evaluated as emulsifiers, producing a final mixture of sucroesters and glycerides called sucrose glycerides (food ingredient number: E474) (Desai & Grüning, 1999; Parker et al., 1976).
All these contacting agents have been used in concentrations from 1 to 30%wt and high temperatures between 130 and 180°C (Fitremann et al., 2003; Galleymore et al., 1981). The most commonly used catalyst is potassium carbonate at concentrations between 5 and 12%wt (Parker et al., 1977). Molar feed ratio of sucrose/FAME can be between 0,1 to 2 (James & Smith, 2012). As a result of these conditions and the presence of impurities, sucrose might exhibit a slight decrease on its melting point. In fact, melting of sucrose mixed with emulsifiers can occur ca. 20°C below melting point of pure sucrose (Feuge, et al., 1973). A main drawback of the reaction is the degradation of sucrose even before melting, causing an undesired color in the final product.
Despite several studies dealt with the use of emulsifiers to promote the solvent-free reaction, the influence of concentration and type of emulsifier on the transesterification process has not been evaluated. Moreover, the influence of the temperature on the solvent-free reaction has never been reported on open literature. This information is required for early decision-making during the process design and economics assessment of sucroesters production. In this study, sucrose palmitate (SE), potassium palmitate (KP) or glycerol monostearate (GMS) were evaluated as contacting agents in the solvent-free transesterification of sucrose and methyl palmitate. The impact of type of contacting agent, concentration, and reaction temperature over the conversion and esterification degree were established.
Experimental
Chemicals and reagents
Sucrose (99,0%wt) was purchased from Panreac AppliChem GmbH(Germany). Methyl palmitate (97%wt) was purchased from Sigma-Aldrich Co.LLC (USA). Potassium palmitate for analysis was purchased from Wako Pure Chemical Ind. Ltd. (Japan). Sucrose palmitate (90%wt) for analysis was purchased from Alfa Aesar (USA). Commercial food grade sucroesters were purchased from Modernist Pantry LLC (USA). Glycerol monostearate for analysis was purchased from Alfa Aesar (USA). Anhydrous potassium carbonate (99%wt) for analysis was purchased from Alfa Aesar (USA). HPLC grade acetonitrile (ACN) and dimethyl sulfoxide (DMSO) were purchased from Panreac AppliChem GmbH (Germany).
Sucrose esters of palmitic acid used as standards were obtained by a preparative chromathographic method that allowed toseparate monopalmitate, dipalmitate, tripalmitate and tetrapalmitate sucrose esters. Identification of these compounds was made by NMR and FTIR. Results of this analysis will be presented elsewhere.
Sucroester synthesis
The conditions of the complete set of experiments using three different emulsifiers are showed in Table 1. Mixtures of 2,5:1 molar ratio of methyl palmitate:sucrose, potassium carbonate (5%wt), and emulsifier at concentrations ranging from 5%wt to 15%wt, were homogenized at 16000rpm and 50°C prior to reactions. The synthesis was carried out in a 150ml glass-jacketed reactor coupled to a condenser and a methanol collector (Figure 2). The reaction mixture was placed in the reactor, degasified from 50°C to the reaction temperature at 0,33bar. It was maintained under agitation at 700rpm with a magnetic stirrer during the whole reaction time. Operating temperature was maintained with a thermal circulation bath (Polystat, Cole Parmer). Condenser temperature was kept at -5°C by a water-propylenglycol circulation bath (Julabo MC, Julabo) in order to recover produced methanol. Samples of reaction (ca. 0,5mL) were withdrawn at different reaction times during the reaction period, using a pre-heated plastic 3mL pipette. They were immediately dissolved in a suitable solvent to avoid solidification or fractionation within the pipette.

Figure 2. Experimental setting for transesterification of FAME and sucrose to produce sucroester.
