Use of EMG biofeedback for basic activities of daily living training in stroke patients. Pilot randomized clinical trial
Uso de biofeedback electromiográfico durante el entrenamiento de las actividades básicas de la vida diaria en pacientes con accidente cerebrovascular. Ensayo clínico aleatorizado piloto
DOI:
https://doi.org/10.15446/revfacmed.v64n3.56213Palabras clave:
Neurorretroalimentación, Rehabilitación, Actividades cotidianas, Accidente cerebrovascular, Terapia Ocupacional (en)Neurorretroalimentación, Rehabilitación, Actividades cotidianas, Accidente cerebrovascular, Terapia Ocupacional (es)
Descargas
Introduction: Sequels in stroke patients include hemiparesis and dependency for performing basic activities of daily living (BADL). EMG biofeedback has yielded some benefits but has been limited to repetitive movement, therefore, it is insufficient for current task-oriented neurorehabilitation paradigms.
Objective: To assess whether the application of EMG biofeedback in upper limbs during BADL training improves motor, occupational and satisfaction performances compared to BADL training without this feedback.
Materials and methods: A pilot randomized clinical trial was conducted with stroke patients of more than six months of evolution, who showed hemiparesis and no cognitive deterioration. These patients were randomly classified into two groups: control group, who underwent conventional occupational therapy (COT), and experimental group, who underwent COT+EMG-BF. Patients were given 10 therapy sessions. Entry, evaluation and data analysis were masked.
Results: Seven patients were included in each group, showing the same initial clinical and demographic characteristics (p>0.05). The group that underwent COT+EMG-BF showed a significantly better performance in all assessments. For example, the Barthel scale obtained a median of 100 points [85-100] for the COT+EMG-BF group versus 85 [80-90] for the control group (p<0.05), whereas ARAT score was 42 [40-47] points versus 20 [15-38] (p=0.03), respectively.
Conclusion: The combination of COT+EMG-BF for BADL may be considered as an alternative for treatment of stroke patients.
Introducción. Las principales secuelas del accidente cerebrovascular (ACV) son la hemiparesia y la dependencia en actividades básicas de la vida diaria (ABVD). El biofeedback electromiográfico (BF-EMG) ha mostrado beneficios, pero su uso se ha centrado en el entrenamiento de movimientos aislados, lo que difiere del paradigma actual en rehabilitación.
Objetivo. Evaluar si la aplicación de BF-EMG durante el entrenamiento de ABVD mejora el nivel de independencia, el funcionamiento motor y la satisfacción en el desempeño comparado con entrenamiento de ABVD sin esta técnica.
Materiales y métodos. Ensayo clínico piloto en pacientes de 18 a 70 años con hemiparesia secundaria a ACV isquémico crónico. Los pacientes fueron aleatorizados en dos grupos: un grupo control con terapia ocupacional convencional (TOC) o un grupo experimental con TOC + BF-EMG. Se enmascaró el enrolamiento, la evaluación y el análisis de datos.
Resultados. Se reclutaron siete pacientes en cada grupo, control y experimental, con las mismas características demográficas y clínicas iniciales (p>0.05). El grupo TOC+BF-EMG mostró mejor rendimiento en todas las evaluaciones. En el índice de Barthel se obtuvo una mediana de 100 puntos (85-100) para el grupo TOC + BF-EMG en comparación a 85 (80-90) del grupo control (p<0.05), mientras que en el test de Arat fueron 42 (40-47) puntos frente a 20 (15-38) (p=0.03), respectivamente.
Conclusión. La combinación de TOC + BF-EMG en ABVD podría considerarse una alternativa en el tratamiento de personas con ACV.
original research
DOI: https://doi.org/10.15446/revfacmed.v64n3.56213
Use of EMG biofeedback for basic activities of daily living
training in stroke patients. Pilot randomized clinical trial
Uso de biofeedback electromiográfico durante el entrenamiento de las actividades básicas de la vida diaria en pacientes con accidente cerebrovascular. Ensayo clínico aleatorizado piloto
Received: 15/03/2016. Accepted: 10/05/2016.
Maricel Garrido-Montenegro1 • Evelyn Álvarez-Espinoza1-2 • Sebastián Vergara-Ruiz1
1 Hospital Clínico Universidad de Chile - Physical Medicine and Rehabilitation Service - Santiago - Chile.
2 Universidad Central de Chile - Faculty of Health Sciences - Occupational Therapy School - Santiago - Chile.
Corresponding author: Maricel Garrido-Montenegro. Hospital Clínico Universidad de Chile. Calle Luis Thayer Ojeda No. 1080,
apartment 21. Phone number: +56 942502855. Santiago. Chile. Email: maricel.garrido.m@gmail.com.
| Abstract |
Introduction: Sequels in stroke patients include hemiparesis and dependency for performing basic activities of daily living (BADL). EMG biofeedback has yielded some benefits but has been limited to repetitive movement, therefore, it is insufficient for current task-oriented neurorehabilitation paradigms.
Objective: To assess whether the application of EMG biofeedback in upper limbs during BADL training improves motor, occupational and satisfaction performances compared to BADL training without this feedback.
Materials and methods: A pilot randomized clinical trial was conducted with stroke patients of more than six months of evolution, who showed hemiparesis and no cognitive deterioration. These patients were randomly classified into two groups: control group, who underwent conventional occupational therapy (COT), and experimental group, who underwent COT+EMG-BF. Patients were given 10 therapy sessions. Entry, evaluation and data analysis were masked.
Results: Seven patients were included in each group, showing the same initial clinical and demographic characteristics (p>0.05). The group that underwent COT+EMG-BF showed a significantly better performance in all assessments. For example, the Barthel scale obtained a median of 100 points [85-100] for the COT+EMG-BF group versus 85 [80-90] for the control group (p<0.05), whereas ARAT score was 42 [40-47] points versus 20 [15-38] (p=0.03), respectively.
