Progressive multifocal leukoencephalopathy caused by the John Cunningham virus: a case report
Leucoencefalopatía multifocal progresiva causada por el virus John Cunningham. Reporte de un caso
DOI:
https://doi.org/10.15446/revfacmed.v72n1.107913Palabras clave:
Leukoencephalopathy, Progressive Multifocal, JC Virus, Opportunistic Infections (en)Leucoencefalopatía multifocal progresiva, Virus JC, Infecciones oportunistas (es)
Descargas
Introduction: Progressive multifocal leukoencephalopathy (PML) is a demyelinating, multifactorial disease that can be caused by the John Cunningham virus (JCV) and occurs mainly in people with HIV/AIDS.
Case presentation: A 28-year-old man with HIV (without antiretroviral treatment [ART]) and a history of disseminated Kaposi's sarcoma visited the emergency room of a tertiary care hospital in Popayán (Cauca) in May 2021 due to headache, nausea, emesis, asthenia, adynamia, and occasional insomnia. During his first hospital stay, based on imaging findings and the results of a cerebrospinal fluid (CSF) culture, he was diagnosed with meningeal cryptococcosis. After 28 days, he was discharged since he showed a favorable response to maintenance treatment (oral fluconazole) and was indicated to start ART. However, two months later, during a new hospitalization, and considering his clinical manifestations, imaging findings, and CSF culture results, PML was suspected, so a PCR test for the detection of JCV in CSF was requested, confirming the diagnosis. In the following months, the patient experienced progressive physical and cognitive deterioration and finally died in August 2022.
Conclusions: The case presented here is of great relevance for the evaluation of patients with an HIV diagnosis and significant involvement of their immune system, as it broadens the spectrum of opportunistic infections that should be considered. It also provides the necessary tools to elucidate the clinical manifestations and the diagnostic approach to PML in the presence of HIV.
Introducción. La leucoencefalopatía multifocal progresiva (LMP) es una enfermedad desmielinizante y multifactorial que es causada por el virus John Cunningham (VJC) y que ocurre principalmente en personas con VIH/sida.
Presentación del caso. Hombre de 28 años con VIH (sin tratamiento antirretroviral [TAR]) y antecedente de sarcoma de Kaposi diseminado quien en mayo de 2021 asistió al servicio de urgencias de un hospital de tercer nivel de atención de Popayán (Colombia) por cefalea, náuseas, emesis, astenia, adinamia e insomnio ocasional. Durante la primera hospitalización, y con base en los hallazgos imagenológicos y del cultivo del líquido cefalorraquídeo (LCR), fue diagnosticado con criptococosis meníngea, siendo dado de alta a los 28 días por respuesta favorable al tratamiento de mantenimiento (fluconazol vía oral), con indicación de iniciar TAR. Dos meses después, durante una nueva hospitalización y con base en las manifestaciones clínicas y los hallazgos imagenológicos y del cultivo de LCR, se sospechó LMP, por lo que se solicitó prueba de PCR para VJC en LCR, con lo cual se confirmó el diagnóstico. En los siguientes meses el paciente presentó un progresivo deterioro físico y cognitivo y finalmente falleció en agosto de 2022.
Conclusiones. El caso aquí presentado es de gran pertinencia para la evaluación de pacientes con diagnóstico de VIH y compromiso significativo de su sistema inmune, pues amplía el espectro de infecciones oportunistas que se deben tener en cuenta en estos pacientes; además, brinda las herramientas necesarias para esclarecer las manifestaciones clínicas y el abordaje diagnóstico de la LMP en presencia de VIH.
Progressive multifocal leukoencephalopathy caused by the John Cunningham virus: a case report
Leucoencefalopatía multifocal progresiva causada por el virus John Cunningham. Reporte de un caso
Dario Fernando Muñoz-Mora1 Víctor Hugo Lozano-Fernández2
Víctor Hugo Lozano-Fernández2 Angie Katherine Uribe-Caldón2
Angie Katherine Uribe-Caldón2
1 Universidad Nacional de Colombia - Bogotá Campus - Faculty of Medicine - Department of Internal Medicine - Bogotá D.C. - Colombia.
2 Universidad del Cauca - Faculty of Health Sciences - Department of Internal Medicine - Popayán - Colombia.
Open access
Received: 21/03/2023
Accepted: 15/09/2023
Corresponding author: Dario Fernando Muñoz-Mora. Departamento de Medicina Interna, Facultad de Medicina, Universidad Nacional de Colombia. Bogotá D.C. Colombia. Email: darmunozmo@unal.edu.co.