Source: Authors
Analytical methods
Most reported methods for quantification of sucroesters involve non-isocratic HPLC separations under isothermal conditions coupled to light-scattering or charged aerosol detectors (Lie & Pedersen, 2013; Ritthitham et al., 2009; Wang et al., 2006). However, we implemented a standardized method using isocratic conditions and a RI detector. Quantification of methyl palmitate and sucrose palmitate with different esterification degrees was performed by HPLC analysis (Dionex UltiMate 3000, Thermo Scientific, USA) equipped with Shodex RI-101 detector (Showa Denko K.K., Japan) and with a LiChrospher100 RP-18 5,0 μm column (125 x 4,0 mm Merck, Germany). The system operated at 40°C using and Isocratic flow of ACN/DMSO 90/10 v/v as the mobile phase at 0,5mL/min. The samples were dissolved (ca. 50mg/mL) using ACN/DMSO 60/40 v/v, and passed through a 0,45μm nylon filter (Membrane Solutions) and an aliquot of 20μL was injected. Chromeleon 7.1 software was used for data acquisition and processing. A typical chromatogram of sucrose palmitate and methyl palmitate used as standard is shown in Figure 3.
Results and Discussion
The selected contacting agents were selected because they could be found as impurities in crude FAMEs (Gerpen, 2005, Narváez, et al., 2007), and they are problematic for drop-in-fuel applications (Meher et al., 2006). Results obtained in the synthesis of sucroesters using the different contacting agents are summarized in Table 1. As expected, in all experiments the viscosity of the reactive mixture markedly increased as reaction proceeds; this could be an indirect indicator of sucroester formation or sugar caramelization (i.e. for process control) (Deshpande et al., 2013; Le-Coent et al., 2003; Zhao et al., 2014). Reactions were carried out until viscosity hindered proper stirring, so the heat transfer was no longer homogeneous. At this point the last sample was taken and the experiment was ended. Besides the change in viscosity, the color of reactive mixture changed from white into dark brown, while passing through a spectrum of light cream to light brown. This was reported in previous studies as indication of sugar caramelization (Fitremann et al., 2007).

Figure 3. HPLC chromatogram of a mixture 50:50 of sucrose palmitate and methyl palmitate ester. Peak identifications: (S) Solvent; (1) Sucrose monopalmitate. (2) Sucrose dipalmitate. (3) Sucrose dipalmitate. (4) Methyl palmitate. (5) Sucrose tripalmitate. (6) Sucrose tetrapalmitate.
Source: Authors
For each experiment, the methyl palmitate conversion, the final content of sucroesters, and the amount of monoester in ester product were calculated from HPLC results. Conversions obtained during experiments ranged from 14 to 58% and the total reaction time varied depending on temperature and emulsifier’s concentration. These results agree with similar studies using similar emulsifiers (max. conversion ~ 40%; James & Smith, 2012). The final monoester content in sucroester ranged between 53 and 86%wt, which corresponds to surfactants with HLB between 10 and 16 (Nelen & Cooper, 2004).
Table 1. Results of reaction during sucroesters production using emulsifiers as contacting agent at different operating conditions.
|
Run |
Temp. (°C) |
Emulsif. |
% wt Emulsif. |
Total reaction time (h) |
Final |
Final |
Final |
|
1* |
140 |
None |
0 |
6,5 |
6 |
0,3 |
94,4 |
|
2* |
140 |
SE |
5 |
4,8 |
4 |
1 |
70 |
|
3* |
140 |
KP |
5 |
4,8 |
2 |
3 |
66 |
|
4* |
140 |
GMS |
5 |
6,5 |
6 |
0,1 |
0 |
|
5 |
100 |
SE |
5 |
47,4 |
23 |
11 |
86 |
|
6 |
100 |
SE |
15 |
47,4 |
40 |
19 |
54 |
|
7 |
120 |
SE |
5 |
42,8 |
53 |
30 |
55 |
|
8 |
120 |
SE |
10 |
42,8 |
43 |
26 |
74 |
|
9 |
120 |
SE |
15 |
20,9 |
41 |
29 |
53 |
|
10 |
120 |
KP |
5 |
26,2 |
58 |
25 |
70 |
|
11 |
120 |
KP |
10 |
23,3 |
45 |
17 |
83 |
|
12 |
120 |
KP |
15 |
20,2 |
39 |
27 |
75 |
|
13 |
120 |
GMS |
5 |
51,9 |
39 |
34 |
55 |
|
14 |
120 |
GMS |
10 |
23,3 |
33 |
11 |
81 |
|
15 |
120 |
GMS |
15 |
22,0 |
35 |
28 |
66 |
|
16 |
140 |
SE |
5 |
2,7 |
14 |
14 |
68 |
|
17 |
140 |
SE |
15 |
3,3 |
40 |
28 |
71 |
*Without catalyst
The selectivity to the mono substitution is particularly high comparing with previous reports that indicate a maximum monoester content of 40%wt (James & Smith, 2012). Monoester fraction reported here refers to the mixture of sucrose monoester isomers that can be obtained. According to the literature (Otomo, 2009), monoesters will be mainly a mixture of 6-O-Palmitoylsucrose (more than 70%), 6´-O-Palmitoylsucrose and 1´-O-Palmitoylsucrose.
