Dolabellane diterpenes from the Caribbean soft corals Eunicea laciniata and Eunicea asperula and determination of their anti HSV-1 activity
Dolabellanos de los octocorales caribeños Eunicea laciniata y Eunicea asperula y determinación de su actividad anti VHS-1
DOI:
https://doi.org/10.15446/rev.colomb.quim.v46n1.62830Palabras clave:
Soft corals, Eunicea, dolabellane diterpenes, antivirals (en)octocorales, Eunicea, dolabellanos, antivirales (es)
Descargas
Los dolabellanos son diterpenos con importante actividad antiviral, la mayor parte de los estudios se han realizado con compuestos aislados de algas pardas del género Dictyota. Los corales blandos son también una importante fuente de dolabellanos, pero el potencial antiviral de éstos ha sido muy poco estudiado. Como parte de nuestra búsqueda de compuestos bioactivos a partir de fuentes marinas, se llevó a cabo el estudio químico de los dolabellanos presentes en los octocorales Eunicea laciniata y Eunicea asperula, recolectados en Santa Marta, Caribe colombiano. Los dolabellanos 1-6 fueron aislados del octocoral E. laciniata mientras que en E. asperula se encontraron los compuestos 2, 4 y 5. La elucidación estructural se llevó a cabo mediante experimentos de RMN, espectrometría de masas, rotación óptica y posterior comparación con reportes previos en la literatura. El análisis por CG-EM evidenció que la dolabellatrienona (2) se puede transformar en los compuestos 4 y 5 como producto del almacenamiento prolongado, no obstante, tales compuestos también estuvieron presentes en los extractos de los organismos estudiados. El compuesto 6 inhibió la replicación del VHS-1 (73,7% de inhibición en células infectadas a una concentración de 50 μM) sin efecto citotóxico (CC50 = 959), mostrando una citotoxicidad similar al Aciclovir®, un control positivo, por lo cual se perfila como un candidato para la realización de estudios adicionales sobre el potencial de los dolabellanos como agentes antivirales.

Network of Scientific Journals from Latin America and the Caribbean, Spain and Portugal
Orgánica y Bioquímica
Dolabellane diterpenes from the Caribbean soft corals Eunicea laciniata and Eunicea asperula and determination of their anti HSV-1 activity
Dolabellanos de los octocorales caribeños Eunicea laciniata y Eunicea asperula y determinación de su actividad anti VHS-1
Dolabellanos dos octocorais caribenhos Eunicea laciniata e Eunicea asperula e determinação de sua atividade anti HSV-1
Dolabellane diterpenes from the Caribbean soft corals Eunicea laciniata and Eunicea asperula and determination of their anti HSV-1 activity
Revista Colombiana de Química, vol. 46, no. 1, 2017
Universidad Nacional de Colombia
Received: 01 July 2016
Accepted: 04 October 2016
Abstract: Dolabellane diterpenes have considerable antiviral activity, most studies have been focused towards compounds isolated from Dictyota brown algae. Although soft corals are also a significant source of these diterpenes, their antiviral potential has not been studied in detail. With the aim of assessing the biological activity of marine sources, we evaluated the dolabellane content in the soft corals Eunicea laciniata and E. asperula collected in Santa Marta, Colombian Caribbean. Dolabellanes 1-6 were isolated from E. laciniata while compounds 2, 4 and 5 were isolated from E. asperula. All compounds were identified by NMR, GC-EIMS, optical rotation and comparison with previously reported dolabellanes. GC-EIMS analyses showed that dolabellatrienone (2) transforms into compounds 4 and 5 as oxidation products upon prolonged storage; however, those compounds were also naturally present in the extract of the studied organisms. Pure dolabellanes were tested in vitro in antiviral assays against HSV-1. Compound 6 inhibited virus replication in infected cells (73.7% of inhibition at 50 µM) without cytotoxic effect (CC50 = 959), showing similar activity to the positive control Acyclovir®. Thus, compound 6 is an interesting candidate for further studies of dolabellanes as antivirals.
Keywords: Soft corals, Eunicea, dolabellane diterpenes, antivirals.
Resumen: Los dolabellanos son diterpenos con importante actividad antiviral, la mayor parte de los estudios se han realizado con compuestos aislados de algas pardas del género Dictyota. Los corales blandos son también una importante fuente de dolabellanos, pero el potencial antiviral de éstos ha sido muy poco estudiado. Como parte de nuestra búsqueda de compuestos bioactivos a partir de fuentes marinas, se llevó a cabo el estudio químico de los dolabellanos presentes en los octocorales Eunicea laciniata y Eunicea asperula, recolectados en Santa Marta, Caribe colombiano. Los dolabellanos 1-6 fueron aislados del octocoral E. laciniata mientras que en E. asperula se encontraron los compuestos 2, 4 y 5. La elucidación estructural se llevó a cabo mediante experimentos de RMN, espectrometría de masas, rotación óptica y posterior comparación con reportes previos en la literatura. El análisis por CG-EM evidenció que la dolabellatrienona (2) se puede transformar en los compuestos 4 y 5 como producto del almacenamiento prolongado, no obstante, tales compuestos también estuvieron presentes en los extractos de los organismos estudiados. El compuesto 6 inhibió la replicación del VHS-1 (73,7% de inhibición en células infectadas a una concentración de 50 µM) sin efecto citotóxico (CC50 = 959), mostrando una citotoxicidad similar al Aciclovir®, un control positivo, por lo cual se perfila como un candidato para la realización de estudios adicionales sobre el potencial de los dolabellanos como agentes antivirales.
Palabras clave: octocorales, Eunicea, dolabellanos, antivirales.
Resumo: Os dolabellanos são diterpenos que têm mostrado uma importante atividade antiviral, os estudos neste campo estam centrados nos compostos isolados de algas do gênero Dictyota. Por outro lado, a atividade antiviral dos dolabellanos isolados de octocorais não tem sido estudada, apesar de ser a segunda principal fonte natural desses diterpenos. Dessa maneira, como parte de nossa investigação de compostos bioativos a partir de fontes marinhas, foi realizado o estudo químico dos octocorais Eunicea laciniata e Eunicea asperula, coletados em Santa Marta, Caribe Colombiano. O estudo químico dos dois organismos permitiu o isolamento dos dolabellanos 1-6 de E. laciniata, enquanto que para E. aspérula foram identificados os compostos 2, 4 e 5. A elucidação estrutural foi realizada mediante experimentos de RMN, espectrometria de massas, rotação óptica e posterior comparação com os dados da literatura. A análise por GC-MS evidenciou que a dolabelatrienona (2) pode gerar os compostos 4 e 5 como produto de degradação, a partir de um armazenamento prolongado. No entanto, os compostos também estavam presentes nos extratos dos organismos estudados. O composto 6 mostrou uma citotoxicidade similar ao Aciclovir®, um controle positivo, numa porcentagem de inibição da replicação do HVS-1 (73,7% de inibição em células infectadas na concentração de 50 µM) sem efeito citotóxico (CC50 = 959), o que torna esse composto um potencial candidato para o desenvolvimento de antivirais.
Palavras-chave: octocorais, Eunicea, dolabellanos, antivirais.
Introduction
Marine organisms are a prolific source of structurally diverse and bioactive compounds ( 1 ). Interestingly, during the 2002-2011 period, 132 new compounds of marine origin with anti-HIV activity on different stages of the replication cycle were reported ( 2 ). Viral diseases are hard to treat and vaccine development against these diseases is still a major challenge and continues to elude the majority of drug discovery programs. Highly active antiretroviral therapy (HAART) is considered the best alternative of treatment against viral infectious diseases ( 3 , 4 ). HAART´s main components for treating AIDS for instance, are nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs), although new targets are constantly explored ( 5 ).
This kind of therapy has turned AIDS from being a mortal disease to becoming a treatable chronic condition, improving life quality and expectation. On the other side, Acyclovir® and its related compounds constitutes the first alternative for treating genital and oral herpes infections caused by Herpes Simplex Viruses 1 and 2 due their unsurpassed capacity to attenuate the symptoms and manifestations of these infections ( 6 ). As mentioned earlier, although HAART is not a definite cure for viral infections, it allows management of viral diseases and improves the life quality of patients and is widely used for treating AIDS. However, the emergence of resistant strains and the various side effects reported for antivirals used in HAART together with lack of efficacy of some of these substances, constitute a major challenge to overcome in order to improve this therapy ( 7 , 8 ).
Dolabellanes are diterpenes that have been isolated from natural sources such as plants ( 9 - - 12 ), fungi ( 13 ), and several marine organisms, mainly brown algae, cnidarians, and sponges ( 14 ). Structurally, they are produced by the ring formation between positions 1,11 and 10,14 in the geranyl-geraniol pyrophosphate to constitute a bicyclic [9.3.0] fused ring. Diterpenes usually exhibit a very interesting range of biological activity including antitumoral ( 14 ), cytotoxic ( 15 , 16 ) antiparasitary ( 17 ), besides a remarkable antiviral activity. Different dolabellanes with antiviral properties have been isolated from brown algae from the genus Dictyota ( 18 , 19 ). Dolabellanetriol isolated from Dictyota friabilis (as D. pfaffii) is the most studied so far, due to its potent inhibition of the human immunodeficiency virus (HIV-1) and the herpes simplex virus (HSV-1) ( 18 , 20 ). Studies concerning the mechanism of action of dolabellanetriol concluded that the molecule acts as a non-competitive HIV-1 reverse transcriptase inhibitor and classifies it into the non-nucleoside reverse transcriptase inhibitors category (NNRTIs) ( 21 ). However, despite the significant evidence on the antiviral activity of dolabellane diterpenes, very little has been reported on the antiviral properties of dolabellanes isolated from marine sources other than brown algae.