Keywords: Leukoencephalopathy, Progressive Multifocal; JC Virus; Opportunistic Infections (MeSH).
Palabras clave: Leucoencefalopatía multifocal progresiva; Virus JC; Infecciones oportunistas (DeCS).
How to cite: Muñoz-Mora DF, Lozano Fernández VH, Uribe-Caldón AK. Progressive multifocal leukoencephalopathy caused by the John Cunningham virus: a case report. Rev. Fac. Med. 2024;72(1):e107913. English. doi: https://doi.org/10.15446/revfacmed.v72n1.107913.
Cómo citar: Muñoz-Mora DF, Lozano Fernández VH, Uribe-Caldón AK. [Leucoencefalopatía multifocal progresiva causada por el virus John Cunningham. Reporte de un caso]. Rev. Fac. Med. 2024;72(1):e107913. English. doi: https://doi.org/10.15446/revfacmed.v72n1.107913.
Copyright: Copyright: ©2024 Universidad Nacional de Colombia. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, as long as the original author and source are credited.
Abstract
Introduction: Progressive multifocal leukoencephalopathy (PML) is a demyelinating, multifactorial disease that can be caused by the John Cunningham virus (JCV) and occurs mainly in people with HIV/AIDS.
Case presentation: A 28-year-old man with HIV (without antiretroviral treatment [ART]) and a history of disseminated Kaposi's sarcoma visited the emergency room of a tertiary care hospital in Popayán (Cauca) in May 2021 due to headache, nausea, emesis, asthenia, adynamia, and occasional insomnia. During his first hospital stay, based on imaging findings and the results of a cerebrospinal fluid (CSF) culture, he was diagnosed with meningeal cryptococcosis. After 28 days, he was discharged since he showed a favorable response to maintenance treatment (oral fluconazole) and was indicated to start ART. However, two months later, during a new hospitalization, and considering his clinical manifestations, imaging findings, and CSF culture results, PML was suspected, so a PCR test for the detection of JCV in CSF was requested, confirming the diagnosis. In the following months, the patient experienced progressive physical and cognitive deterioration and finally died in August 2022.
Conclusions: The case presented here is of great relevance for the evaluation of patients with an HIV diagnosis and significant involvement of their immune system, as it broadens the spectrum of opportunistic infections that should be considered. It also provides the necessary tools to elucidate the clinical manifestations and the diagnostic approach to PML in the presence of HIV.
Resumen
Introducción. La leucoencefalopatía multifocal progresiva (LMP) es una enfermedad desmielinizante y multifactorial que es causada por el virus John Cunningham (VJC) y que ocurre principalmente en personas con VIH/sida.
Presentación del caso. Hombre de 28 años con VIH (sin tratamiento antirretroviral [TAR]) y antecedente de sarcoma de Kaposi diseminado quien en mayo de 2021 asistió al servicio de urgencias de un hospital de tercer nivel de atención de Popayán (Colombia) por cefalea, náuseas, emesis, astenia, adinamia e insomnio ocasional. Durante la primera hospitalización, y con base en los hallazgos imagenológicos y del cultivo del líquido cefalorraquídeo (LCR), fue diagnosticado con criptococosis meníngea, siendo dado de alta a los 28 días por respuesta favorable al tratamiento de mantenimiento (fluconazol vía oral), con indicación de iniciar TAR. Dos meses después, durante una nueva hospitalización y con base en las manifestaciones clínicas y los hallazgos imagenológicos y del cultivo de LCR, se sospechó LMP, por lo que se solicitó prueba de PCR para VJC en LCR, con lo cual se confirmó el diagnóstico. En los siguientes meses el paciente presentó un progresivo deterioro físico y cognitivo y finalmente falleció en agosto de 2022.
Conclusiones. El caso aquí presentado es de gran pertinencia para la evaluación de pacientes con diagnóstico de VIH y compromiso significativo de su sistema inmune, pues amplía el espectro de infecciones oportunistas que se deben tener en cuenta en estos pacientes; además, brinda las herramientas necesarias para esclarecer las manifestaciones clínicas y el abordaje diagnóstico de la LMP en presencia de VIH.