Conclusion: The combination of COT+EMG-BF for BADL may be considered as an alternative for treatment of stroke patients.
Keywords: Neurofeedback; Rehabilitation; Activities of Daily Living; Stroke; Occupational Therapy (MeSH).
Garrido-Montenegro M, Álvarez-Espinoza E, Vergara-Ruiz S. Use of EMG biofeedback for basic activities of daily living training in stroke patients. Pilot randomized clinical trial. Rev. Fac. Med. 2016;64(3):477-83. English. doi: https://doi.org/10.15446/revfacmed.v64n3.56213.
| Resumen |
Introducción. Las principales secuelas del accidente cerebrovascular (ACV) son la hemiparesia y la dependencia en actividades básicas de la vida diaria (ABVD). El biofeedback electromiográfico (BF-EMG) ha mostrado beneficios, pero su uso se ha centrado en el entrenamiento de movimientos aislados, lo que difiere del paradigma actual en rehabilitación.
Objetivo. Evaluar si la aplicación de BF-EMG durante el entrenamiento de ABVD mejora el nivel de independencia, el funcionamiento motor y la satisfacción en el desempeño comparado con entrenamiento de ABVD sin esta técnica.
Materiales y métodos. Ensayo clínico piloto en pacientes de 18 a 70 años con hemiparesia secundaria a ACV isquémico crónico. Los pacientes fueron aleatorizados en dos grupos: un grupo control con terapia ocupacional convencional (TOC) o un grupo experimental con TOC + BF-EMG. Se enmascaró el enrolamiento, la evaluación y el análisis de datos.
Resultados. Se reclutaron siete pacientes en cada grupo, control y experimental, con las mismas características demográficas y clínicas iniciales (p>0.05). El grupo TOC+BF-EMG mostró mejor rendimiento en todas las evaluaciones. En el índice de Barthel se obtuvo una mediana de 100 puntos (85-100) para el grupo TOC + BF-EMG en comparación a 85 (80-90) del grupo control (p<0.05), mientras que en el test de Arat fueron 42 (40-47) puntos frente a 20 (15-38) (p=0.03), respectivamente.
Conclusión. La combinación de TOC + BF-EMG en ABVD podría considerarse una alternativa en el tratamiento de personas con ACV.
Palabras clave: Neurorretroalimentación; Rehabilitación; Actividades cotidianas; Accidente cerebrovascular; Terapia Ocupacional (DeCS).
Garrido-Montenegro M, Álvarez-Espinoza E, Vergara-Ruiz S. [Uso de biofeedback electromiográfico durante el entrenamiento de las actividades básicas de la vida diaria en pacientes con accidente cerebrovascular. Ensayo clínico aleatorizado piloto]. Rev. Fac. Med. 2016;64(3):477-83. English. doi: https://doi.org/10.15446/revfacmed.v64n3.56213.
Introduction
According to the World Health Organization (WHO), cardiovascular diseases are the leading cause of death worldwide; among them, stroke is the third cause of disability-adjusted life year (DALY) (1). Most of the patients who survive have an incomplete motor recovery after six months; the main sequel is hemiparesis (50%), followed by cognitive, gait, affective, sensory and language deficits (2). A year after the stroke, 60% of patients present some kind of dependence. This figure increases to 66% at three years (3), which affects both families and the State socially and economically.
The effects of strokes alter the occupational performance of patients by making them unable to meet the expectations of their role and the demands of the activities performed on a daily basis. For example, changes in basic activities of daily living (BADL), which consist of body self-care tasks, show a dependence rate of 58.6% at six months and 50% at one year. Bathing, going upstairs and dressing are the most affected and the most difficult activities to achieve independently (4). For occupational therapists, training in BADL is paramount, but there is little evidence of techniques or tools that can facilitate this process.
Electromyographic biofeedback (EMG-BF) is recommended for the treatment of various conditions after a stroke (5), making patients more aware of muscle activity and helping to control the level of muscle contraction applied. However, the technique is used in the training of specific and repetitive movements, outside the context of functional activities of daily living, therefore, it is still insufficient for current paradigms of neurorehabilitation and the concept of rehabilitation for occupational therapy (OT). Therefore, this research uses the EMG-BF during the execution of BADL, explicitly including feedback in a functional and motivating therapeutic process. It also proposes a hypothesis consisting of the use of EMG-BF during BADL training for significantly improving the level of independence, motor functioning and performance satisfaction compared with BADL training without EMG-BF.
As discussed above, the objective of this research was to evaluate whether the application of EMG-BF in the upper limbs during BADL training improves the level of independence, motor functioning and satisfaction in performance, compared to training BADL without EMG-BF.
Materials and methods
Design, selection and description of participants
A randomized pilot clinical study was conducted with stroke patients. Two intervention procedures were proposed: a control group that underwent conventional occupational therapy (COT) and an experimental group that was treated with conventional occupational therapy plus EMG-BF (COT+EMG-BF).
Independence in BADL is a relevant parameter to be assessed during rehabilitation studies after a stroke (3,4); therefore, the sample size was calculated based on this variable and on another study of EMG-BF for which the Barthel index was used (6).
This research aimed to achieve an improvement of 40% of independence during BADL, with a statistical power of 80%, in a sample size of 14 patients per group —control and experimental—. However, as this is a pilot study, 50% of the sample was included, that is, seven patients per group. The sample was made up of patients suffering from the effects of a stroke, who lived in the Commune of Santiago, Chile, for eight consecutive months.
For selection, a non-probabilistic intentional sampling was conducted, considering the following inclusion criteria: age between 18 and 70 years old, hemiparesis as consequence of unilateral ischemic stroke of at least six months and a maximum of three years of evolution at the time inclusion, only one stroke or previous events without effects, active range of motion ≥20° for wrist extension and >10° for finger extension (7). Meanwhile, the exclusion criteria were sensory and perceptual disturbances, cognitive impairment in the Folstein Mini-Mental State Examination, affective disorders without treatment and painful shoulder or shoulder subluxation.