Brown algae are an important source of dolabellane diterpenes of marine origin ( 14 ), but soft corals also constitute a prolific source of such compounds, and in several cases significant ecological roles have been attributed to those ( 22 ). Most dolabellane diterpenes have been isolated from the Alcyonacean genus Clavularia and the Octocorallian genus Eunicea ( 14 ). The Colombian Caribbean harbors are large and possess diverse communities of Eunicea soft corals ( 23 ). In some of our previous studies, the soft coral Eunicea laciniata collected in Santa Marta, yielded the dolabellane diterpenes β-araneosene (1) and dolabellatrienone (2) as major compounds in the organic extract ( 24 ). Recently, we reported the anti-HIV activity of those compounds and some semisynthetic derivatives ( 25 ). Natural compounds showed low antiviral activity and they did not have considerable cytotoxic activity. On the other hand, semisynthetic derivatives were 100 times more active compared with their precursors inhibiting HIV-1 replication without a considerable increase in their cytotoxicity. Thus, dolabellane diterpenes are very interesting candidates for the development of new antivirals.
As a part of our ongoing research on antivirals from marine sources, herein we show the characterization of six dolabellane diterpenes from the soft corals Eunicea laciniata and Eunicea asperula, collected at Santa Marta, Colombian Caribbean as well as the evaluation of their antiviral activity assessed as the inhibition of HSV-1 replication and cytotoxic activity.
Materials and methods
General
Optical rotation was measured on a ADP440+, Bellingham +Stanley polarimeter. 1H and 13C-NMR spectra were recorded on a Bruker AVANCE 400 (400 MHz) and Bruker AVANCE (300 MHz) spectrometers as dilute solutions in CDCl3 (δH 7.26 δC 77.0) at room temperature, unless stated otherwise. Chemical shields are quoted in parts per million (ppm) relative to residual solvent peaks and coupling constants are quoted in Hertz. Kovats index determination and mass spectra analyses were performed on an Agilent Technologies 7890B chromatograph equipped with a HP-5MS column (30 m x 0.25 mm x 0.25 µm) and an Agilent 5977A MSD mass detector using electronic impact ionization (70 eV) at 290 °C. Mass detection range was selected between m/z 20 to 800.
For each analysis, 1 µL of a 1 mg/mL solution in acetone was injected in split mode (1:30) at an injector temperature of 260 °C. Helium 5.0 grade was used as carrier gas maintaining a 1 mL/min flux. Temperature program started at 40 °C maintaining this temperature for 1 min, followed by a temperature ramp as follows: 6 °C/min until 100 °C, 4 °C/min until 260 °C, 10 °C/min until 290°C, maintaining at 290 °C for 15 min. Kovats indexes were calculated based on a mixture of paraffins from C10 to C25 (Dr Ehrenstorfer, Augsburg, Germany).
Thin layer chromatography was developed on aluminum plates precoated with silica gel 60G F254 from Merck. Plates were visualized under ultraviolet light (λ = 254 nm) and 5% cerium ammonium sulfate with 10% sulfuric acid in methanol followed by heating. Silica gel (0.043–0.060 mm, Merck), Sephadex LH-20 (GE Healthcare Life Siences), and HyperSep™ Diol cartridges (Thermo Scientific™) were employed for column chromatography separations. Solvents of analytical quality were employed and they were acquired from Merck.
Animal material
The octocorals Eunicea laciniata and Eunicea asperula were collected in reef flats along the coast of Santa Marta, Colombian Caribbean by scuba diving in December, 2011; at an approximate depth of 10 m. The Ministerio de Ambiente, Vivienda y Desarrollo Territorial granted permission for research on marine organisms (Contratos de Acceso a Recurso Genético No 99, 100 and 109). According to the permissions, soft corals were cut carefully and the collected quantities were not enough to cause an ecological impact. Small fragments were cut out of large colonies with sharp scissors, leaving the remaining colony intact. Fresh material was frozen immediately after collection and remained frozen until extraction. Collected organisms were identified by Mónica Puyana and voucher specimens were deposited at the invertebrate collection of ICN (Instituto de Ciencias Naturales, Universidad Nacional de Colombia), coded as ICN-MHN-CR-106 and PO0267, respectively.
Organism extraction and compound isolation
The sample of Eunicea laciniata was cut in small pieces and extracted with dichloromethane (DCM) four times. The extract was filtered and concentrated under vacuum yielding a dark green oil (13.8 g). The extract was separated by flash column chromatography (20 cm x 6 cm) eluting with 500 mL mobile phases of increasing polarity (hexane:toluene 90:10 to 50:50, toluene, toluene:ethyl acetate 50:50, ethyl acetate, ethyl acetate:methanol 50:50, methanol) to yield nine fractions (FL1 to FL9) according to their TLC profile. The fractions were monitored by 1H-NMR looking for signals assignable to methyl groups and double bound protons, which are common for diterpenes. This analysis allowed us identifying fractions FL1 and FL5 as those with a high diterpene content.
FL1 (869 mg, hex:tol 90:10) was a yellow oil. Its 1H-NMR and APT spectra showed it was constituted by the dolabellane β-araneosene (1). Fraction FL5 (7400 mg, tol and hex:tol 50 50) was further purified by column chromatography eluting with hex:EtOAc (100:0 to 50:50) to yield six fractions (FL5.1 to FL5.6). Subfraction FL5.3 corresponded to pure dolabellatrienone (2) (1780 mg). Subfraction FL5.5 (522 mg) was separated through a Diol cartridge with hex:EtOAc mixtures (80:20 to 0:100) to yield seven fractions (FL5.5.1 to FL5.5.7). Subfraction FL5.5.2 was separated using column chromatography to yield eight fractions (FL5.5.2.1 to FL5.5.2.8). Subfraction FL5.5.2.3 (35.4 mg) was finally purified by a Sephadex LH-20 column eluting with MeOH to yield pure compound 3 (18.4 mg). Subfraction FL5.5.2.5 corresponded to pure compound 4 (34.8 mg). Subfraction FL5.5.2.7 was purified by a Sephadex LH-20 column eluting with MeOH to yield pure compound 5 (18.8 mg). Subfraction FL5.6 was fractionated using a Diol cartridge eluting with hex:EtOAc mixtures (70:30 to 0:100) to obtain seven subfractions (FL5.6.1 to FL5.6.7). Subfraction FL5.6.3 (32.7 mg) was separated through silica gel column chromatography eluting with hex:EtOAc (70:30) to obtain pure compound 6 (7.1 mg).
The sample of Eunicea asperula (174 g dry weight) was extracted and fractionated using the same methodology previously explained for E. laciniata. The crude extract (3.2 g) was obtained as a dark green oil. Silica gel flash chromatography yielded nine fractions (FA1 to FA9) based on their TLC profiles. Fraction FA2 (243.9 mg, toluene) was separated through column chromatography eluting with hex:EtOAc (90:10 to 60:40) to obtain pure compound 2 (80 mg). Fraction FA4 (1480 mg, tol:EtOAc 50:50) was fractionated with a DIOL cartridge eluting with hex:EtOAc (90:10 to 50:50) yielding five subfractions (FA4.1 to FA4.5). Fraction FA4.2 corresponded to pure compound 4 (880 mg). Fraction FA4.4 was separated through column chromatography eluting with a gradient of hex:EtOAc (90:10 to 70:30) to obtain pure compound 5 (20.3 mg).
Compound 1: β-araneosene; yellowish oil; -32.0 (c 0.6, CHCl3); GC Retention time 38.88 min (KI 2011); EIMS m/z (relative intensity) 272 [M]+ (6), 257 [M- CH3]+ (4), 243 [M- C2H5]+ (0.2), 229 [M- C3H7]+ (14), 216 (22), 201 (8), 189 (24), 175 (9), 161 (28), 147 (14), 136 (62), 121 (100), 107 (51), 79 (36), 67 (36), 55 (25); 1H-NMR (400 MHz, CDCl3) δH 5.25 (1H, dd, J = 11.0, 4.8 Hz, H-3), 4.90 (1H, d, J = 10.4 Hz, H-7), 1.65 (3H, s, H-17), 1.63 (3H, s, H-19/20), 1.60 (3H, s, H-19/20), 1.45 (3H, s, H-16), 1.13 (3H, s, H-15); 13C-NMR (100 MHz, CDCl3) δ 142.5 (C-12), 134.8 (C-4), 132.5 (C-8), 129.4 (C-7), 126.0 (C-3), 122.0 (C-18), 48.4 (C-1), 42.0 (C-11), 40.3 (C-2), 40.0 (C-9), 38.7 (C-14), 38.2 (C-5), 42.0 (C-11), 28.3 (C-13), 27.9 (C-10), 24.3 (C-6), 23.7 (C-15), 21.7 (C-19), 21.3 (C-20), 16.3 (C-16), 15.3 (C-17). Spectroscopic properties are consistent with those published for the total synthesis of β-araneosene by Corey and Kingsbury ( 26 ).
Compound 2: (1R,3E,7E,11S)-13-keto-dolabella-3,7,12(18)-triene; Yellowish oil; 15.0 (0.9 c, CHCl3); GC Retention time 44.74 min (KI 2272); EIMS m/z (relative intensity) 286 [M]+ (8), 271 [M- CH3]+ (7), 253 [M- CH3- H2O]+ (0.4), 230 (5), 203, (11), 163 (43), 150 (100), 135 (65), 121 (36), 107 (56), 91 (77), 79 (75), 67 (85), 55 (52); 1H-NMR (400 MHz, CDCl3) δH 5.22 (1H, dd, J = 11.1, 4.6 Hz, H-3), 4.91 (1H, d, J = 10.3 Hz, H-7), 2.81 (1H, d, J = 11.6 Hz, H-11), 2.37 (1H, d, J = 18.5 Hz, H-14a), 2.08 (1H, d, J = 18.5 Hz, H-14b), 2.20 (3H, br s, H-20), 1.81 (3H, s, H-19), 1.62 (3H, s, H-17), 1.43 (3H, s, H-16), 1.21 (3H, s, H-15). 13C-NMR (100 MHz, CDCl3) δC 207.2 (C-13), 148.2 (C-18), 138.0 (C-12), 135.7 (C-4), 131.7 (C-8), 130.3 (C-7), 124.8 (C-3), 54.8 (C-14), 41.5 (C-11), 41.0 (C-1), 40.1 (C-2), 39.8 (C-5), 38.1 (C-9), 28.0 (C-10), 24.5 (C-19), 24.2 (C-6), 23.1 (C-15), 21.4 (C-20), 16.1 (C-17), 15.5 (C-16). Spectroscopic properties are consistent with data published for dolabellatrienone obtained by total synthesis ( 27 ).