Case report
Introduction
Progressive multifocal leukoencephalopathy (PML) is a demyelinating, often lethal disease caused by lytic infection of glial cells in patients with severe immunosuppression in whom demyelination extensively and asymmetrically affects the encephalic white matter.1,2 In 1958, the disease was first described as a disease of unknown viral etiology,3 and it was only in 1971 that the John Cunningham virus (JCV) was identified as the cause.4
PML occurs in immunocompromised individuals, especially in patients with human immunodeficiency virus (HIV) (75-80%).2,5 However, cases have also been reported in patients with hematologic cancer (10%);5 primary immunodeficiencies (severe combined immunodeficiency, idiopathic CD4+ cell lymphopenia, CD8+ cell lymphopenia); immunosuppression caused by cancer treatments and chronic systemic inflammatory diseases (e.g. natalizumab use for multiple sclerosis); organ transplant recipients; and individuals with humoral immunodeficiency such as pregnant women, the elderly, and individuals with cirrhosis and kidney failure.2,5
In the case of AIDS patients, before the discovery of highly active antiretroviral therapy (HAART), Lang et al.,6 in a study using autopsy data from 135 AIDS patients who died in Switzerland between 1981 and 1987, reported in 1989 that the prevalence of PML was 7%. Similarly, Martínez-Martin & Diéz-Tejedor,7 in a study conducted between 1984 and 1988 in 10 neurology departments of Spanish hospitals, found a mortality rate of 8% for PML in these patients.
Primary infection with JCV usually occurs in childhood and remains latent in different tissues.8 Moreover, the reported seroprevalence of anti-JCV antibodies varies between 39% and 91%, depending on the population studied.8,9
This article describes the case of a patient with HIV who was diagnosed with PML based on clinical and imaging findings and cerebrospinal fluid (CSF) polymerase chain reaction (PCR) test results.
Case presentation
A 28-year-old man with a history of disseminated Kaposi’s sarcoma and HIV infection since 2017, who was not receiving ART of his own volition, visited the emergency room of a tertiary care hospital in Popayán (Colombia) in May 2021 due to the presentation of holocranial headache (predominant symptom), nausea, emesis, asthenia, adynamia, decreased appetite, and occasional insomnia during the last 3 months. Physical examination did not reveal any abnormal findings or neurological involvement. On the same day of admission, a complete blood count showed leukopenia (1 200/mm3), neutropenia (506/mm3), lymphopenia (436/mm3), and moderate microcytic hypochromic anemia (9.2g/dL), while a simple cranial computed tomography (CT) scan ruled out hemorrhagic lesions, space-occupying lesions, or alterations of the ventricular system.
Due to suspicion of neuroinfection (patient with HIV and headache), a lumbar puncture (LP) was performed on the second day of stay in the emergency room, and the following findings were reported: high CSF protein concentrations, hypoglycorrhachia, presence of yeast encapsulated in India ink, positive Cryptococcus spp. antigen in CSF, and Cryptococcus spp. isolate in culture. In addition, an HIV follow-up test performed on the same day reported CD4 T lymphocytes at 45 cells/mm3 (7.1%) and a viral load of 321 000 copies of HIV RNA per milliliter.
In view of these results, after three days of stay in the emergency room, a diagnosis of meningeal cryptococcosis was made, so a transfer to the hospital was ordered and induction treatment with amphotericin B deoxycholate (45mg intravenously every 24 hours) and flucytosine (750mg intravenously every 6 hours) was started. After 14 days of treatment, these drugs were suspended and treatment with fluconazole (400mg orally every 24 hours) was started to initiate the consolidation phase, achieving a satisfactory response consisting of a decrease in the initial symptoms and a negative result for Cryptococcus spp. in the follow-up CSF culture, performed 15 days after starting the induction treatment.
After 28 days of hospitalization and taking into account the favorable response to fluconazole consolidation phase therapy, the patient was discharged with indication to start ART with abacavir/lamivudine/dolutegravir (ABC/3TC/DTG) 6 to 8 weeks after discharge.
One month after discharge, the patient returned to the emergency room due to tinnitus, emesis, and subjective vertigo. During this visit, a cranial CT scan was performed and no abnormalities were observed, so outpatient symptomatic management with acetaminophen and ibuprofen was indicated. Thirty days later, the patient visited the emergency room again with a severe headache. This time, the physical examination showed no meningeal signs, but the cranial CT scan showed hydrocephalus and involutional brain changes that were not typical of the patient’s age. Therefore, he was hospitalized and that same day he underwent therapeutic LP to reduce intracranial pressure, which resulted in clinical improvement and CSF collection for cultures, which were negative.
On the second day of this new hospitalization, the patient presented a generalized tonic-clonic seizure, so a cranial CT scan was performed, which showed hypodensities in the white matter of the right precentral gyrus with cortical enhancement, probably associated with a local inflammatory process. For this reason, magnetic resonance imaging (MRI) of the brain with contrast was performed to further investigate these findings, which were confirmed. At the same time, studies were carried out in search of other viral tropisms for the central nervous system (CNS), including immunoglobulin G and immunoglobulin M tests for cytomegalovirus, toxoplasma blood test, and non-treponemal test for syphilis, all with negative results.