The content of sucroester on the final mixture was between 11 and 34%wt, and they are similar to those obtained when transesterification is carried out with solvents as reacting media. According to a recent report (Deshpande et al., 2013), reaction carried out at 120°C using dimethyl formamide (DMF) as solvent, showed an 85% of conversion in two hours, with a low content of sucroester in the reactor (ca.26%wt), since nearly 65%wt of the mixture was solvent. This indicates that the solvent-free process can exhibit good productivity while reducing the required separation operations. All the reaction profiles (Figures 4 and 5) were obtained from heterogeneous reacting media. Consequently, the concentration profiles have sigmoid shapes showing an induction time (lag period) that varied with reaction temperature and emulsifier’s concentration. This indicates that both operation parameters, temperature and emulsifier’s concentration, influence reaction rate. Obtained profiles were used to calculate time needed to reach 20% of conversion of methyl palmitate under different reaction conditions (a surrogate indicator of reaction rate). This value of conversion corresponds to the half of the maximal conversion reported for this kind of solvent-free reaction systems. These results are shown in Figures 6 and 8, and allow comparing transesterification rate at intermediate conversion. In addition, using results from Table 1, productivity of sucroester was calculated as mass of sucroester produced per hour and kg of reactive mixture (Figures 7, 9 and 10). Obtained productivities ranged between 2,3 and 84,4 g SE/h kg. The maximum productivity obtained is similar to that obtained when using solvents as contacting agents (~100 g SE/h kg).
Influence of emulsifier’s concentration
Kinetic profiles of methyl palmitate and sucroesters show that transesterification reaction rate increases with the content of emulsifier. As shown in Figure 8, reaction time to achieve 20% of methyl palmitate conversion is reduced when using higher amount of emulsifier. In the same way, productivity of transesterification is improved when using 15%wt of emulsifier (Figure 9). However, these relations are not linear, and it appears to be a minimum amount of emulsifier needed to speed up reactions and to increase productivity.
On the other hand, the content of monoester in the final product is the highest for all experiments when using 10%wt emulsifying agent. At the same time, the total content of sucroester in final mixture is the lowest at this condition (lower productivity). Generally, lower monoester content was obtained when less methyl palmitate conversion was achieved. This is expected since first reaction occurring is the production of sucrose monoester, which acts as emulsifier and also reacts forming sucrose diester and other polyesters, so that concentration of monoester should be higher in the first stage of the transesterification.


Figure 4. Kinetic profiles in sucrose palmitate production by transesterification of methyl palmitate and sucrose at 120°C using (up) potassium palmitate or (down) glycerol monoestearate as emulsifiers, at different concentrations (15 wt%,
10 wt % and
5 wt%).
Source: Authors

Figure 5. Kinetic profiles during sucrose palmitate production by transesterification of methyl palmitate and sucrose using sucrose palmitate as emulsifier at (a) 140°C, (b) 120°C and (c) 100°C, and at different concentrations (15 wt%,
10 wt % and
5 wt%).