Compound 3: Colorless oil; 1H-NMR (400 MHz, CDCl3) δH 5.11 (1H, d, J = 11.0 Hz), 2.72 (1H, t, J = 3.4 Hz), 1.70 (3H, s), 1.22 (3H, s), 1.14 (3H, d, J = 7.0 Hz), 0.97 (3H, d, J = 7.0 Hz), 0.88 (3H, s). 13C-NMR (100 MHz, CDCl3) δC 219.2, 135.1, 125.8, 60.4, 60.2, 59.8, 58.6, 52.0, 42.0, 40.3, 38.1, 33.8, 27.9, 26.3, 25.3, 22.8, 21.3, 18.1, 17.4, 16.8.
Compound 4: (1R,3E,7R,8R,11S)-7,8-epoxy-13-keto-dolabella-3,12(18)-diene; White solid; -7.81 (0.58 c, CHCl3); GC Retention time 48.17 min (KI 2439); EIMS m/z (relative intensity) 302 [M]+ (6), 284 [M- H2O]+ (0.9), 259 [M- C3H7]+ (2), 241[M- C3H7- H2O]+ (1), 219 (9), 201 (5), 189 (9), 175 (10), 163 (24), 150 (75), 136 (53), 121 (52), 107 (62), 93 (98), 79 (88), 67 (87), 55 (100); 1H-NMR (400 MHz, CDCl3) δH 5.41 (1H, dd, J = 11.3, 4.6 Hz, H-3), 2.90 (1H, d, J = 8.7 Hz, H-7), 2.67 (1H, d, J = 12.7 Hz, H-11), 2.39 (1H, d, J = 18.6 Hz, H-14a), 2.12 (1H, d, J = 18.6 Hz, H-14b), 2.25 (3H, br d, J = 0.8 Hz, H-20), 1.92 (3H, s, H-19), 1.55 (3H, s, H-16), 1.35 (3H, s, H-17), 1.17 (3H, s, H-15); 13C-NMR (100 MHz, CDCl3) δC 206.8 (C-13), 149.4 (C-18), 137.6 (C-4), 136.1 (C-12), 124.8 (C-3), 65.8 (C-7), 60.6 (C-8), 54.4 (C-14), 42.1 (C-11), 41.1 (C-1), 39.8 (C-2), 38.0 (C-9), 36.9 (C-5), 27.5 (C-6), 25.1 (C-19), 23.4 (C-15), 22.9 (C-10), 21.9 (C-20), 17.7 (C-17), 15.7 (C-16). Spectroscopic data are consistent with those published for a dolabellane isolated previously from Eunicea sp. ( 17 ).
Compound 5: Claenone; colorless oil; -10.66 (1.14 c, CHCl3); GC Retention time 48.23 min (KI 2442); EIMS m/z (relative intensity) 302 [M]+ (3), 269 [M- CH3- H2O]+ (1), 245 (2), 220 (7), 203 (6), 177 (11), 164 (8), 163 (26), 150 (91), 135 (52), 121 (36), 107 (65), 91 (90), 79 (99), 67 (100), 55 (85); 1H-NMR (300 MHz, CDCl3) δH 5.10 (1H, d, J = 10.9 Hz, H-7), 2.97 (1H, dd, J = 11.0, 2.9 Hz, H-3), 2.92 (1H, d, J = 11.6 Hz, H-11), 2.39 (1H, d, J = 18.4 Hz, H-14a), 2.09 (1H, d, J = 18.4 Hz, H-14b), 2.18 (3H, s, H-20), 1.82 (3H, s, H-19), 1.72 (3H, s, H-17), 1.39 (3H, s, H-16), 1.13 (3H, s, H-15); 13C-NMR (75 MHz, CDCl3) δC 206.5 (C-13), 149.0 (C-18), 137.2 (C-12), 133.1 (C-8), 128.4 (C-7), 64.0 (C-3), 61.6 (C-4), 55.6 (C-14), 42.4 (C-11), 40.9 (C-1), 38.6 (C-9), 37.7 (C-2), 37.3 (C-5), 27.4 (C-6), 24.6 (C-15), 24.5 (C-10), 23.4 (C-15), 21.4 (C-20), 16.6 (C-17), 15.5 (C-16). Spectroscopic properties are consistent with those reported for the dolabellane claenone obtained by total synthesis ( 27 ).
Compound 6: (1R,3R,4R,7R,8R,11S)-di-3,4:7,8-epoxy-13-keto-dolabell-12(18)-ene. White solid; -21.14 (1.14 c, CHCl3); GC Retention time 50.61 min (Calculated KI 2561); EIMS m/z (relative intensity) 318 [M]+ (48), 275 [M- C3H7]+ (7), 257 [M- C3H7- H2O]+ (0.6), 233 (1), 217 (10), 203 (8), 189 (13), 175 (14), 161 (22), 149 (100), 135 (73), 121 (62), 109 (69), 93 (76), 79 (63), 67 (51), 55 (72); 1H-NMR (400 MHz, CDCl3) δH 3.05 (1H, dd, J = 11.1, 2.7 Hz, H-3), 2.96 (1H, d, J = 7.8 Hz, H-7), 2.81 (1H, d, J = 12.6 Hz, H-11), 2.44 (1H, d, J = 18.5 Hz, H-14α), 2.24 (3H, s, H-20), 2.13 (1H, d, J = 18.6 Hz, H-14β), 1.94 (3H, s, H-19), 1.43 (3H, s, H-16), 1.33 (3H, s, H-17), 1.21 (3H, s, H-15); 13C-NMR (100 MHz, CDCl3) δC 205.5 (C-13), 149.6 (C-18), 137.0 (C-12), 63.7(C-7), 63.0 (C-3), 60.6 (C-8), 60.3 (C-4), 54.7 (C-14), 42.9 (C-11), 40.2 (C-2), 37.8 (C-1), 37.2 (C-5), 36.6 (C-9), 27.8 (C-10), 25.2 (C-19), 23.6 (C-15), 23.3 (C-6), 21.6 (C-20), 17.5 (C-17), 15.6 (C-16). NMR data are identical to a dolabellane from Eunicea sp. previously reported ( 17 ).
Cell cultures and viral strains
African green monkey kidney cells (Vero cells) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco™) containing 4.5 g/L glucose, 2 g/L sodium bicarbonate, 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin (Gibco™) and 2.5 µg/mL anphotericine B supplemented with fetal bovine serum (FBS, Sigma). Vero cells were maintained at 37 °C under 5% CO2 atmosphere. In order to prepare virus stocks, Vero cells were infected with HSV-1 susceptible to Acyclovir® (KOS strain) at a multiplicity of infection (MOI) of 0.1 (28). After 24 hours of postinfection (p.i.), cells were lysed by three cycles of freezing and thawing, centrifuged at 400 x g at 4 °C for 20 min. The viral titration was made by the plaque reduction assay ( 29 ).
Evaluation of antiviral and cytotoxic activity
Monolayers of Vero cells placed in 24-well plates (2 x 105 per well) were infected with HSV-1 at MOI of 1 for 1 h at 37 °C under 5% CO2 atmosphere. After this period, the cells were treated with different concentrations of the compounds. After incubation for 24 h, cells were lysed by three cycles of freezing and thawing for viral titration. Cytotoxicity was evaluated by the MTT method and the results were analyzed comparing the absorbance of treated wells with that of non-treated controls ( 30 ).