Four days into this last hospitalization, when the patient had already been on ART with ABC/3TC/DTG for 8 weeks, HIV monitoring studies were performed, finding CD4 T lymphocytes at 140 cells/mm3 and viral load of 1 151 copies of HIV RNA per milliliter. On the following day, the patient presented with episodes of localized myoclonias in the left upper limb and intensification of headache with nuchal rigidity. That same day, levetiracetam (500mg orally every 12 hours) was started to treat the abnormal movements, and due to signs of increased intracranial pressure, a new therapeutic LP was performed for CSF drainage, as well as another MRI. On MRI, there were signs of PML that were best seen on the T2 sequence (Figure 1) and on the fluid-attenuated inversion recovery (T2/FLAIR) sequence (Figure 2). In view of this finding, a PCR test for JCV in CSF was requested. In the following two weeks, the patient’s clinical condition worsened due to left hemiparesis, ipsilateral myoclonias, and persistent headache, so multiple therapeutic LP’s were performed to reduce intracranial pressure.
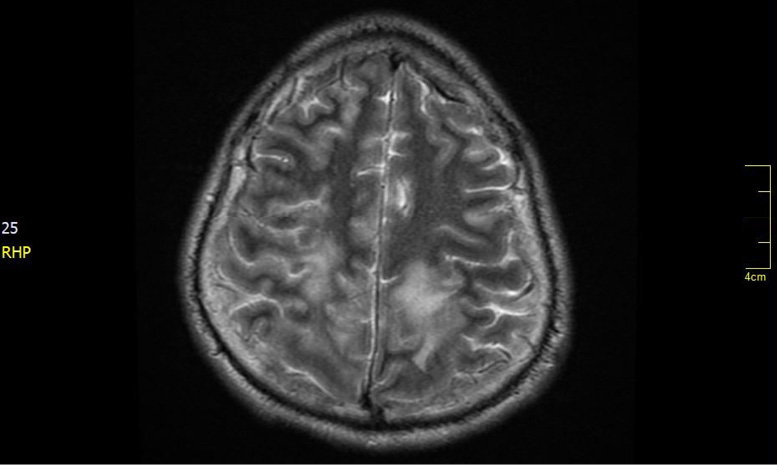
Figure 1. T2-weighted magnetic resonance imaging. Bilateral parenchymal hyperintensities (greater right involvement) are observed in the subcortical white matter in the parietal regions.
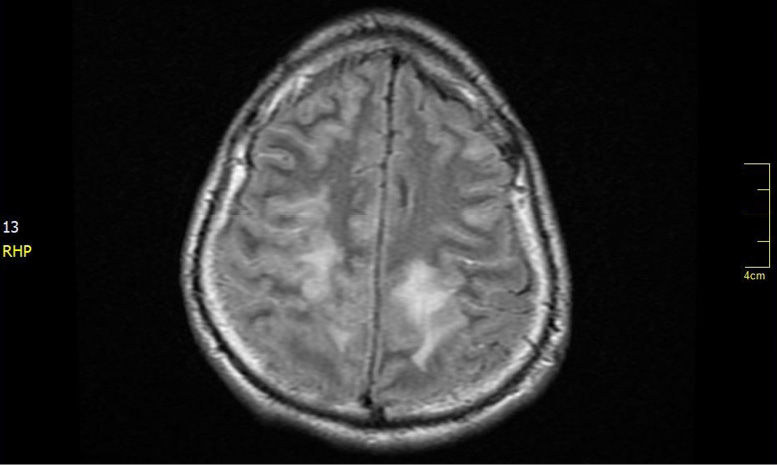
Figure 2. T2-weighted-fluid-attenuated inversion recovery nuclear magnetic resonance. Bilateral parenchymal hyperintensities (greater right involvement) are observed in the subcortical white matter in the parietal regions.
In the course of admission and discharge from this last hospitalization, the progression of physical limitation and cognitive impairment became noticeable, to the point that the patient became totally dependent on a caregiver. Furthermore, according to the report of the PCR test for JCV in CSF, which was available one month after its performance, the patient was positive for JCV (viral load DNA <10 copies/mL). This was interpreted as a possible reactivation of the virus due to the patient’s immunodeficiency status, thus confirming the diagnosis of PML due to JCV. Accordingly, the use of maraviroc was requested as the only alternative treatment. However, his health care provider never authorized it and the patient died in August 2022.