After signing the informed consent, patients were randomly assigned to one group. This process was conducted by a third party unrelated to the study and to the patients, who did not know the clinical record and maintained allocation concealment to researchers. For distribution, random number sequences generated by computer were used. All patients were enrolled by an internist and the assignment was made by an occupational therapist.
Data collection methods
Participants in both groups were evaluated before and after completing the intervention. To assess the primary outcome (level of independence) the Barthel index, which has a score range from 0 to 100 points, was used (8-12). The following tests were used for secondary outcomes: 1) Instrumental Activities of Daily Living (IADL), with score 0-8 (13); 2) Action Research Arm Test (ARAT) to assess manipulative ability with a score of 0-56 (14); 3) Motor Activity Log (MAL) to know self-perception of quality and use of the affected upper limbs in daily activities with score 0-5 (15,16); 4) Mini-Mental State Folstein to detect cognitive impairment during enrollment with score of 0-30 (17), and 5) Canadian Occupational Performance Measure (COPM) to quantify the self-perceived change in occupational performance and satisfaction with a score of 0-10 (18). Selected guidelines are consistent with correct psychometric measures (12,14,16,18).
The evaluators were two occupational therapists who had no contact with the patients and who were masked to the group of subjects. These therapists were familiar with the aforementioned tests and unified the application criteria through training with patients.
Procedures
The procedures followed the ethical standards of the Declaration of Helsinki and were reviewed and approved by the ethics committee of Universidad Central de Chile.
It is important to clarify that EMG-BF was used during the execution of BADL by patients and not during repetitive training of analytical movements. Both control and experimental groups attended 10 60-minutes intervention sessions, three times a week, and two evaluation sessions, one at the beginning and the other at the end of the study. Depending on the level of importance attributed to COPM assessment, two BADL were selected to be worked with each participant; these activities had to meet two criteria: being a bimanual activity and promoting the use of gravitational muscles of the upper limbs.
The session for the control group was structured as follows: 1) patient preparation (5 minutes) which involved explaining the activities during the session and removing all elements that may hinder the work; 2) low-impact therapeutic activities that promoted flexion, abduction and external rotation of shoulder, elbow extension, forearm supination, wrist extension, and extension and abduction of long fingers and thumbs (10 minutes); 3) BADL training with activation and facilitation of movement in shoulder, elbow, wrist and fingers accordingly (40 minutes) and 4) relaxation of the exercised segment (5 minutes).
For the experimental group, each session was structured as follows: 1) preparation of the patient, and apart from the activities performed by the control group, activities like skin cleansing and application of electrodes were added (5 minutes); 2) low-impact therapeutic activities that promoted flexion, abduction and external rotation of shoulder, elbow extension, forearm supination, wrist extension and extension and abduction of long fingers and thumbs (10 minutes); 3) BADL training with activation and facilitation of movement in shoulder, elbow, wrist and fingers, accordingly, using EMG-BF in the selected antigravity muscles (30 minutes); 4) ADL training without using EMG-BF (10 minutes), and 5) relaxation of the exercised segment (5 minutes). The feedback received by the patient through a visual and auditory stimulus was positive.
Four occupational therapists participated in the interventions; their criteria were unified through a written set of practice guidelines and classroom training for using EMG-BF. To verify the implementation of the intervention stages, each patient had a treatment log sheet where the auditor could check compliance.
The persons responsible for enrollment, assessment, recording and analysis of data were masked. Given the characteristics of the intervention, the occupational therapist could not be masked.
Data analysis
The descriptive statistical analysis of the variables was obtained with the median p25-p75. The significance level used in the analysis was p<0.05 with two tails. To compare variables between the two groups, the Wilcoxon test was used. Data also were analyzed and tested again by an outside statistician using the SPSS 19 software.
Results
For eight months, 90 patients were evaluated; 14 of them were recruited and randomized —7 in the control group and 7 in the trial group— without losing any of them during follow-up (Figure 1).
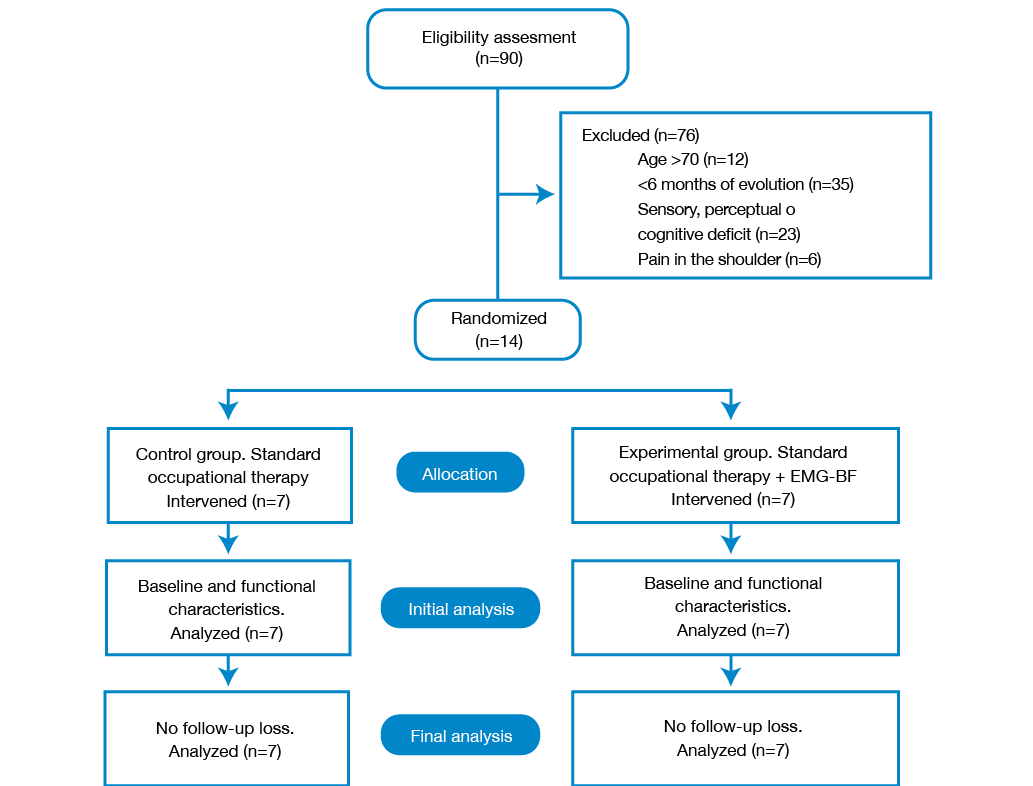
Figure 1. Flow chart of the study. Source: Own elaboration based on the data obtained in the study.