Results and discussion
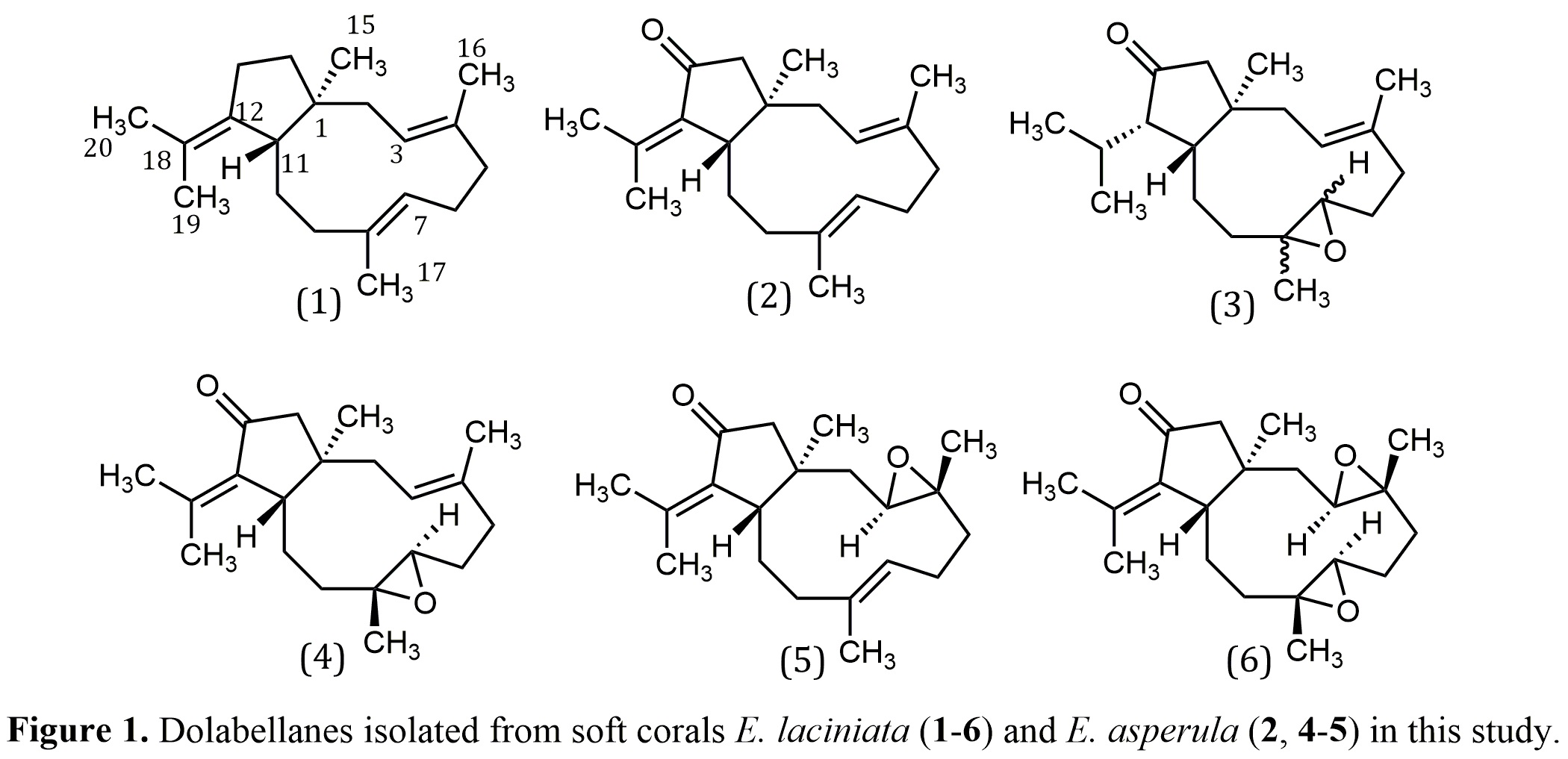
To select the most promising soft corals in terms of dolabellane content, we previously ran TLC plates of crude extracts obtained from a large collection of soft corals, available in our group, collected at various sites of the Colombian Caribbean. Based on TLC profiles, the most promising crude extracts were fractionated through a SPE Diol cartridge and fractions were further analyzed by gas chromatography electronic impact mass spectrometry (GC-EIMS). GCMS analyses showed a major chromatographic peak (RT: 44.7 min, KI: 2271 and m/z: 286) in two of the extracts. These signals were attributed to the previously known compound dolabellatrienone (2) due to the high degree of coincidence in the Kovats index and EIMS spectra. The promising extracts were obtained from the soft corals Eunicea laciniata and E. asperula from Santa Marta. Therefore, extracts from both organisms were submitted to several steps of purification by column chromatography yielding six compounds in total that are shown in Figure 1
Compound 1 was isolated as a yellowish oil and was the major component in fraction FL1 obtained from E. laciniata. GC-MS analysis indicated that compound 1 was pure enough and was identified without further purification steps. The analysis showed a major peak with a molecular ion at m/z = 272 corresponding to [M]+ and consistent with a C20H32 molecular formula. Compound 1 1H-NMR spectrum had signals in δH 5.25 (1H, dd, J = 11.0, 4.8 Hz, H-3) and 4.90 (1H, br d, J = 10.4 Hz, H-7) corresponding to olefinic protons and five additional signals were observed in δH 1.65 (3H, s, H-17), 1.63 (3H, s, H-19), 1.60 (3H, s, H-20), 1.45 (3H, s, H-16) and 1.13 (3H, s, H-15) corresponding to methyl groups.
On the other hand, the APT spectrum of 1 showed twenty signals, which were assigned as five methyls, seven methylenes (all sp3), three methines (one sp3 and two sp2), and five quaternary carbons (one sp3 and four sp2). All this information suggested that compound 1 was a dolabellane diterpene with two trisubstituted and one tetrasubstituted double bonds. Comparison of spectral data were consistent with those reported for the known dolabellane β-araneosene obtained by total synthesis ( 26 ) and allowed us to identify compound 1. The stereochemistry at the ring fusion was defined as 1S, 11R based on the similar value of optical rotation comparable to that obtained by total synthesis.
Compound 2 was isolated as a yellow oil. 1H-NMR and APT spectra were quite similar to those obtained for compound 1. However, minimal differences suggested the presence of additional functional groups. 1H-NMR spectrum had five methyl signals but two of them were in δH 2.20 (3H, br s, H-20) and 1.81 (3H, s, H-19) while the APT spectrum showed a signal in δC 207.2 (C-13) together with a deshielded methylene in δC 54.8 (CH2-14). These observations were consistent with the presence of a conjugated ketone carbonyl and justified the observed signals. The spectroscopic properties and experimental optical rotation ( +15.0 (c 0.9 CHCl3)) were consistent with those reported in dolabellatrienone (2) ( +32.0 (c 0.14 CHCl3)), a dolabellane previously isolated from several Eunicea species and also obtained by total synthesis ( 27 ).
Compound 3 was isolated as a colorless oil. 1H-NMR signals in δH 5.11 (1H, d, J = 9.1 Hz) and 2.72 (1H, t, J =3.4 Hz) corresponded with an olefinic proton and an oxygenated methine, respectively. Additionally, five methyl signals were also identified in δH 1.70 (3H, s), 1.22 (3H, s), 1.14 (1H, d, J = 7.0 Hz), 0.97 (1H, d, J = 7.0 Hz), 0.88 (3H, s). The APT spectrum showed signals for five methyls (δC 16.8, 17.4, 18.1, 21.3 and 22.8), six methylenes, (25.3, 26.3, 33.8, 38.1, 40.3, 58.6), five methines, one olefinic, one oxygenated (δC 27.9, 42.0, 60.2, 60.4 and 125.8), and four quaternary carbons, one oxygenated (δC 59.8), one olefinic (δC 135.1), and a ketone carbonyl (δC 219.2). We inferred that compound 3 was a dolabellane diterpene with a trisubstituted double bond and an epoxide group, and the structural elucidation process was conducted by 2D-NMR spectroscopy.
However, compound 3 decomposed during the experiments suggesting an unexpected lability and a low probability of application as an antiviral compound. Despite this, we proposed a planar structure for compound 3 based on information deduced from 1D-NMR spectra and comparison with other dolabellanes. We observed two doublet methyls with chemical shifts and coupling constants quite similar to a previous reported dolabellane without a double bound between positions C-12 and C-18 ( 31 ). Moreover, signals at δC 219.2 and 60.2 indicated the presence of a ketone with and an alfa methine, and they were also consistent with those reported in the known compound. The position of the double bond position was deduced taking advantage of the high degree of conservation in the chemical shifts for double bonds in dolabellanes. We have observed in many dolabellanes that the chemical shift for carbon atoms in a double bond between C-3 and C-4 differs from that showed by a double bond between C-7 and C-8 (17, 31). Compound 3 had the expected signals for a double bond between C-3 and C-4 while the epoxide group was located between C-7 and C-8. Figure 2 shows the planar structure of compound 3 and some related structures that were useful to our elucidation purposes.

Figure 2
Compound 4 was isolated as a white solid. The EIMS spectrum showed a molecular ion corresponding to [M]+ in m/z = 302. 1H and 13C-NMR (100 MHz, CDCl3) spectra showed similarities with dolabellatrienone (2) but there were differences that indicated the presence of an additional functional group in 4. It was observed one signal at δH 2.90 (1H, br d, J = 8.7 Hz, H-7) together with signals at δC 65.8 (CH-7) and 60.6 (C-8) which corresponded to an epoxide functionality. As previously explained, the double bond was located between C-3 and C-4 due to the observed chemical shifts for the trisubstituted double bond in δC 124.8 (CH-3) and 137.6 (C-4). The experimental optical rotation and spectroscopic properties were consistent with those reported for the compound (1R,3E,7R,8R,11S)-7,8-epoxy-13-keto-dolabella-3,12(18)-diene and allowed us identifying compound 4 ( 32 ).
Compound 5 exhibited 1H-NMR, APT, and EIMS spectral data almost identical to compound 4, with minimal differences that indicated they were structural isomers. The observed signals at δH 2.97 (1H, dd, J = 11.0, 2.9 Hz, H-3), δC 64.0 (CH-3), 61.6 (C-4) allowed confirming the presence of an epoxide group while signals at δC 133.1 (C-8) and 128.4 (CH-7) located the double bond between C-7 and C-8. Compound 5 was identified by comparison with the spectroscopic properties and optical rotation values reported in the total synthesis of the dolabellane claenone ( 27 ).
Compound 6 showed an EIMS with a molecular ion at m/z = 318, which was consistent with a C20H30O3 molecular formula. The 1H-NMR spectrum showed signals at 3.05 (1H, dd, J = 11.1, 2.7 Hz, H-3) and 2.96 (1H, d, J = 7.8 Hz, H-7) while the APT spectrum showed four signals for oxygenated carbons at δC 63.7 (C-7), 63.0 (CH-3), 60.6 (CH-8) and 60.3 (C-4). This information confirmed the presence of two epoxide groups and indicated that compound 6 was a diepoxide analogue of 4 and 5. The remaining signals and experimental optical rotation allowed us to identify compound 6 as (1R,3R,4R,7R,8R,11S)-di-3,4:7,8-epoxy-13-keto-dolabell-12(18)-ene, a dolabellane previously isolated from an unidentified Eunicea species and whose absolute stereochemistry was determined previously ( 17 ).
Compounds 1-6 were isolated from Eunicea laciniata whereas only compounds 2, 4, and 5 were isolated from E. asperula, where 4 was the major compound, and 2 and 5 were minor components. Previous works on the chemistry of this particular species, showed that E. asperula collected in Tobago yielded cembrane and asperketal diterpenes ( 33 ). Although variations in chemical profiles is not unusual between organisms collected at different places, the diterpene core usually remains the same ( 34 , 35 ). Interestingly, our sample yielded dolabellane diterpenes suggesting it might be a different chemotype, assuming, in both studies, that taxonomic identity is correct.