Source: Authors
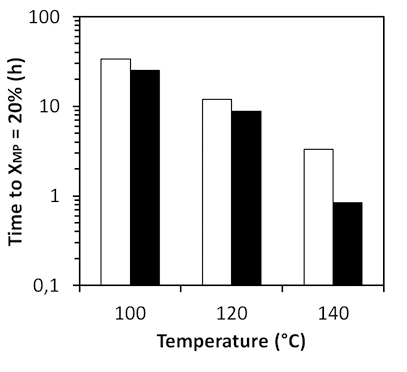
Figure 6. Time of reaction required to reach 20% conversion of methyl palmitate in sucroester production at 100, 120 and 140°C using as emulsifier sucroester at 5%wt and
15%wt.

Source: Authors
Figure 7. Productivity of solvent-free sucroester production at 100, 120 and 140°C using as emulsifier sucroester at 5%wt and
15%wt.

Source: Authors
Figure 8. Time of reaction required to reach 20% conversion of methyl palmitate in sucroester production at 120°C using as emulsifier potassium palmitate (KP), sucroester (SE) or glycerol monostearate (GMS) 5%wt,
10%wt and
15%wt.
Source: Authors
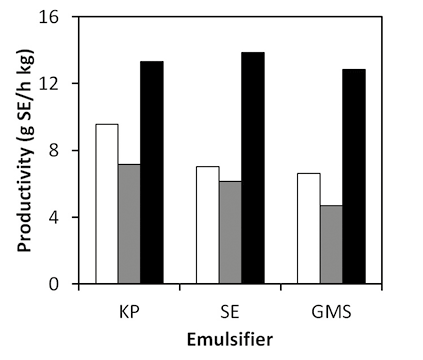
Figure 9. Productivity of solvent-free sucroester production at 120°C using as emulsifier potassium palmitate (KP), sucroester (SE) or glycerol monos-tearate (GMS) 5%wt,
10%wt and
15%wt.
Source: Authors
Influence of type of emulsifier
Evaluated contacting agents have different emulsifying properties, so that different effects on the reaction were expected. Potassium palmitate is an anionic surfactant highly soluble in water and tends to form o/w emulsions. Glycerol monostearate is a non-ionic surfactant soluble in oil and tends to form w/o emulsions. Sucroester used is a non-ionic surfactant with approximately 70% of monoester, so that is soluble in water and tends to form o/w emulsions. Since continuous phase of reactive system is non-polar, it could be expected that an oil-soluble emulsifier was more appropriate to induce a stable sucrose-in-FAME suspension. However, Zhao et al. 2014 reported that solubility of sucrose in FAME is higher using sucroesters with high HLB.
From Figure 8, it can be observed that when using sucroester as emulsifier, reaction time to achieve 20% of conversion is the lowest. Regarding reaction productivities (Figure 9), though all contacting agents performed similarly, the use of potassium palmitate provides the highest productivities. The catalyst activity of this emulsifier on the transesterification promotes higher productivities. This is also observed in Figure 10, where performance of reactions without catalyst is shown. Productivities of reactions without catalyst are considerably lower than when using 5%wt of catalyst (around 30 g SE/h kg). However, productivity of reaction at 140°C using potassium palmitate as emulsifier without catalyst is almost the same of reaction at 120°C with 5%wt of catalyst. This evidences the catalytic activity of this emulsifier, which can be considered an advantage over other contacting agents. Moreover, when comparing results of productivities in reactions without catalyst, it can be observed that productivities are higher when high HLB emulsifiers are used. This is in agreement with solubility reports, in which a higher HLB provided higher sucrose solubility in FAME. Results of Figure 10 show that emulsifiers with low HLB, such as glycerol monostearate, perform similarly to reactive systems without any contacting agent. Emulsifiers with high HLB will be more likely to interact with sucrose due to their high hydrophilicity. Consequently, such emulsifiers are able to effectively improve contact and solubility of sucrose, and to provide higher productivity of transesterification reaction.

Figure 10. Productivity of solvent-free sucroester production at 140°C using different emulsifiers and no catalyst.