No dangerous actions occurred in any of the patients in both groups. The 14 patients were included in the intention-to-treat population and their demographic, clinical and functional baseline characteristics did not differ (Table 1).
Table 1. Demographic, clinical and functional characteristics of study participants.
|
Demographic characteristics |
Control (n=7) |
Experimental (n=7) |
p value |
|
|
Age |
55 (37-63) |
48 (35-60) |
0.770 |
|
|
Female (%) |
4 (57%) |
3 (43%) |
0.792 |
|
|
Years of schooling |
11 (10-12) |
12 (12-12) |
0.693 |
|
|
Current work (%) |
No (100%) |
No (100%) |
1 |
|
|
Laterality |
Left |
2 (29%) |
1 (14.30%) |
0.785 |
|
Right |
5 (71%) |
6 (85.7%) |
0.783 |
|
|
Clinical characteristics |
Control (n=7) |
Experimental (n=7) |
p value |
|
|
Stroke location |
Left hemisphere (%) |
3 (43%) |
3 (43%) |
1 |
|
Right hemisphere (%) |
4 (57%) |
4 (57%) |
1 |
|
|
Months of evolution |
18 (14-21) |
14 (9-20) |
0.537 |
|
|
Associated therapies |
Physiotherapy and kinesiology (%) |
4 (57%) |
5 (100%) |
1 |
|
Speech therapy (%) |
3 (43%) |
2 (20%) |
0.847 |
|
|
Functional characteristics |
Control (n=7) |
Experimental (n=7) |
p value |
|
|
Barthel index |
80 (75-80) |
80 (75-85) |
1 |
|
|
IADL |
3 (2-3) |
3 (2-3) |
1 |
|
|
ARAT |
15 (10-30) |
15 (10-30) |
1 |
|
|
MAL amount |
1 (0-2.16) |
1.16 (0.4-1.33) |
0.920 |
|
|
MAL quality |
0.9 (0-1) |
0.3 (0.26-0.66) |
0.537 |
|
|
COPM performance |
2 (1-2.5) |
1.5 (1.5-2) |
0.777 |
|
|
COPM satisfaction |
2 (1.5-2.25) |
1.5 (1.25-2) |
0.429 |
|
IADL: Instrumental Activity Daily Living; ARAT: Action Research Arm Test; MAL: Motor Activity Log; COPM: Canadian Occupational Performance Measure; median = p25-p75. Source: Own elaboration based on the data obtained in the study.
The protocol of the experimental group was modified because 10 and not 5 minutes were used for the conditioning of the patient, since the skin cleaning and electrode placement procedures took longer than expected. All other the steps of the protocol remained unchanged in both the control and the experimental group, which was verified through the treatment log sheet filled by the occupational therapist after each session.
The results related to independence show that the experimental group achieved significantly higher levels than the control group. BADL were evaluated using the Barthel index; the experimental group received 15 points more than the median of the control group, reaching 100 points (p<0.05). Also, 57% of patients in the experimental group reached the highest level of independence unlike the control group, where no patients achieved this level (Table 2 and Figure 2). This result shows a statistical power of 81%.
Table 2. Final results of evaluations.
|
Occupational performance |
Control (n=7) |
Experimental (n=7) |
p value |
|
Barthel index |
85 (80-90) |
100 (85-100) |
0.038 |
|
Independent patients (%) |
0 (0%) |
4 (57%) |
|
|
IADL |
4 (3-4) |
6 (5-6) |
0.001 |
|
Motor performance |
Control (n=7) |
Experimental (n=7) |
p value |
|
ARAT |
20 (15-38) |
40 (35-45) |
0.026 |
|
Performance satisfaction |
Control (n=7) |
Experimental (n=7) |
p value |
|
MAL amount |
1.33 (0.13-2.33) |
3.16 (2.5-3.66) |
0.017 |
|
MAL quality |
0.5 (0.16-1) |
1.33 (1-2) |
0.011 |
|
COPM performance |
2 (1-3) |
3.5 (3-3.5) |
0.004 |
|
COPM satisfaction |
2 (2-2.5) |
3.5 (3-4) |
0.011 |
IADL: Instrumental Activity Daily Living; ARAT: Action Research Arm Test; MAL: Motor Activity Log; COPM: Canadian Occupational Performance Measure; median = p25-p75. Source: Own elaboration based on the data obtained in the study.
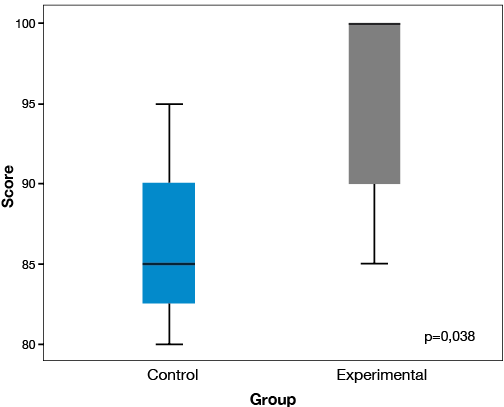
Figure 2. Final Barthel index. Source: Own elaboration based on the data obtained in the study.