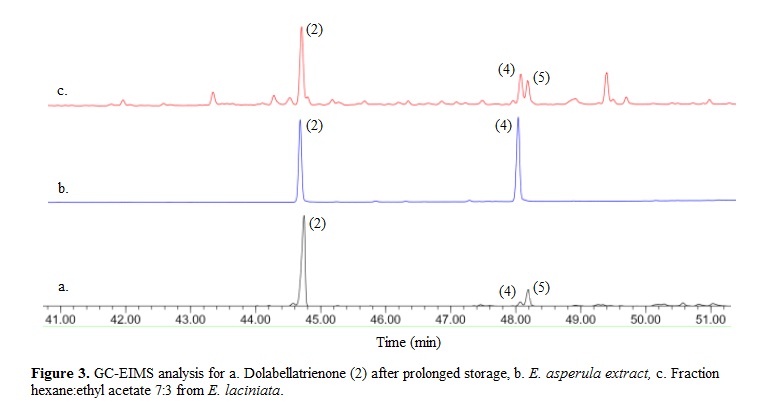
Previous studies with E. laciniata have shown the high dolabellane content of this species and the considerable number of compounds with variable oxygenation grade. Samples collected in Puerto Rico, Bahamas, Tobago, Honduras, and Colombia have reported the isolation of dolabellanes β-araneosene (1) and dolabellatrienone (2) ( 14 , 24 ). In this work we report the isolation of compounds 3-6 from E. laciniata which were not previously described from this species. Nevertheless, it was found that following purification, compound 2 experiments an oxidation process to yield a mixture of compounds 4 and 5. Figure 3a shows a GC-EIMS analysis for compound 2 (initially pure) and after several weeks of storage. The chromatogram shows a major peak corresponding to compound 2 and two minor peaks with Kovats index and EIMS coincident with 4 and 5.

Figure 3
Interestingly, compound 5 has been reported as a natural product only from a Clavularia soft coral collected in Japan ( 36 ). Considering this, the isolation of compounds 4 and 5 in this study suggested that these could be artifacts produced during storage of the extract and fractions. Alternatively, the presence of those compounds in a particular sample could reflect a different chemotype. In order to establish if compounds 4 and 5 were storage artifacts, a new GC-EIMS analysis was conducted after extraction and fractionation of a fresh specimen. As an additional precaution, fractions were kept under argon atmosphere and frozen at temperature below zero, dissolving them just before the chromatographic analysis. In E. laciniata, compounds 2, 4 and 5 were identified in the fraction that eluted with hex:EtOAc 70:30 (Figure 3c). Compound 6 was not found in this fraction nor in those of greater polarity. We conclude that although minimal quantities of compounds 4 and 5 are present in the extracts from E. laciniata, the absence of compound 6 suggests that both monoepoxides are mainly oxidation products of (2) in this soft coral. On the other hand, compound 2 and the major compound 4 were found in E. asperula but compound 5 was not (Figure 3b), suggesting that compound 5 is the only oxidation product of (2) in E. asperula.
Vero cells have been used to evaluate anti-HSV-1 activity of a wide range of natural products, including dolastane and dolabellane diterpenes ( 20 ). Cytotoxic activity in Vero cells was determined for compounds 2, 4, 5, 6 with Acyclovir® as a positive control. We did not test compound 1 since it was not pure enough to run the assays. Compounds 2 (CC50 = 146 µM) and 5 (CC50 = 467 µM) showed a greater cytotoxic effect as compared to Acyclovir® (CC50 = 960 µM) while compounds 4 (CC50 = 526 µM) and 6 (CC50 = 959 µM) had comparable values.
An important feature for any antiviral compound is that it must not be toxic and must be selective to infected cells. For this reason, we decided to evaluate antiviral activity only for compounds 4 and 6 in an HSV-1 inhibition assay using concentration of 50 µM for each compound. Compound 4 showed a 42.6% of inhibition while compound 6 exhibited a 73.7% of inhibition. Considering that 6 also has a remarkable in vitro anti-HIV-1 activity ( 25 ), it is a potential candidate to perform further studies to assess its full potential as an antiviral agent as well as understanding its mechanism of action against viruses including HSV-1.
Conclusions
Six dolabellane diterpenes from the soft corals Eunicea laciniata and E. asperula, collected in Santa Marta, Colombian Caribbean Sea, were isolated in this work. Compounds 1, 2, 4-6 were identified by spectroscopic means and compared with previously reported dolabellanes. Compound 3 was not fully characterized due to its decomposition, suggesting it had a high degree of reactivity. Dolabellatrienone (2) and (1R,3E,7R,8R,11S)-7,8-epoxy-13-keto-dolabella-3,12(18)-diene (4) were the major compounds isolated from E. laciniata and E. asperula, respectively. To the best of our knowledge, this is the first study of E. asperula collected in the Colombian Caribbean and dolabellanes were found as the major diterpenes. If is considered that variations in the family of isolated diterpenes do not depend on location, either this work studied a different chemotype or there were problems with taxonomical characterization. However, further studies should be developed in order to confirm this hypothesis.
GC-EIMS analyses proved that prolonged storage of pure dolabellatrienone (2) induces a spontaneous oxidation to yield compounds 4 and 5. This finding evidences a need to improve the manipulation and storage conditions of extracts and fractions obtained from those organisms in order to minimize the artifact formation and increase the reproducibility of chemical studies.
Biological assays showed that compound 6 inhibited HSV-1 replication at a concentration of 50 µM and was not cytotoxic. Although the remaining compounds were more toxic than Acyclovir®, their antiviral activity was determined to calculate selectivity indexes and establish their potential as antivirals. These results encourage us to continue the study of dolabellanes of marine origin and evaluating their antiviral activity.
Aknowledgments
Financial support was provided by Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación Francisco José de Caldas through the “Programa jóvenes investigadores” as well as the DIB–Universidad Nacional de Colombia and Fundación para la promoción de la investigación y la tecnología (FPIT) Banco de la Republica. Maria Leonisa Sanchez Nuñez, Izabel Christina Nunes de Palmer Paixão and Valeria Laneuville Teixeira are grateful to CNPq (Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq) and FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro) for financial support. The Ministerio de Ambiente, Vivienda y Desarrollo Territorial granted permission for research on marine organisms (Contratos de Acceso a Recurso Genético No 99, 100 and 109).
Marine natural products.
1. Blunt, J. W.; Copp, B. R.; Keyzers, R. A.; Munro, M. H. G.; Prinsep, M. R.; Faulkner, D. J. Marine natural products. Nat. Prod. Rep. 2016, 33 (3), 382–431. DOI: https://doi.org/10.1039/C5NP00156K.
2. Zhou, X.; Liu, J.; Yang, X. W.; Liu, Y. Marine Natural Products with Anti-HIV Activities in the Last Decade. Curr. Med. Chem. 2013, 20 (7), 953-973. DOI: https://doi.org/10.2174/0929867311320070009.
3. De Clercq, E. Antivirals: past, present and future. Biochem. Pharmacol. 2013, 85 (6), 727-744. DOI: https://doi.org/10.1016/j.bcp.2012.12.011.
4. Looney, D.; Ma, A.; Johns, S. HIV therapy-the state of art. Curr. Top. Microbiol. Immunol. 2015, 389, 1-29. DOI: https://doi.org/10.1007/82_2015_440.
5. Kinch, M. S.; Patridge, E. An analysis of FDA-approved drugs for infectious disease: HIV/AIDS drugs. Drug. Discov. Today. 2014, 19 (10), 1510-1513. DOI: https://doi.org/10.1016/j.drudis.2014.05.012.
6. James, S. H.; Prichard, M. N. Current and future therapies for herpes simplex virus infections: mechanism of action and drug resistance. Curr. Opin. Virol. 2014, 8, 54-61. DOI: https://doi.org/10.1016/j.coviro.2014.06.003.
7. Hawkins, T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Res. 2010, 85 (1), 201-209. DOI: https://doi.org/10.1016/j.antiviral.2009.10.016.
8. Margolis, A. M.; Heverling, H.; Pham, P. A.; Stolbach, A. A review of the toxicity of HIV medications. J. Med. Toxicol. 2014, 10 (1), 26-39. DOI: https://doi.org/10.1007/s13181-013-0325-8.
9. Yodsaoue, O.; Sonprasit, J.; Karalai, C.; Ponglimanont, C.; Tewtrakul, S.; Chantrapromma, S. Diterpenoids and triterpenoids with potential anti-inflammatory activity from the leaves of Aglaia odorata. Phytochemistry 2012, 76, 83-91. DOI: https://doi.org/10.1016/j.phytochem.2012.01.015.
10. Aboushoer, M. I.; Fathy, H. M.; Abdel-Kader, M. S.; Goetz, G.; Omar, A. A. Terpenes and flavonoids from an Egyptian collection of Cleome droserifolia. Nat. Prod. Res. 2010, 24 (7), 687-696. DOI: https://doi.org/10.1080/14786410903292433.
11. Cai, X. H.; Wang, Y. Y.; Zhao, P. J.; Li, Y.; Luo, X. D. Dolabellane diterpenoids from Aglaia odorata. Phytochemistry 2010, 71 (8-9), 1020-1024. DOI: https://doi.org/10.1016/j.phytochem.2010.03.005.
12. Yoshimitsu, H.; Miyashita, H.; Nishida, M.; Mineno, T.; Nohara, T. Dolabellane Diterpene and Three Cycloartane Glycosides from Thalictrum squarrosum. Chem. Pharm. Bull. 2010, 58 (8), 1043-1046. DOI: https://doi.org/10.1248/cpb.58.1043.
13. Hinkley, S. F.; Mazzola, E. P.; Fettinger, J. C.; Lam, Y.-F.; Jarvis, B. B. Atranones A–G, from the toxigenic mold Stachybotrys chartarum. Phytochemistry 2000, 55 (6), 663-673. DOI: https://doi.org/10.1016/S0031-9422(00)00231-4.
14. Rodríguez, A. D.; González, E.; Ramírez, C. The structural chemistry, reactivity, and total synthesis of dolabellane diterpenes. Tetrahedron, 1998, 54 (39), 11683-11729. DOI: https://doi.org/10.1016/S0040-4020(98)83033-0.
15. Wang, S.-K.; Huang, M.-J.; Duh, C.-Y. Cytotoxic constituents from the formosan soft coral Clavularia inflata var. luzoniana. J. Nat. Prod. 2006, 69 (10), 1411-1416. DOI: https://doi.org/10.1021/np0601253.
16. Duh, C.-Y.; Chia, M.-C.; Wang, S.-K.; Chen, H.-J.; El-Gamal, A. A. H.; Dai, C.-F. Cytotoxic dolabellane diterpenes from the Formosan soft coral Clavularia inflata. J. Nat. Prod. 2001, 64 (8), 1028-1031. DOI: https://doi.org/10.1021/np010106n.