Discussion
PML is a disease secondary to JCV reactivation. This virus infects oligodendrocytes and astrocytes, causing a lytic infection that results in the destruction of the myelin sheath and demyelination of the affected areas of the white matter; however, this pathophysiological process can also occur within the cortical gray matter.10 JCV is a human double-stranded DNA virus of the genus Polyomavirus that can cause other diseases such as immune reconstitution inflammatory syndrome (IRIS) associated with PML, granule cell neuropathy, nephropathy, encephalopathy, meningitis, and encephalitis.7,10,11
According to White & Khalili,12 some studies have detected JCV DNA in tissue from the tonsils and oropharynx region, suggesting a possible oral or respiratory route of infection, but genomic sequences of this virus have also been detected in epithelial cells of the gastrointestinal tract and esophagus, suggesting that the gastrointestinal tract may also be a gateway for the virus. Other possible modes of transmission include urino-oral, transplacental, and blood transfusion transmission; contact with semen; and organ transplantation.2,13,14 Virions presumably move by the hematogenous route from the primary site of infection to secondary sites such as the kidney, bone marrow-derived cells (including B lymphocytes and CD4+ cells), and brain, where focal areas of infection or latency are established.13,14
JCV infection occurs through the binding of viral particles to sialic acid residues and the serotonin 5HT2a receptor of the host cell. Following this binding, the virus uses a clathrin-dependent endocytosis mechanism to be transported to the cytoplasm and subsequently travel to the endoplasmic reticulum. The VP1 protein facilitates its entry into the host cell nucleus, where viral assembly occurs. The virus utilizes the host cell’s replicative machinery to finally release its viral products after cell lysis.5,14
At first, PML is asymptomatic, then, depending on the affected area, its clinical manifestations are heterogeneous, but the main ones include progressive cortical symptoms. However, patients frequently present with hemiparesis, ataxia, gait disturbance, limb weakness, hemianopsia, coordination difficulties, aphasia, and cognitive changes. Furthermore, demyelination and inflammation of the cerebral cortex may facilitate epileptogenesis, resulting in a high prevalence of seizures.13,15 In the case reported here, the patient presented left hemiparesis, left upper limb myoclonias, and progressive cognitive impairment. In addition, cell counts on CSF analysis are normal in up to 70% of patients with PML,16 while 55% have high CSF protein concentrations.13 These findings suggest that a normal CSF cell count and protein profile are fully compatible with active PML.
Detection of JCV DNA in CSF using PCR has become the cornerstone of PML diagnosis, with a specificity of 92-100% and a sensitivity of 72-92% in HIV patients not receiving antiretroviral drug treatment.13 However, according to Grebenciucova & Berger,16 the sensitivity of newer PCR methods is at least 95%. In the present case, given the symptomatology, imaging findings and CSF culture results (high CSF protein concentrations), PML was suspected and a PCR test for JCV in CSF was requested, confirming the diagnosis.
MRI is the imaging test of choice to detect brain changes associated with PML. Typical imaging features are located in the frontal and parieto-occipital regions, are usually subcortical or juxtacortical, and involve U-fibers; cortical involvement is very rare.13,17 T2 sequences, in particular T2/FLAIR, where hypertensive multifocal lesions should be checked for, are very sensitive for lesion detection and are the most important for confirming the diagnosis of PML. T1 sequences show isointense (early stage) and hypointense (advanced stages) lesions that demonstrate the degree of tissue destruction as a function of signal intensity and allow the identification of lamellar necrosis.18
Likewise, MR spectroscopy in these patients shows a profile with decreased N-acetyl-aspartate peak suggesting axonal damage, increased choline peaks suggesting cell membrane and myelin breakdown, and lactate peaks.2,18 In the present case, T2 and T2/FLAIR sequences showed bilateral parenchymal hyperintensities (greater right involvement) in the subcortical white matter in the parietal regions.
It should be noted that even though the diagnosis of classic PML was confirmed in the present case, the patient may also meet criteria for IRIS associated with PML given the lack of initial clinical and imaging data and the fact that findings compatible with PML were observed after the initiation of combination ART, when a decrease in HIV viral load and a considerable increase in CD4 T lymphocytes in blood had been achieved.5 Of note, in HIV patients, the two main forms of PML presentation have been described as classic and IRIS-associated variant, the latter as an exuberant inflammatory response to a dead, dying, or viable pathogen from a persistent infection.19
It is worth pointing out that there are several diagnostic criteria for PML that include clinical features, radiological findings, and positive PCR result for JCV in CSF (Table 1). However, the diagnosis can also be made based on histopathological criteria, which are very relevant when there is clinical-radiological suspicion. When PCR for JCV is negative for a possible false negative, definitive diagnosis is based on the presence of the typical histopathological triad (demyelination, bizarre astrocytes, and enlarged oligodendroglial nuclei on brain biopsy), coupled with the techniques to show the presence of JCV by electron microscopy/immunohistochemistry or PCR positivity.19-21
Serological screening alone does not identify all individuals infected with JCV, given the presence of seronegative subjects with PCR detectable JCV-DNA in blood or urine. However, new tools such as microRNA quantification in blood, urine, and CSF can identify these patients,19 although further studies are still needed to determine their potential diagnostic yield. In our case, the findings (clinical, imaging, and PCR test for JCV) found in the patient matched the criteria compatible with a definitive diagnosis of PML.