The experimental group showed an increase of three points in its median for the IADL evaluated using the IADL proposal by Lawton & Brody (13), in contrast with the single point obtained by the control group (p=0009) (Table 2).
The experimental group also obtained significant improvements in motor functioning, unlike the control group, with final results of 40 points (Med=35-45) in the ARAT tests versus 20 (Med=15-38), respectively (p=0.026) (Figure 3).

Figure 3. ARAT test results. Source: Own elaboration based on the data obtained in the study.
The results obtained in the self-perception assessments show significant differences in the experimental group in all aspects measured by MAL and COPM tests, in quantity, quality, performance and satisfaction. The results of the MAL test regarding quantity showed scores of 3.16 (Med=2.5-3.66) and 1.33 (Med=0.13-2.33) for the experimental and the control groups, respectively (p<0.05) (Table 2). In addition, according to the MAL test on quality, significant differences were found in the experimental group (p<0.05) (Table 2). Regarding COPM, the greatest statistical difference was obtained in the performance assessment, where the experimental group received a score of 3.5 (Med=3-3.5) versus 2 (Med=1-3) of the control group (p=0.004) (Table 2).
Finally, although patients of the experimental group achieved higher levels of satisfaction in the performance than the control group in COPM, the scores were similar (p<0.05) (Table 2).
Discussion
The novelty of this research relates to the evaluation of the EMG-BF application during training of basic BADL. This approach attempts to maximize the probabilities offered by a treatment that combines the use of EMG-BF with task-oriented therapy, a key contemporary paradigm in terms of vision, and a historical paradigm regarding motor learning in the work of the occupational therapist (19).
The use of the EMG-BF technique during the execution of a significant BADL was a major contribution to increasing independence, which achieved better results than those obtained in another study of EMG-BF in upper limbs (6). Not only progress was achieved in the selected activities, but also in other basic and even instrumental activities.
This is consistent with other studies on strokes that incorporate motivational activities such as games or virtual reality and that support the importance of considering the patient’s motivation in the rehabilitation process because motor learning not only requires practice, but also motivation with functional objective activities for brain reorganization (20).
BADL were selected as the basis of treatment because these are the activities with the highest degree of alteration and dependence six months after the stroke (4); likewise, patients were allowed to make their own choice of BADL to work on, prioritizing them according to the self-perceived degree of importance. This motivated patients to take a more active role in treatment decisions and increase their independence, which is considered a positive factor in the clinical and functional evolution (21).
Moreover, the consequences associated with stroke often produce, to a greater or lesser extent, changes in the necessary sensory feedback for the control and motor learning, limiting the ability of patients to acquire, change or adjust skills because the actual functioning of their bodies is not perceived correctly. In this sense, other studies report the benefits of using EMG-BF in the rehabilitation of hemiparesis in upper and lower limbs after a stroke in contrast with conventional management (22-25), as well as the importance of simultaneous visual or auditory feedback for promoting better performance (26). Generally, and except for a couple of experiences of case studies in cases of gait training (27,28), the technique has been integrated into therapies whose work and measuring results are based on analytic movement or its components, without ensuring the transfer of individual movements to the complexity of an everyday activity.
In this study, the use of EMG-BF during the execution of a BADL provided an amplification of muscle signals through the visual-auditory feedback that compensated the feedback deficit of patients, allowing them a greater awareness and precision in muscle activation control during an activity. Awareness about muscle function during a motivating activity plays an important role in the acquisition of movement patterns because, if a process is hidden or is not perceived by the nervous system, it is less capable of correcting any problems in the quality of performance (29). Therefore, feedback, the use of therapeutic activities oriented to tasks, as well as motivation, play a fundamental role in motor learning and have shown to be a contribution to treatment (20,30,31).
In addition to the benefits achieved in BADL, finding better performance of ADLs was not expected since this aspect is not evaluated by other studies; this suggests that patients, having greater awareness of their motor skills, can transfer learning more effectively to other activities. This is seen in the independence achieved in activities such as meal preparation, housekeeping and purchases.
In relation to advances in motor performance, the results show that patients in the experimental group improved the functional mobility of the affected upper limb, a key factor for incorporation into everyday unimanual and bimanual activities, e.g. using a knife and fork, holding a glass, grabbing cleaning products, etc. The scores obtained at the end of the intervention are similar to other techniques of upper limbs rehabilitation after stroke, such as mirror therapy or restriction of movement therapy (32,33). In this regard, the results suggest that there is a relationship between the benefits obtained in objective motor evaluation (ARAT) and better subjective perception by patients, both on the incorporation of the affected upper limb in daily activities, and quality perception of their movements, one of the objectives of the study. In the same vein, the COPM test showed significant progress in the perception and satisfaction of performance in the chosen BADL, essential factors for achieving successful outcomes in rehabilitation (34).
The structure adopted in the intervention protocol favored the learning of patients. However, the time initially estimated for each session (one hour) was insufficient, since, on average, the sessions were one hour and five minutes long due to a longer time for conditioning of the patient (10 and not 5 minutes), an aspect to improve in future studies. It should also be noted that the EMG-BF surface did not allow obtaining reliable data on small upper limb muscles, which was possibly related to the fine motor tong activities being referred as more difficult by patients, for example, using keys to open doors, buttoning/unbuttoning, putting on/taking off bras, zipping/unzipping.
Finally, the potential limitations when generalizing the study results must be recognized taking into account the limited sample of individuals with chronic stroke that was tested. The results are only applicable to people with similar characteristics to the participant group. A greater number of patients is necessary to extrapolate the results, but this is still a significant and interesting alternative to the conventional use of EMG-BF.
Conclusion
The use of EMG-BF during BADL yielded significant progress in all defined parameters: motor, occupational and satisfaction levels, constituting a complement to the rehabilitation treatment of people with sequelae of stroke.