17. Wei, X.; Rodríguez, A. D.; Baran, P.; Raptis, R. G. Dolabellane-Type Diterpenoids with Antiprotozoan Activity from a Southwestern Caribbean Gorgonian Octocoral of the Genus Eunicea. J. Nat. Prod. 2010, 73 (5), 925-934. DOI: https://doi.org/10.1021/np100074r.
18. Barbosa, J. P.; Pereira, R. C.; Abrantes, J. L.; Cirne dos Santos, C.C.; Rebello, M. A.; Frugulhetti, I. C. et al. In vitro Antiviral Diterpenes from the Brazilian Brown Alga Dictyota pfaffii. Planta Med. 2004, 70 (09), 856-860. DOI: https://doi.org/10.1055/s-2004-827235
19. Pardo-Vargas, A.; de Barcelos Oliveira, I.; Stephens, P.R.S.; Cirne-Santos, C.C.; de Palmer Paixão, I.C.N.; Ramos, F.A. et al. Dolabelladienols A–C, New Diterpenes Isolated from Brazilian Brown Alga Dictyota pfaffii. Mar. Drugs, 2014, 12, 4247-4259. DOI: https://doi.org/10.3390/md12074247.
20. Abrantes, J. L.; Barbosa, J.; Cavalcanti, D.; Pereira, R. C.; Frederico Fontes, C. L.; Teixeira, V. L. et al. The effects of the diterpenes isolated from the Brazilian brown algae Dictyota pfaffii and Dictyota menstrualis against the herpes simplex type-1 replicative cycle. Planta Med. 2010, 76 (4), 339-44. DOI: https://doi.org/10.1055/s-0029-1186144.
21. Cirne-Santos, C. C.; Souza, T. M.; Teixeira, V. L.; Fontes, C. F.; Rebello, M. A.; Castello-Branco, L. R. et al. The dolabellane diterpene Dolabelladienetriol is a typical noncompetitive inhibitor of HIV-1 reverse transcriptase enzyme. Antiviral Res. 2008, 77 (1), 64-71. DOI: https://doi.org/10.1016/j.antiviral.2007.08.006.
22. Coll, J. C. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 1992, 92 (4), 613–631. DOI: https://doi.org/10.1021/cr00012a006.
23. Sánchez, J. A. Systematics of the candelabrum gorgonian corals (Eunicea Lamouroux; Plexauridae; Octocorallia; Cnidaria). Zool. J. Linn. Soc. 2009, 157 (2), 237-263. DOI: https://doi.org/10.1111/j.1096-3642.2008.00515.x.
24. Cuadrado, C.; Castellanos, L.; Osorno, O. E.; Ramos, F. A.; Duque, C.; Puyana, M. Estudio Químico y evaluación de la actividad antifouling del octocoral caribeño Eunicea laciniata. Quim. Nova. 2010, 33 (3), 656-661. DOI: https://doi.org/10.1590/S0100-40422010000300033.
25. Pardo-Vargas, A.; Ramos, F. A.; Cirne-Santos, C. C.; Stephens, P. R.; Paixão, I. C. P.; Teixeira, V. L. et al. Semi-synthesis of oxygenated dolabellane diterpenes with highly in vitro anti-HIV-1 activity. Bioorg. Med. Chem. Lett., 2014, 24 (18), 4381-4383. DOI: https://doi.org/10.1016/j.bmcl.2014.08.019.
26. Kingsbury, J. S.; Corey, E. J. Enantioselective Total Synthesis of Isoedunol and β-Araneosene Featuring Unconventional Strategy and Methodology. J. Am. Chem. Soc. 2005, 127 (40), 13813-13815. DOI: https://doi.org/10.1021/ja055137+.
27. Miyaoka, H.; Isaji, Y.; Mitome, H.; Yamada, Y. Total synthesis of the dolabellane marine diterpenoids, claenone, palominol and dolabellatrienone. Tetrahedron, 2003, 59 (1), 61-75. DOI: https://doi.org/10.1016/S0040-4020(02)01474-6.
28. Esquenazi, D.; Wigg, M. D.; Miranda, M. M. F. S.; Rodrigues, H. M.; Tostes, J. B. F.; Rozental, S. et al. Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn. (Palmae) husk fiber extract. Res. Microbiol. 2002, 153 (10), 647-652. DOI: https://doi.org/10.1016/S0923-2508(02)01377-3.
29. Kuo, Y.-C.; Chen, C.-C.; Tsai, W.-J.; Ho, Y.-H. Regulation of herpes simplex virus type 1 replication in Vero cells by Psychotria serpens: relationship to gene expression, DNA replication, and protein synthesis. Antiviral Res. 2001, 51 (2), 95-109. DOI: https://doi.org/10.1016/S0166-3542(01)00141-3
30. Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65 (1), 55-63. DOI: https://doi.org/10.1016/0022-1759(83)90303-4
31. Shin, J.; Fenical, W. Structures and reactivities of new dolabellane diterpenoids from the Caribbean gorgonian Eunicea laciniata. J. Org. Chem. 1991, 56 (10), 3392-3398. DOI: https://doi.org/10.1021/jo00010a039.
32. Look, S. A.; Fenical, W., New bicyclic diterpenoids from the Caribbean gorgonian octocoral Eunicea calyculata. J. Org. Chem. 1982, 47 (21), 4129-4134. DOI: https://doi.org/10.1021/jo00142a024.
33. Shin, J.; Fenical, W. Asperketals A-F, new diterpenoids of the dilophol class from the Caribbean gorgonian Eunicea asperula. J. Org. Chem. 1988, 53 (14), 3271-3276. DOI: https://doi.org/10.1021/jo00249a024.
34. Puyana, M.; Narvaez, G.; Paz, A.; Osorno, O.; Duque, C. Pseudopterosin Content Variability of the Purple Sea Whip Pseudopterogorgia elisabethae at the Islands of San Andres and Providencia (Sw Caribbean). J. Chem. Ecol. 2004, 30 (6), 1183-1201. DOI: https://doi.org/10.1023/B:JOEC.0000030271.73629.26.
35. Reina, E.; Puentes, C.; Rojas, J.; Garcia, J.; Ramos, F. A.; Castellanos, L. et al. Fuscoside E: a strong anti-inflammatory diterpene from Caribbean octocoral Eunicea fusca. Bioorg. Med. Chem. Lett. 2011, 21 (19), 5888-91. DOI: https://doi.org/10.1016/j.bmcl.2011.07.092.
36. Mori, K.; Iguchi, K.; Yamada, N.; Yamada, Y.; Inouye, Y. Bioactive marine diterpenoids from Japanese soft coral of Clavularia sp. Chem. Pharm. Bull. 1988, 36 (8), 2840-2852. DOI: https://doi.org/10.1248/cpb.36.2840.
Recibido: 1 de julio de 2016; Aceptado: 4 de octubre de 2016
Abstract
Dolabellane diterpenes have considerable antiviral activity, most studies have been focused towards compounds isolated from Dictyota brown algae. Although soft corals are also a significant source of these diterpenes, their antiviral potential has not been studied in detail. With the aim of assessing the biological activity of marine sources, we evaluated the dolabellane content in the soft corals Eunicea laciniata and E. asperula collected in Santa Marta, Colombian Caribbean. Dolabellanes 1-6 were isolated from E. laciniata while compounds 2, 4 and 5 were isolated from E. asperula. All compounds were identified by NMR, GC-EIMS, optical rotation and comparison with previously reported dolabellanes. GC-EIMS analyses showed that dolabellatrienone (2) transforms into compounds 4 and 5 as oxidation products upon prolonged storage; however, those compounds were also naturally present in the extract of the studied organisms. Pure dolabellanes were tested in vitro in antiviral assays against HSV-1. Compound 6 inhibited virus replication in infected cells (73.7% of inhibition at 50 µM) without cytotoxic effect (CC50 = 959), showing similar activity to the positive control Acyclovir®. Thus, compound 6 is an interesting candidate for further studies of dolabellanes as antivirals.
Keywords
Soft corals, Eunicea, dolabellane diterpenes, antivirals.Resumen
Los dolabellanos son diterpenos con importante actividad antiviral, la mayor parte de los estudios se han realizado con compuestos aislados de algas pardas del género Dictyota. Los corales blandos son también una importante fuente de dolabellanos, pero el potencial antiviral de éstos ha sido muy poco estudiado. Como parte de nuestra búsqueda de compuestos bioactivos a partir de fuentes marinas, se llevó a cabo el estudio químico de los dolabellanos presentes en los octocorales Eunicea laciniata y Eunicea asperula, recolectados en Santa Marta, Caribe colombiano. Los dolabellanos 1-6 fueron aislados del octocoral E. laciniata mientras que en E. asperula se encontraron los compuestos 2, 4 y 5. La elucidación estructural se llevó a cabo mediante experimentos de RMN, espectrometría de masas, rotación óptica y posterior comparación con reportes previos en la literatura. El análisis por CG-EM evidenció que la dolabellatrienona (2) se puede transformar en los compuestos 4 y 5 como producto del almacenamiento prolongado, no obstante, tales compuestos también estuvieron presentes en los extractos de los organismos estudiados. El compuesto 6 inhibió la replicación del VHS-1 (73,7% de inhibición en células infectadas a una concentración de 50 µM) sin efecto citotóxico (CC50 = 959), mostrando una citotoxicidad similar al Aciclovir®, un control positivo, por lo cual se perfila como un candidato para la realización de estudios adicionales sobre el potencial de los dolabellanos como agentes antivirales.