Table 1. Clinical and radiological criteria, and PCR test result.
|
Certainty of PML diagnosis |
Compatible clinical symptoms |
Compatible imaging |
PCR test for John Cunningham virus in cerebrospinal fluid |
|
Definitive |
+ |
+ |
+ |
|
Probable |
+ |
- |
+ |
|
- |
+ |
+ |
|
|
Possible |
+ |
+ |
-/NR |
|
- |
- |
+ |
|
|
No PML |
- |
- |
- |
|
+ |
- |
- |
|
|
- |
+ |
- |
PML: progressive multifocal leukoencephalopathy.; PCR: polymerase chain reaction; NR: not referenced.
Regarding the treatment for PML in HIV patients, it has been established that ART should be optimized to increase 1-year survival. Moreover, the following factors have been associated with increased mortality in these patients: CD4+ cell count <100 cells/uL at the start of highly active ART, presence of hematologic cancer, having received organ transplants, having idiopathic immune deficits, and not readily achieving immune reconstitution.22
Similarly, several treatment strategies have been proposed to inhibit viral replication with nucleoside analogues such as cytarabine and cidofovir and the topoisomerase inhibitor topotecan, as well as treatments that block viral entry into glial cells such as mirtazapine (5-HT2A receptor antagonist) and others that block viral infection and replication in vitro such as mefloquine. However, none of these strategies has demonstrated convincingly to prolong survival or decrease neurological disability.5 On the other hand, innovative therapies have been proposed, including immune checkpoint inhibitors (pembrolizumab and nivolumab) and allogeneic transplantation of polyomavirus-specific T cells.22,23
Regarding the management of IRIS associated with PML, drugs such as CCR5 antagonists (e.g. maraviroc) have been used to selectively limit immune cell trafficking in the CNS, but the results have not demonstrated clear benefits.5 In our patient, despite initiation of ART, the symptomatic progression of PML continued with its natural course until he died. It should be noted that although maraviroc was requested as the only therapeutic alternative at that time, its delivery was not possible due to administrative problems in obtaining the drug.
Conclusion
CJV infection and the development of PML is a possibility in patients with HIV and a serious compromise of their immune system. The PML diagnosis is made mainly based on clinical and imaging criteria and the result of a CSF PCR test, and its treatment is based on the optimization of ART because treatments targeted against the virus have not yielded favorable results in terms of survival and improvement of neurological disability. In addition, there is insufficient evidence on new therapeutic targets such as immune checkpoint inhibitors and allogeneic transplantation of specific T cells, although their future results are promising.
The case reported here is of great relevance for the evaluation of patients with a diagnosis of HIV and significant compromise of their immune system, as it broadens the spectrum of opportunistic infections that should be considered in these patients. It also provides the necessary tools to clarify the clinical manifestations and diagnostic approach to PML in the presence of HIV.
Ethical considerations
For the preparation and publication of this case report, written informed consent was obtained from the patient.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
None stated by the authors.
References
1.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425-37. https://doi.org/d2kfc5.
2.Morcillo-Muñoz AF, Acosta-Fajardo HA, Ariza-Varón MA, Ramos-Romero M. Leucoencefalopatía multifocal progresiva. Acta Neurol Colomb. 2021;37(1 Suppl 1):47-54. https://doi.org/mkzw.
3.Åström KE, Mancall EL, Richardson EP Jr. Progressive multifocal leuko-encephalopathy: A hitherto unrecognized complication o chronic lymphatic leukæmia and Hodgkin’s disease. Brain. 1958;81(1):93-111. https://doi.org/fgqqph.
4.Padgett BL, Walker DL, Zurhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;297(7712):1257-60. https://doi.org/d65r6v.
5.Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2021;17(1):37-51. https://doi.org/ghs4gt.