Source: Authors
On the other hand, a higher degree of substitution was observed in the final product (HLB ca. 10) when sucroester was used as emulsifier. This is caused by the initial sucroester added to the reactive mixture, which are liquid at reaction temperature and easily reacts to form poly-substituted esters. A product with the higher content of monoester (HLB ca. 16) was obtained when potassium palmitate was used as emulsifier. When using glycerol monostearate, a sucroester with high content of monoester (HLB ca. 14) was also obtained. This information can be useful for tuning sucroester esterification degree and for proposing the production of sucroester with different applications in one single process by using a different contact agent.
Conclusions
Solvent-free sucroester production was studied using sucroester, potassium palmitate or glycerol monostearate as emulsifiers to improve reactant’s compatibility. Observed conversions were around 40%, with around 23%wt of sucroester in final reactive mixture and 68%wt of monoester content in sucroester. Reaction times were shorter when temperature was 140°C and emulsifier’s concentration was 15%wt. However, in process design both operation parameters should be optimized, since high temperatures promote sucrose decomposition and higher content of emulsifiers might be not useful. Mono-substitution was the highest at 10% of emulsifiers; however, this amount of emulsifier provided the lower productivities. Higher conversions, as well as higher monoester content (HLB ca. 16), were obtained when potassium palmitate was used as emulsifier. A more substituted sucroester (HLB ca. 10) is obtained when sucroester is used as emulsifier. Results of this study can be used for preliminary process design in a solvent-free production of sucroesters with different emulsifying properties.
Acknowledgements
This work was financially supported by the “División de Investigación de la Universidad Nacional de Colombia, Sede Bogotá, through the program Convocatoria del programa nacional de proyectos para el fortalecimiento de la investigación, la creación y la innovación en posgrados de la Universidad Nacional de Colombia 2013-2015”, under the project “Estudio del sistema de transesterificación entre sacarosa y ésteres metílicos de ácidos grasos para la producción de ésteres de sacarosa como surfactantes bioderivados”, and Colciencias, through the program “Convocatoria 617 para apoyo a proyectos de doctorado en Colombia”.
References
Baker, I. J. a., Matthews, B., Suares, H., Krodkiewska, I., Furlong, D. N., Grieser, F., & Drummond, C. I. (2000). Sugar fatty acid ester surfactants: Structure and ultimate aerobic biodegradability. Journal of Surfactants and Detergents, 3(1), 1–11. http://doi.org/10.1007/s11743-000-0107-2
Desai, N., & Grüning, B. (1999). Process for the preparation of sucrose fatty acid esters. Germany.
Deshpande, P. S., Deshpande, T. D., Kulkarni, R. D., & Mahulikar, P. P. (2013). Synthesis of Sucrose–Coconut Fatty Acids Esters: Reaction Kinetics and Rheological Analysis. Industrial & Engineering Chemistry Research, 52(43), 15024–15033. http://doi.org/10.1021/ie401524g
Farone, W. A., Serfass, R., & Robert, S. (1996). Sugar-ester manufacturing process. United States.
Ferrer, M., Soliveri, J., Plou, F. J., Nieves, L., Reyes-duarte, D., & Christensen, M. (2005). Synthesis of sugar esters in solvent mixtures by lipases from Thermomyces lanuginosus and Candida antarctica B , and their antimicrobial properties. Enzyme and Microbial Technology, 36, 391–398. http://doi.org/10.1016/j.enzmictec.2004.02.009
Feuge, R. O., Zeringue, H. J., & Weiss, T. J. (1973). Process for the production of sucrose esters of fatty acids. United States.
Fitremann, J., Bouchu, A., & Queneau, Y. (2003). Synthesis and Gelling Properties of N-Palmitoyl-L-Phenylalanine Sucrose Esters. Langmuir, 19, 9981–9983.
Fitremann, J., Queneau, Y., Maitre, J., & Bouchu, A. (2007). Co-melting of solid sucrose and multivalent cation soaps for solvent-free synthesis of sucrose esters. Tetrahedron Letters, 48, 4111–4114. http://doi.org/10.1016/j.tetlet.2007.04.015
Galleymore, H. R., James, K., Jones, H. F., & Bhardwaj, C. L. (1981). Process for the production of a surfactant containing sucrose esters. United States.