Contributions
Álvarez-Espinoza and Garrido-Montenegro participated in the conception, design, analysis and interpretation of results; Vergara-Ruiz participated in patient recruitment and data collection; all authors drafted, wrote and contributed to the revision of the text, as well as in the review and approval of the final version.
Ethical principles for medical research
Both the main author and the other authors declare that they have full access to all data in the study and take responsibility for the integrity of the data and the accuracy of its analysis.
Conflict of interests
None stated by the authors.
Funding
None stated by the authors.
Acknowledgements
To Daniela Salgado, Pamela Salcidua and Sebastián Tobar, occupational therapists, for their cooperation during the execution of the study.
References
1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197-223.
http://doi.org/f2f8rw.
2.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J. Stroke Cerebrovasc. Dis. 2003;12(3):119-26. http://doi.org/czqppx.
3.Patel MD, Tilling K, Lawrence E, Rudd AG, Wolfe CD, McKevitt C. Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing. 2006;35(3):273-9. http://doi.org/bfnxx8.
4.Kong KH, Lee J. Temporal recovery of activities of daily living in the first year after ischemic stroke: a prospective study of patients admitted to a rehabilitation unit. NeuroRehabilitation. 2014;35(2):221-6.
http://doi.org/bqcd.
5.Ottawa Panel, Khadilkar A, Phillips K, Jean N, Lamothe C, Milne S, et al. Ottawa panel evidence-based clinical practice guidelines for post-stroke rehabilitation. Top Stroke Rehabil. 2006;13(2):1-269.
http://doi.org/dzdq8p.
6.Doğan-Aslan M, Nakipoğlu-Yüzer GF, Doğan A, Karabay I, Özgirgin N. The Effect of Electromyographic Biofeedback Treatment in Improving Upper Extremity Functioning of Patients with Hemiplegic Stroke. J. Stroke Cerebrovasc. Dis. 2012;21(3):187-92. http://doi.org/d3w42r.
7.Fritz SL, Light KE, Patterson TS, Behrman AL, Davis SB. Active finger extension predicts outcomes after constraint-induced movement therapy for individuals with hemiparesis after stroke. Stroke. 2005;36(6):1172-7. http://doi.org/frsgdv.
8.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int. Disabil. Stud. 1988;10(2):61-3. http://doi.org/dr6t27.
9.Gresham GE, Phillips TF, Labi ML. ADL status in stroke: relative merits of three standard indexes. Arch. Phys. Med. Rehabil. 1980;61(8):355-8.
10.Loewen SC, Anderson BA. Predictors of stroke outcome using objective measurement scales. Stroke. 1990;21(1):78-81. http://doi.org/bwd929.
11.Lyden PD, Hantson L. Assessment scales for the evaluation of stroke patients. J. Stroke Cerebrovasc. Dis. 1998;7(2):113-27. http://doi.org/c9jq34.
12.Hsueh IP, Lee MM, Hsieh CL. Psychometric characteristics of the Barthel activities of daily living index in stroke patients. J. Formos Med. Assoc. 2001;100(8):526-532.
13.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-86. http://doi.org/b82qwn.
14.Nijland R, van Wegen E, Verbunt J, van Wijk R, van kordelaar J, Kwakkel G. A comparison of two validated tests for upper limb function after stroke: The Wolf Motor Function Test and the Action Research Arm Test. J. Rehabil. Med. 2010;42(7):694-6. http://doi.org/bkxv2c.
15.Hammer AM, Lindmark B. Responsiveness and validity of the Motor Activity Log in patients during the subacute phase after stroke. Disabil. Rehabil. 2010;32(14):1184-93. http://doi.org/b79gxn.
16.van der, Beckerman H, Knol DL, Vet HCW de, Bouter LM. Clinimetric Properties of the Motor Activity Log for the Assessment of Arm Use in Hemiparetic Patients. Stroke. 2004;35(6):1410-4. http://doi.org/d28gn7 .
17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr Res. 1975;12(3):189-98. http://doi.org/b9jhjp.
18.Dedding C, Cardol M, Eyssen IC, Dekker J, Beelen A. Validity of the Canadian Occupational Performance Measure: a client-centred outcome measurement. Clin. Rehabil. 2004;18(6):660-7. http://doi.org/ds9gj9.
19.Jonsdottir J, Cattaneo D, Regola A, Crippa A, Recalcati M, Rabuffetti M, et al. Concepts of Motor Learning Applied to a Rehabilitation Protocol Using Biofeedback to Improve Gait in a Chronic Stroke Patient: An A-B System Study With Multiple Gait Analyses. Neurorehabil. Neural Repair. 2007;21(2):190-4. http://doi.org/fgtv95.
20.Popović MD, Kostić MD, Rodić SZ, Konstantinović LM. Feedback-mediated upper extremities exercise: increasing patient motivation in poststroke rehabilitation. BioMed Res. Int. 2014;2014:520374. http://doi.org/bqcf.
21.Campbell R, Evans M, Tucker M, Quilty B, Dieppe P, Donovan JL. Why don’t patients do their exercises? Understanding non-compliance with physiotherapy in patients with osteoarthritis of the knee. J. Epidemiol. Community Health. 2001;55(2):132-8. http://doi.org/c4hhvq.
22.Barreca S, Wolf SL, Fasoli S, Bohannon R. Treatment interventions for the paretic upper limb of stroke survivors: a critical review. Neurorehabil. Neural. Repair. 2003;17(4):220-6. http://doi.org/cj3j5h.
23.Moreland JD, Thomson MA, Fuoco AR. Electromyographic biofeedback to improve lower extremity function after stroke: a meta-analysis. Arch. Phys. Med. Rehabil. 1998;79(2):134-40. http://doi.org/dhj3c3.
24.Moreland J, Thomson MA. Efficacy of electromyographic biofeedback compared with conventional physical therapy for upper-extremity function in patients following stroke: a research overview and meta-analysis. Phys. Ther. 1994;74(6):534-43.