6.Lang W, Miklossy J, Deruaz JP, Pizzolato GP, Probst A, Schaffner T, et al. Neuropathology of the acquired immune deficiency syndrome (AIDS): a report of 135 consecutive autopsy cases from Switzerland. Acta Neuropathol. 1989;77(4):379-90. https://doi.org/b8g8q7.
7.Martínez-Martín P, Díez-Tejedor E. Complicaciones neurológicas del SIDA. Panorámica según una encuesta hospitalaria multicéntrica. Arch Neurobiol (Madr). 1989;52(Suppl 1):23-9.
8.Camporro J, Bruno V, Cammarota A, del Castillo M, Alessandro L. Espectro clínico de la leucoencefalopatía multifocal progresiva: diferencias y similitudes en pacientes con y sin virus de la inmunodeficiencia humana. Rev Neurol. 2019;69(4):152-8. https://doi.org/mkz9.
9.Bozic C, Subramanyam M, Richman S, Plavina T, Zhang A, Ticho B. Anti-JC virus (JCV) antibody prevalence in the JCV Epidemiology in MS (JEMS) trial. Eur J Neurol. 2014;21(2):299-304. https://doi.org/f5nzbw.
10.Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and bennett’s principles and practice of infectious diseases. Volume 2. 9th ed. Philadelphia, PA: Elsevier; 2019.
11.Atkinson AL, Atwood WJ. Fifty years of JC Polyomavirus: A brief overview and remaining questions. Viruses. 2020;12(9):969. https://doi.org/gqwv4s.
12.White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy--revisited. J Infect Dis. 2011;203(5):578-86. https://doi.org/dbgv7g.
13.Zhai S, Brew BJ. Progressive multifocal leukoencephalopathy. Handb Clin Neurol. 2018;152:123-37. https://doi.org/mk2n.
14.Pietropaolo V, Prezioso C, Bagnato F, Antonelli G. John Cunningham virus: an overview on biology and disease of the etiological agent of the progressive multifocal leukoencephalopathy. New Microbiol. 2018;41(1):179-86.
15.Bartsch T, Rempe T, Leypoldt F, Riedel C, Jansen O, Berg D, et al. The spectrum of progressive multifocal leukoencephalopathy: a practical approach. Eur J Neurol. 2019;26(4):566-e41. https://doi.org/mk2p.
16.Grebenciucova E, Berger JR. Progressive multifocal leukoencephalopathy. Neurol Clin. 2018;36(4):739-50. https://doi.org/mk2q.
17.Kartau M, Sipilä JO, Auvinen E, Palomäki M, Verkkoniemi-Ahola A. Progressive multifocal leukoencephalopathy: Current insights. Degener Neurol Neuromuscul Dis. 2019;9:109-21. https://doi.org/mk2r.
18.Baldassari LE, Wattjes MP, Cortese ICM, Gass A, Metz I, Yousry T, et al. The neuroradiology of progressive multifocal leukoencephalopathy: a clinical trial perspective. Brain. 2022;145(2):426-40. https://doi.org/gsdf7r.
19.Iannetta M, Zingaropoli MA, D’Abramo A, Oliva A, Mastroianni CM, Vullo V, et al. HIV-associated progressive multifocal leukoencephalopathy: current perspectives. Neurobehav HIV Med. 2016;7:43-52. https://doi.org/mk2t.
20.Lee SY, Ko HC, Kim SI, Lee YS, Son BC. Progressive multifocal leukoencephalopathy diagnosed by brain biopsy, not by the DNA test for JC Virus. Asian J Neurosurg. 2019;14(1):240-4. https://doi.org/mk2v.
21.Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430-8. https://doi.org/f4w6c7.
22.Bernard-Valnet R, Koralnik IJ, Du Pasquier R. Advances in treatment of progressive multifocal leukoencephalopathy. Ann Neurol. 2021;90(6):865-73. https://doi.org/mk2w.
23.Möhn N, Grote-Levi L, Hopfner F, Eiz-Vesper B, Maecker-Kolhoff B, Warnke C, et al. Innovative therapeutic concepts of progressive multifocal leukoencephalopathy. J Neurol. 2022;269(5):2403-13. https://doi.org/mk2x.
Referencias
Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425-37. https://doi.org/d2kfc5.
Morcillo-Muñoz AF, Acosta-Fajardo HA, Ariza-Varón MA, Ramos-Romero M. Leucoencefalopatía multifocal progresiva. Acta Neurol Colomb. 2021;37(1 Suppl 1):47-54. https://doi.org/mkzw.
Åström KE, Mancall EL, Richardson EP Jr. Progressive multifocal leuko-encephalopathy: A hitherto unrecognized complication o chronic lymphatic leukæmia and Hodgkin’s disease. Brain. 1958;81(1):93-111. https://doi.org/fgqqph.