Gerpen, J. Van. (2005). Biodiesel processing and production. Fuel Processing Technology, 86, 1097–1107. http://doi.org/10.1016/j.fuproc.2004.11.005
Hass, H. B., Summit, N. J., Snell, F. D., York, W. C., & Osipow, L. I. (1959). Process for producing Sugar Esters. United States.
James, K., & Smith, J. F. (2009). Process for the production of esters of sugar and sugar derivatives. United States.
James, K., & Smith, J. F. (2012). Process for the production of esters of sugars and sugar derivatives. United States.
Le-Coent, A. L., Tayakout-fayolle, M., Couenne, F., Briancon, S., Lieto, J., Fitremann-Gagnaire, J., … Bouchu, A. (2003). Kinetic parameter estimation and modelling of sucrose esters synthesis without solvent. Chemical Engineering Science, 58, 367–376.
Lie, A., & Pedersen, L. H. (2013). Elution strategies for reversed-phase high-performance liquid chromatography analysis of sucrose alkanoate regioisomers with charged aerosol detection. Journal of Chromatography. A, 1311, 127–33. http://doi.org/10.1016/j.chroma.2013.08.081
Meher, L. C., Sagar, D. V., & Naik, S. N. (2006). Technical aspects of biodiesel production by transesterification — a review. Renewable and Sustainable Energy Reviews, 10, 248–268. http://doi.org/10.1016/j.rser.2004.09.002
Nakamura, K. S., Nagahara, S. H., & Kawaguchi, J. O. (1995). Process for production of high-monoester sucrose higher fatty acid esters. United States.
Narváez, P. C., Rincón, S. M., & Sánchez, F. J. (2007). Kinetics of palm oil methanolysis. Journal of the American Oil Chemists’ Society, 84(10), 971–977. http://doi.org/10.1007/s11746-007-1120-y
Nelen, B. A. P., & Cooper, J. M. (2004). Sucrose esters. In R. J. Whitehurst (Ed.), Emulsifiers in Food Technology (pp. 131– 162). Backwell Publishing Ltd.
sipow, L. I., Rosenblatt, W., & Valley, S. (1969). Esterification of polyhydric compounds in the presence of transparent emulsifying agent. United States.
Otomo, N. (2009). Basic properties of sucrose fatty acid esters and their applications. In D. G. Hayes, D. Kitamoto, D. K. Y. Solaiman, & R. D. Ashby (Eds.), Biobased Surfactants and Detergents. Urbana, Illinois: AOCS Press.
Parker, K. J., James, K., & Hurford, J. (1977). Sucrose Ester Surfactants — A Solventless Process and the Products Thereof. In J. L. Hickson (Ed.), Sucrochemistry (pp. 97– 114). Washington, DC: American Chemical Society.
Ritthitham, S., Wimmer, R., Stensballe, A., & Pedersen, L. H. (2009). Analysis and purification of O-decanoyl sucrose regio-isomers by reversed phase high pressure liquid chromatography with evaporative light scattering detection. Journal of Chromatography. A, 1216(25), 4963– 7. http://doi.org/10.1016/j.chroma.2009.04.054
hi, Y., Li, J., & Chu, Y.-H. (2011). Enzyme-catalyzed regioselective synthesis of sucrose-based esters. Journal of Chemical Technology & Biotechnology, 86(12), 1457– 1468. http://doi.org/10.1002/jctb.2711
Wang, Q., Zhang, S., Zhang, P., Zhu, J., & Yang, J. (2006). Separation and Quantitation of Sucrose Esters Using HPLC with Evaporative Light Scattering Detection. Journal of Liquid Chromatography & Related Technologies, 29(16), 2399–2412. http://doi.org/10.1080/10826070600864874
Zhao, R., Chang, Z., Jin, Q., Li, W., Dong, B., & Miao, X. (2014). Heterogeneous base catalytic transesterification synthesis of sucrose ester and parallel reaction control. International Journal of Food Science & Technology, 49(3), 854–860. http://doi.org/10.1111/ijfs.12376

Attribution 4.0 International (CC BY 4.0) Share - Adapt