Palabras clave
octocorales, Eunicea, dolabellanos, antivirales.Resumo
Os dolabellanos são diterpenos que têm mostrado uma importante atividade antiviral, os estudos neste campo estam centrados nos compostos isolados de algas do gênero Dictyota. Por outro lado, a atividade antiviral dos dolabellanos isolados de octocorais não tem sido estudada, apesar de ser a segunda principal fonte natural desses diterpenos. Dessa maneira, como parte de nossa investigação de compostos bioativos a partir de fontes marinhas, foi realizado o estudo químico dos octocorais Eunicea laciniata e Eunicea asperula, coletados em Santa Marta, Caribe Colombiano. O estudo químico dos dois organismos permitiu o isolamento dos dolabellanos 1-6 de E. laciniata, enquanto que para E. aspérula foram identificados os compostos 2, 4 e 5. A elucidação estrutural foi realizada mediante experimentos de RMN, espectrometria de massas, rotação óptica e posterior comparação com os dados da literatura. A análise por GC-MS evidenciou que a dolabelatrienona (2) pode gerar os compostos 4 e 5 como produto de degradação, a partir de um armazenamento prolongado. No entanto, os compostos também estavam presentes nos extratos dos organismos estudados. O composto 6 mostrou uma citotoxicidade similar ao Aciclovir®, um controle positivo, numa porcentagem de inibição da replicação do HVS-1 (73,7% de inibição em células infectadas na concentração de 50 µM) sem efeito citotóxico (CC50 = 959), o que torna esse composto um potencial candidato para o desenvolvimento de antivirais.
Palavras-chave
octocorais, Eunicea, dolabellanos, antivirais.Introduction
Marine organisms are a prolific source of structurally diverse and bioactive compounds ( 1 ). Interestingly, during the 2002-2011 period, 132 new compounds of marine origin with anti-HIV activity on different stages of the replication cycle were reported ( 2 ). Viral diseases are hard to treat and vaccine development against these diseases is still a major challenge and continues to elude the majority of drug discovery programs. Highly active antiretroviral therapy (HAART) is considered the best alternative of treatment against viral infectious diseases ( 3 , 4 ). HAART´s main components for treating AIDS for instance, are nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs), although new targets are constantly explored ( 5 ).
This kind of therapy has turned AIDS from being a mortal disease to becoming a treatable chronic condition, improving life quality and expectation. On the other side, Acyclovir® and its related compounds constitutes the first alternative for treating genital and oral herpes infections caused by Herpes Simplex Viruses 1 and 2 due their unsurpassed capacity to attenuate the symptoms and manifestations of these infections ( 6 ). As mentioned earlier, although HAART is not a definite cure for viral infections, it allows management of viral diseases and improves the life quality of patients and is widely used for treating AIDS. However, the emergence of resistant strains and the various side effects reported for antivirals used in HAART together with lack of efficacy of some of these substances, constitute a major challenge to overcome in order to improve this therapy ( 7 , 8 ).
Dolabellanes are diterpenes that have been isolated from natural sources such as plants ( 9 - - 12 ), fungi ( 13 ), and several marine organisms, mainly brown algae, cnidarians, and sponges ( 14 ). Structurally, they are produced by the ring formation between positions 1,11 and 10,14 in the geranyl-geraniol pyrophosphate to constitute a bicyclic [9.3.0] fused ring. Diterpenes usually exhibit a very interesting range of biological activity including antitumoral ( 14 ), cytotoxic ( 15 , 16 ) antiparasitary ( 17 ), besides a remarkable antiviral activity. Different dolabellanes with antiviral properties have been isolated from brown algae from the genus Dictyota ( 18 , 19 ). Dolabellanetriol isolated from Dictyota friabilis (as D. pfaffii) is the most studied so far, due to its potent inhibition of the human immunodeficiency virus (HIV-1) and the herpes simplex virus (HSV-1) ( 18 , 20 ). Studies concerning the mechanism of action of dolabellanetriol concluded that the molecule acts as a non-competitive HIV-1 reverse transcriptase inhibitor and classifies it into the non-nucleoside reverse transcriptase inhibitors category (NNRTIs) ( 21 ). However, despite the significant evidence on the antiviral activity of dolabellane diterpenes, very little has been reported on the antiviral properties of dolabellanes isolated from marine sources other than brown algae.
Brown algae are an important source of dolabellane diterpenes of marine origin ( 14 ), but soft corals also constitute a prolific source of such compounds, and in several cases significant ecological roles have been attributed to those ( 22 ). Most dolabellane diterpenes have been isolated from the Alcyonacean genus Clavularia and the Octocorallian genus Eunicea ( 14 ). The Colombian Caribbean harbors are large and possess diverse communities of Eunicea soft corals ( 23 ). In some of our previous studies, the soft coral Eunicea laciniata collected in Santa Marta, yielded the dolabellane diterpenes β-araneosene (1) and dolabellatrienone (2) as major compounds in the organic extract ( 24 ). Recently, we reported the anti-HIV activity of those compounds and some semisynthetic derivatives ( 25 ). Natural compounds showed low antiviral activity and they did not have considerable cytotoxic activity. On the other hand, semisynthetic derivatives were 100 times more active compared with their precursors inhibiting HIV-1 replication without a considerable increase in their cytotoxicity. Thus, dolabellane diterpenes are very interesting candidates for the development of new antivirals.
As a part of our ongoing research on antivirals from marine sources, herein we show the characterization of six dolabellane diterpenes from the soft corals Eunicea laciniata and Eunicea asperula, collected at Santa Marta, Colombian Caribbean as well as the evaluation of their antiviral activity assessed as the inhibition of HSV-1 replication and cytotoxic activity.
Materials and methods
General
Optical rotation was measured on a ADP440+, Bellingham +Stanley polarimeter. 1H and 13C-NMR spectra were recorded on a Bruker AVANCE 400 (400 MHz) and Bruker AVANCE (300 MHz) spectrometers as dilute solutions in CDCl3 (δH 7.26 δC 77.0) at room temperature, unless stated otherwise. Chemical shields are quoted in parts per million (ppm) relative to residual solvent peaks and coupling constants are quoted in Hertz. Kovats index determination and mass spectra analyses were performed on an Agilent Technologies 7890B chromatograph equipped with a HP-5MS column (30 m x 0.25 mm x 0.25 µm) and an Agilent 5977A MSD mass detector using electronic impact ionization (70 eV) at 290 °C. Mass detection range was selected between m/z 20 to 800.
For each analysis, 1 µL of a 1 mg/mL solution in acetone was injected in split mode (1:30) at an injector temperature of 260 °C. Helium 5.0 grade was used as carrier gas maintaining a 1 mL/min flux. Temperature program started at 40 °C maintaining this temperature for 1 min, followed by a temperature ramp as follows: 6 °C/min until 100 °C, 4 °C/min until 260 °C, 10 °C/min until 290°C, maintaining at 290 °C for 15 min. Kovats indexes were calculated based on a mixture of paraffins from C10 to C25 (Dr Ehrenstorfer, Augsburg, Germany).
Thin layer chromatography was developed on aluminum plates precoated with silica gel 60G F254 from Merck. Plates were visualized under ultraviolet light (λ = 254 nm) and 5% cerium ammonium sulfate with 10% sulfuric acid in methanol followed by heating. Silica gel (0.043–0.060 mm, Merck), Sephadex LH-20 (GE Healthcare Life Siences), and HyperSep™ Diol cartridges (Thermo Scientific™) were employed for column chromatography separations. Solvents of analytical quality were employed and they were acquired from Merck.
Animal material
The octocorals Eunicea laciniata and Eunicea asperula were collected in reef flats along the coast of Santa Marta, Colombian Caribbean by scuba diving in December, 2011; at an approximate depth of 10 m. The Ministerio de Ambiente, Vivienda y Desarrollo Territorial granted permission for research on marine organisms (Contratos de Acceso a Recurso Genético No 99, 100 and 109). According to the permissions, soft corals were cut carefully and the collected quantities were not enough to cause an ecological impact. Small fragments were cut out of large colonies with sharp scissors, leaving the remaining colony intact. Fresh material was frozen immediately after collection and remained frozen until extraction. Collected organisms were identified by Mónica Puyana and voucher specimens were deposited at the invertebrate collection of ICN (Instituto de Ciencias Naturales, Universidad Nacional de Colombia), coded as ICN-MHN-CR-106 and PO0267, respectively.
Organism extraction and compound isolation
The sample of Eunicea laciniata was cut in small pieces and extracted with dichloromethane (DCM) four times. The extract was filtered and concentrated under vacuum yielding a dark green oil (13.8 g). The extract was separated by flash column chromatography (20 cm x 6 cm) eluting with 500 mL mobile phases of increasing polarity (hexane:toluene 90:10 to 50:50, toluene, toluene:ethyl acetate 50:50, ethyl acetate, ethyl acetate:methanol 50:50, methanol) to yield nine fractions (FL1 to FL9) according to their TLC profile. The fractions were monitored by 1H-NMR looking for signals assignable to methyl groups and double bound protons, which are common for diterpenes. This analysis allowed us identifying fractions FL1 and FL5 as those with a high diterpene content.
FL1 (869 mg, hex:tol 90:10) was a yellow oil. Its 1H-NMR and APT spectra showed it was constituted by the dolabellane β-araneosene (1). Fraction FL5 (7400 mg, tol and hex:tol 50 50) was further purified by column chromatography eluting with hex:EtOAc (100:0 to 50:50) to yield six fractions (FL5.1 to FL5.6). Subfraction FL5.3 corresponded to pure dolabellatrienone (2) (1780 mg). Subfraction FL5.5 (522 mg) was separated through a Diol cartridge with hex:EtOAc mixtures (80:20 to 0:100) to yield seven fractions (FL5.5.1 to FL5.5.7). Subfraction FL5.5.2 was separated using column chromatography to yield eight fractions (FL5.5.2.1 to FL5.5.2.8). Subfraction FL5.5.2.3 (35.4 mg) was finally purified by a Sephadex LH-20 column eluting with MeOH to yield pure compound 3 (18.4 mg). Subfraction FL5.5.2.5 corresponded to pure compound 4 (34.8 mg). Subfraction FL5.5.2.7 was purified by a Sephadex LH-20 column eluting with MeOH to yield pure compound 5 (18.8 mg). Subfraction FL5.6 was fractionated using a Diol cartridge eluting with hex:EtOAc mixtures (70:30 to 0:100) to obtain seven subfractions (FL5.6.1 to FL5.6.7). Subfraction FL5.6.3 (32.7 mg) was separated through silica gel column chromatography eluting with hex:EtOAc (70:30) to obtain pure compound 6 (7.1 mg).