Padgett BL, Walker DL, Zurhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;297(7712):1257-60. https://doi.org/d65r6v.
Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2021;17(1):37-51. https://doi.org/ghs4gt.
Lang W, Miklossy J, Deruaz JP, Pizzolato GP, Probst A, Schaffner T, et al. Neuropathology of the acquired immune deficiency syndrome (AIDS): a report of 135 consecutive autopsy cases from Switzerland. Acta Neuropathol. 1989;77(4):379-90. https://doi.org/b8g8q7.
Martínez-Martín P, Díez-Tejedor E. Complicaciones neurológicas del SIDA. Panorámica según una encuesta hospitalaria multicéntrica. Arch Neurobiol (Madr). 1989;52(Suppl 1):23-9.
Camporro J, Bruno V, Cammarota A, del Castillo M, Alessandro L. Espectro clínico de la leucoencefalopatía multifocal progresiva: diferencias y similitudes en pacientes con y sin virus de la inmunodeficiencia humana. Rev Neurol. 2019;69(4):152-8. https://doi.org/mkz9.
Bozic C, Subramanyam M, Richman S, Plavina T, Zhang A, Ticho B. Anti-JC virus (JCV) antibody prevalence in the JCV Epidemiology in MS (JEMS) trial. Eur J Neurol. 2014;21(2):299-304. https://doi.org/f5nzbw.
Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and bennett’s principles and practice of infectious diseases. Volume 2. 9th ed. Philadelphia, PA: Elsevier; 2019.
Atkinson AL, Atwood WJ. Fifty years of JC Polyomavirus: A brief overview and remaining questions. Viruses. 2020;12(9):969. https://doi.org/gqwv4s.
White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy--revisited. J Infect Dis. 2011;203(5):578-86. https://doi.org/dbgv7g.
Zhai S, Brew BJ. Progressive multifocal leukoencephalopathy. Handb Clin Neurol. 2018;152:123-37. https://doi.org/mk2n.
Pietropaolo V, Prezioso C, Bagnato F, Antonelli G. John Cunningham virus: an overview on biology and disease of the etiological agent of the progressive multifocal leukoencephalopathy. New Microbiol. 2018;41(1):179-86.
Bartsch T, Rempe T, Leypoldt F, Riedel C, Jansen O, Berg D, et al. The spectrum of progressive multifocal leukoencephalopathy: a practical approach. Eur J Neurol. 2019;26(4):566-e41. https://doi.org/mk2p.
Grebenciucova E, Berger JR. Progressive multifocal leukoencephalopathy. Neurol Clin. 2018;36(4):739-50. https://doi.org/mk2q.
Kartau M, Sipilä JO, Auvinen E, Palomäki M, Verkkoniemi-Ahola A. Progressive multifocal leukoencephalopathy: Current insights. Degener Neurol Neuromuscul Dis. 2019;9:109-21. https://doi.org/mk2r.
Baldassari LE, Wattjes MP, Cortese ICM, Gass A, Metz I, Yousry T, et al. The neuroradiology of progressive multifocal leukoencephalopathy: a clinical trial perspective. Brain. 2022;145(2):426-40. https://doi.org/gsdf7r.
Iannetta M, Zingaropoli MA, D’Abramo A, Oliva A, Mastroianni CM, Vullo V, et al. HIV-associated progressive multifocal leukoencephalopathy: current perspectives. Neurobehav HIV Med. 2016;7:43-52. https://doi.org/mk2t.
Lee SY, Ko HC, Kim SI, Lee YS, Son BC. Progressive multifocal leukoencephalopathy diagnosed by brain biopsy, not by the DNA test for JC Virus. Asian J Neurosurg. 2019;14(1):240-4. https://doi.org/mk2v.
Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430-8. https://doi.org/f4w6c7.
Bernard-Valnet R, Koralnik IJ, Du Pasquier R. Advances in treatment of progressive multifocal leukoencephalopathy. Ann Neurol. 2021;90(6):865-73. https://doi.org/mk2w.
Möhn N, Grote-Levi L, Hopfner F, Eiz-Vesper B, Maecker-Kolhoff B, Warnke C, et al. Innovative therapeutic concepts of progressive multifocal leukoencephalopathy. J Neurol. 2022;269(5):2403-13. https://doi.org/mk2x.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2024 Revista de la Facultad de Medicina

Esta obra está bajo una licencia Creative Commons Reconocimiento 3.0 Unported.
-





