25.Schleenbaker RE, Mainous AG. Electromyographic biofeedback for neuromuscular reeducation in the hemiplegic stroke patient: a meta-analysis. Arch. Phys. Med. Rehabil. 1993;74(12):1301-4. http://doi.org/d5vc54.
26.Parker J, Mountain G, Hammerton J. A review of the evidence underpinning the use of visual and auditory feedback for computer technology in post-stroke upper-limb rehabilitation. Disabil. Rehabil. Assist. Technol. 2011;6(6):465-72. http://doi.org/d9685s.
27.Jonsdottir J, Cattaneo D, Recalcati M, Regola A, Rabuffetti M, Ferrarin M, et al. Task-oriented biofeedback to improve gait in individuals with chronic stroke: motor learning approach. Neurorehabil. Neural Repair. 2010;24(5):478-85. http://doi.org/bgh25v.
28.Richards CL, Malouin F, Bravo G, Dumas F, Wood-Dauphinee S. The role of technology in task-oriented training in persons with subacute stroke: a randomized controlled trial. Neurorehabil. Neural Repair. 2004;18(4):199-211. http://doi.org/dqhjwn.
29.Nelson LA. The role of biofeedback in stroke rehabilitation: past and future directions. Top Stroke Rehabil. 2007;14(4):59-66. http://doi.org/frdwhm.
30.Huang H, Wolf SL, He J. Recent developments in biofeedback for neuromotor rehabilitation. J. Neuroeng. Rehabil. 2006;3(1):11.
http://doi.org/bjtzj3.
31.Woodford H, Price C. EMG biofeedback for the recovery of motor function after stroke. Cochrane Database Syst Rev. 2007;(2):CD004585. http://doi.org/b5ktmd.
32.LinKC, Chen YT, Huang PC, Wu CY, Huang WL, Yang HW, et al. Effect of mirror therapy combined with somatosensory stimulation on motor recovery and daily function in stroke patients: a pilot study. J. Formos Med. Assoc. 2014;113(7):422-8. http://doi.org/bqcg.
33.McIntyre A, Viana R, Janzen S, Mehta S, Pereira S, Teasell R. Systematic review and meta-analysis of constraint-induced movement therapy in the hemiparetic upper extremity more than six months post stroke. Top Stroke Rehabil. 2012;19(6):499-513. http://doi.org/bqch.
34.Chen CY, Neufeld PS, Feely CA, Skinner CS. Factors influencing compliance with home exercise programs among patients with upper-extremity impairment. Am. J. Occup. Ther. 1999;53(2):171-80. http://doi.org/bqcj.

César Alexander Eslava Franco
“Mapas anatómicos”
Universidad Nacional de Colombia
Referencias
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197-223.
http://doi. org/f2f8rw.
Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J. Stroke Cerebrovasc. Dis. 2003;12(3):119-26. http://doi. org/czqppx.
Patel MD, Tilling K, Lawrence E, Rudd AG, Wolfe CD, McKevitt C. Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing. 2006;35(3):273-9. http://doi. org/bfnxx8.
Kong KH, Lee J. Temporal recovery of activities of daily living in the first year after ischemic stroke: a prospective study of patients admitted to a rehabilitation unit. NeuroRehabilitation. 2014;35(2):221-6.
http://doi. org/bqcd.
Ottawa Panel, Khadilkar A, Phillips K, Jean N, Lamothe C, Milne S, et al. Ottawa panel evidence-based clinical practice guidelines for post-stroke rehabilitation. Top Stroke Rehabil. 2006;13(2):1-269.
http://doi. org/dzdq8p.
Doğan-Aslan M, Nakipoğlu-Yüzer GF, Doğan A, Karabay I, Özgirgin N. The Effect of Electromyographic Biofeedback Treatment in Improving Upper Extremity Functioning of Patients with Hemiplegic Stroke. J. Stroke Cerebrovasc. Dis. 2012;21(3):187-92. http://doi. org/d3w42r.
Fritz SL, Light KE, Patterson TS, Behrman AL, Davis SB. Active finger extension predicts outcomes after constraint-induced movement therapy for individuals with hemiparesis after stroke. Stroke. 2005;36(6):1172-7. http://doi. org/frsgdv.
Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int. Disabil. Stud. 1988;10(2):61-3. http://doi. org/dr6t27.
Gresham GE, Phillips TF, Labi ML. ADL status in stroke: relative merits of three standard indexes. Arch. Phys. Med. Rehabil. 1980;61(8):355-8.
Loewen SC, Anderson BA. Predictors of stroke outcome using objective measurement scales. Stroke. 1990;21(1):78-81. http://doi. org/bwd929.
Lyden PD, Hantson L. Assessment scales for the evaluation of stroke patients. J. Stroke Cerebrovasc. Dis. 1998;7(2):113-27. http://doi. org/c9jq34.
Hsueh IP, Lee MM, Hsieh CL. Psychometric characteristics of the Barthel activities of daily living index in stroke patients. J. Formos Med. Assoc. 2001;100(8):526-532.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-86. http://doi. org/b82qwn.
Nijland R, van Wegen E, Verbunt J, van Wijk R, van kordelaar J, Kwakkel G. A comparison of two validated tests for upper limb function after stroke: The Wolf Motor Function Test and the Action Research Arm Test. J. Rehabil. Med. 2010;42(7):694-6. http://doi. org/bkxv2c.
Hammer AM, Lindmark B. Responsiveness and validity of the Motor Activity Log in patients during the subacute phase after stroke. Disabil. Rehabil. 2010;32(14):1184-93. http://doi. org/b79gxn.
van der, Beckerman H, Knol DL, Vet HCW de, Bouter LM. Clinimetric Properties of the Motor Activity Log for the Assessment of Arm Use in Hemiparetic Patients. Stroke. 2004;35(6):1410-4. http://doi. org/d28gn7 .
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr Res. 1975;12(3):189-98. http://doi. org/b9jhjp.
Dedding C, Cardol M, Eyssen IC, Dekker J, Beelen A. Validity of the Canadian Occupational Performance Measure: a client-centred outcome measurement. Clin. Rehabil. 2004;18(6):660-7. http://doi. org/ds9gj9.
Jonsdottir J, Cattaneo D, Regola A, Crippa A, Recalcati M, Rabuffetti M, et al. Concepts of Motor Learning Applied to a Rehabilitation Protocol Using Biofeedback to Improve Gait in a Chronic Stroke Patient: An A-B System Study With Multiple Gait Analyses. Neurorehabil. Neural Repair. 2007;21(2):190-4. http://doi. org/fgtv95.
Popović MD, Kostić MD, Rodić SZ, Konstantinović LM. Feedback-mediated upper extremities exercise: increasing patient motivation in poststroke rehabilitation. BioMed Res. Int. 2014;2014:520374. http://doi. org/bqcf.
Campbell R, Evans M, Tucker M, Quilty B, Dieppe P, Donovan JL. Why don’t patients do their exercises? Understanding non-compliance with physiotherapy in patients with osteoarthritis of the knee. J. Epidemiol. Community Health. 2001;55(2):132-8. http://doi. org/c4hhvq.
Barreca S, Wolf SL, Fasoli S, Bohannon R. Treatment interventions for the paretic upper limb of stroke survivors: a critical review. Neurorehabil. Neural. Repair. 2003;17(4):220-6. http://doi. org/cj3j5h.
Moreland JD, Thomson MA, Fuoco AR. Electromyographic biofeedback to improve lower extremity function after stroke: a meta-analysis. Arch. Phys. Med. Rehabil. 1998;79(2):134-40. http://doi. org/dhj3c3.
Moreland J, Thomson MA. Efficacy of electromyographic biofeedback compared with conventional physical therapy for upper-extremity function in patients following stroke: a research overview and meta-analysis. Phys. Ther. 1994;74(6):534-43.
Schleenbaker RE, Mainous AG. Electromyographic biofeedback for neuromuscular reeducation in the hemiplegic stroke patient: a meta-analysis. Arch. Phys. Med. Rehabil. 1993;74(12):1301-4. http://doi. org/d5vc54.
Parker J, Mountain G, Hammerton J. A review of the evidence underpinning the use of visual and auditory feedback for computer technology in post-stroke upper-limb rehabilitation. Disabil. Rehabil. Assist. Technol. 2011;6(6):465-72. http://doi. org/d9685s.
Jonsdottir J, Cattaneo D, Recalcati M, Regola A, Rabuffetti M, Ferrarin M, et al. Task-oriented biofeedback to improve gait in individuals with chronic stroke: motor learning approach. Neurorehabil. Neural Repair. 2010;24(5):478-85. http://doi. org/bgh25v.
Richards CL, Malouin F, Bravo G, Dumas F, Wood-Dauphinee S. The role of technology in task-oriented training in persons with subacute stroke: a randomized controlled trial. Neurorehabil. Neural Repair. 2004;18(4):199-211. http://doi. org/dqhjwn.
Nelson LA. The role of biofeedback in stroke rehabilitation: past and future directions. Top Stroke Rehabil. 2007;14(4):59-66. http://doi. org/frdwhm.
Huang H, Wolf SL, He J. Recent developments in biofeedback for neuromotor rehabilitation. J. Neuroeng. Rehabil. 2006;3(1):11.
http://doi. org/bjtzj3.
Woodford H, Price C. EMG biofeedback for the recovery of motor function after stroke. Cochrane Database Syst Rev. 2007;(2):CD004585. http://doi. org/b5ktmd.
LinKC, Chen YT, Huang PC, Wu CY, Huang WL, Yang HW, et al. Effect of mirror therapy combined with somatosensory stimulation on motor recovery and daily function in stroke patients: a pilot study. J. Formos Med. Assoc. 2014;113(7):422-8. http://doi. org/bqcg.
McIntyre A, Viana R, Janzen S, Mehta S, Pereira S, Teasell R. Systematic review and meta-analysis of constraint-induced movement therapy in the hemiparetic upper extremity more than six months post stroke. Top Stroke Rehabil. 2012;19(6):499-513. http://doi. org/bqch.
Chen CY, Neufeld PS, Feely CA, Skinner CS. Factors influencing compliance with home exercise programs among patients with upper-extremity impairment. Am. J. Occup. Ther. 1999;53(2):171-80. http://doi. org/bqcj.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Mazen M. Yassin, Mohamed N. Saad, Ayman M. Khalifa, Ashraf M. Said. (2024). Advancing clinical understanding of surface electromyography biofeedback: bridging research, teaching, and commercial applications. Expert Review of Medical Devices, 21(8), p.709. https://doi.org/10.1080/17434440.2024.2376699.
2. Andrei Tutu, Dan Trofin, Dragos-Petrica Sardaru, Ilie Onu, Cristiana Amalia Onita, Emilian Bogdan Ignat, Daniela-Marilena Trofin, Ana Onu, Daniela Viorelia Matei. (2026). Enhancing Physiotherapy Outcomes Through Multimodal Interventions in Post-Stroke Rehabilitation. Applied Sciences, 16(4), p.1760. https://doi.org/10.3390/app16041760.
3. Ana Clara Bonini-Rocha, Anderson Lúcio Souza de Andrade, Ruth dos Santos Pereira, André Marques de Moraes, Liana Barbaresco Gomide Matheus, Sérgio Teixeira da Fonseca, Alexandre Lima de Araújo Ribeiro, Wagner Rodrigues Martins. (2023). Biofeedback interventions for short term upper limb function following stroke: A systematic review with meta-analysis. Journal of Hand Therapy, 36(3), p.693. https://doi.org/10.1016/j.jht.2022.05.001.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Revista de la Facultad de Medicina

Esta obra está bajo una licencia Creative Commons Reconocimiento 3.0 Unported.
-