The sample of Eunicea asperula (174 g dry weight) was extracted and fractionated using the same methodology previously explained for E. laciniata. The crude extract (3.2 g) was obtained as a dark green oil. Silica gel flash chromatography yielded nine fractions (FA1 to FA9) based on their TLC profiles. Fraction FA2 (243.9 mg, toluene) was separated through column chromatography eluting with hex:EtOAc (90:10 to 60:40) to obtain pure compound 2 (80 mg). Fraction FA4 (1480 mg, tol:EtOAc 50:50) was fractionated with a DIOL cartridge eluting with hex:EtOAc (90:10 to 50:50) yielding five subfractions (FA4.1 to FA4.5). Fraction FA4.2 corresponded to pure compound 4 (880 mg). Fraction FA4.4 was separated through column chromatography eluting with a gradient of hex:EtOAc (90:10 to 70:30) to obtain pure compound 5 (20.3 mg).
Compound 1: β-araneosene; yellowish oil; -32.0 (c 0.6, CHCl3); GC Retention time 38.88 min (KI 2011); EIMS m/z (relative intensity) 272 [M]+ (6), 257 [M- CH3]+ (4), 243 [M- C2H5]+ (0.2), 229 [M- C3H7]+ (14), 216 (22), 201 (8), 189 (24), 175 (9), 161 (28), 147 (14), 136 (62), 121 (100), 107 (51), 79 (36), 67 (36), 55 (25); 1H-NMR (400 MHz, CDCl3) δH 5.25 (1H, dd, J = 11.0, 4.8 Hz, H-3), 4.90 (1H, d, J = 10.4 Hz, H-7), 1.65 (3H, s, H-17), 1.63 (3H, s, H-19/20), 1.60 (3H, s, H-19/20), 1.45 (3H, s, H-16), 1.13 (3H, s, H-15); 13C-NMR (100 MHz, CDCl3) δ 142.5 (C-12), 134.8 (C-4), 132.5 (C-8), 129.4 (C-7), 126.0 (C-3), 122.0 (C-18), 48.4 (C-1), 42.0 (C-11), 40.3 (C-2), 40.0 (C-9), 38.7 (C-14), 38.2 (C-5), 42.0 (C-11), 28.3 (C-13), 27.9 (C-10), 24.3 (C-6), 23.7 (C-15), 21.7 (C-19), 21.3 (C-20), 16.3 (C-16), 15.3 (C-17). Spectroscopic properties are consistent with those published for the total synthesis of β-araneosene by Corey and Kingsbury ( 26 ).
Compound 2: (1R,3E,7E,11S)-13-keto-dolabella-3,7,12(18)-triene; Yellowish oil; 15.0 (0.9 c, CHCl3); GC Retention time 44.74 min (KI 2272); EIMS m/z (relative intensity) 286 [M]+ (8), 271 [M- CH3]+ (7), 253 [M- CH3- H2O]+ (0.4), 230 (5), 203, (11), 163 (43), 150 (100), 135 (65), 121 (36), 107 (56), 91 (77), 79 (75), 67 (85), 55 (52); 1H-NMR (400 MHz, CDCl3) δH 5.22 (1H, dd, J = 11.1, 4.6 Hz, H-3), 4.91 (1H, d, J = 10.3 Hz, H-7), 2.81 (1H, d, J = 11.6 Hz, H-11), 2.37 (1H, d, J = 18.5 Hz, H-14a), 2.08 (1H, d, J = 18.5 Hz, H-14b), 2.20 (3H, br s, H-20), 1.81 (3H, s, H-19), 1.62 (3H, s, H-17), 1.43 (3H, s, H-16), 1.21 (3H, s, H-15). 13C-NMR (100 MHz, CDCl3) δC 207.2 (C-13), 148.2 (C-18), 138.0 (C-12), 135.7 (C-4), 131.7 (C-8), 130.3 (C-7), 124.8 (C-3), 54.8 (C-14), 41.5 (C-11), 41.0 (C-1), 40.1 (C-2), 39.8 (C-5), 38.1 (C-9), 28.0 (C-10), 24.5 (C-19), 24.2 (C-6), 23.1 (C-15), 21.4 (C-20), 16.1 (C-17), 15.5 (C-16). Spectroscopic properties are consistent with data published for dolabellatrienone obtained by total synthesis ( 27 ).
Compound 3: Colorless oil; 1H-NMR (400 MHz, CDCl3) δH 5.11 (1H, d, J = 11.0 Hz), 2.72 (1H, t, J = 3.4 Hz), 1.70 (3H, s), 1.22 (3H, s), 1.14 (3H, d, J = 7.0 Hz), 0.97 (3H, d, J = 7.0 Hz), 0.88 (3H, s). 13C-NMR (100 MHz, CDCl3) δC 219.2, 135.1, 125.8, 60.4, 60.2, 59.8, 58.6, 52.0, 42.0, 40.3, 38.1, 33.8, 27.9, 26.3, 25.3, 22.8, 21.3, 18.1, 17.4, 16.8.
Compound 4: (1R,3E,7R,8R,11S)-7,8-epoxy-13-keto-dolabella-3,12(18)-diene; White solid; -7.81 (0.58 c, CHCl3); GC Retention time 48.17 min (KI 2439); EIMS m/z (relative intensity) 302 [M]+ (6), 284 [M- H2O]+ (0.9), 259 [M- C3H7]+ (2), 241[M- C3H7- H2O]+ (1), 219 (9), 201 (5), 189 (9), 175 (10), 163 (24), 150 (75), 136 (53), 121 (52), 107 (62), 93 (98), 79 (88), 67 (87), 55 (100); 1H-NMR (400 MHz, CDCl3) δH 5.41 (1H, dd, J = 11.3, 4.6 Hz, H-3), 2.90 (1H, d, J = 8.7 Hz, H-7), 2.67 (1H, d, J = 12.7 Hz, H-11), 2.39 (1H, d, J = 18.6 Hz, H-14a), 2.12 (1H, d, J = 18.6 Hz, H-14b), 2.25 (3H, br d, J = 0.8 Hz, H-20), 1.92 (3H, s, H-19), 1.55 (3H, s, H-16), 1.35 (3H, s, H-17), 1.17 (3H, s, H-15); 13C-NMR (100 MHz, CDCl3) δC 206.8 (C-13), 149.4 (C-18), 137.6 (C-4), 136.1 (C-12), 124.8 (C-3), 65.8 (C-7), 60.6 (C-8), 54.4 (C-14), 42.1 (C-11), 41.1 (C-1), 39.8 (C-2), 38.0 (C-9), 36.9 (C-5), 27.5 (C-6), 25.1 (C-19), 23.4 (C-15), 22.9 (C-10), 21.9 (C-20), 17.7 (C-17), 15.7 (C-16). Spectroscopic data are consistent with those published for a dolabellane isolated previously from Eunicea sp. ( 17 ).
Compound 5: Claenone; colorless oil; -10.66 (1.14 c, CHCl3); GC Retention time 48.23 min (KI 2442); EIMS m/z (relative intensity) 302 [M]+ (3), 269 [M- CH3- H2O]+ (1), 245 (2), 220 (7), 203 (6), 177 (11), 164 (8), 163 (26), 150 (91), 135 (52), 121 (36), 107 (65), 91 (90), 79 (99), 67 (100), 55 (85); 1H-NMR (300 MHz, CDCl3) δH 5.10 (1H, d, J = 10.9 Hz, H-7), 2.97 (1H, dd, J = 11.0, 2.9 Hz, H-3), 2.92 (1H, d, J = 11.6 Hz, H-11), 2.39 (1H, d, J = 18.4 Hz, H-14a), 2.09 (1H, d, J = 18.4 Hz, H-14b), 2.18 (3H, s, H-20), 1.82 (3H, s, H-19), 1.72 (3H, s, H-17), 1.39 (3H, s, H-16), 1.13 (3H, s, H-15); 13C-NMR (75 MHz, CDCl3) δC 206.5 (C-13), 149.0 (C-18), 137.2 (C-12), 133.1 (C-8), 128.4 (C-7), 64.0 (C-3), 61.6 (C-4), 55.6 (C-14), 42.4 (C-11), 40.9 (C-1), 38.6 (C-9), 37.7 (C-2), 37.3 (C-5), 27.4 (C-6), 24.6 (C-15), 24.5 (C-10), 23.4 (C-15), 21.4 (C-20), 16.6 (C-17), 15.5 (C-16). Spectroscopic properties are consistent with those reported for the dolabellane claenone obtained by total synthesis ( 27 ).
Compound 6: (1R,3R,4R,7R,8R,11S)-di-3,4:7,8-epoxy-13-keto-dolabell-12(18)-ene. White solid; -21.14 (1.14 c, CHCl3); GC Retention time 50.61 min (Calculated KI 2561); EIMS m/z (relative intensity) 318 [M]+ (48), 275 [M- C3H7]+ (7), 257 [M- C3H7- H2O]+ (0.6), 233 (1), 217 (10), 203 (8), 189 (13), 175 (14), 161 (22), 149 (100), 135 (73), 121 (62), 109 (69), 93 (76), 79 (63), 67 (51), 55 (72); 1H-NMR (400 MHz, CDCl3) δH 3.05 (1H, dd, J = 11.1, 2.7 Hz, H-3), 2.96 (1H, d, J = 7.8 Hz, H-7), 2.81 (1H, d, J = 12.6 Hz, H-11), 2.44 (1H, d, J = 18.5 Hz, H-14α), 2.24 (3H, s, H-20), 2.13 (1H, d, J = 18.6 Hz, H-14β), 1.94 (3H, s, H-19), 1.43 (3H, s, H-16), 1.33 (3H, s, H-17), 1.21 (3H, s, H-15); 13C-NMR (100 MHz, CDCl3) δC 205.5 (C-13), 149.6 (C-18), 137.0 (C-12), 63.7(C-7), 63.0 (C-3), 60.6 (C-8), 60.3 (C-4), 54.7 (C-14), 42.9 (C-11), 40.2 (C-2), 37.8 (C-1), 37.2 (C-5), 36.6 (C-9), 27.8 (C-10), 25.2 (C-19), 23.6 (C-15), 23.3 (C-6), 21.6 (C-20), 17.5 (C-17), 15.6 (C-16). NMR data are identical to a dolabellane from Eunicea sp. previously reported ( 17 ).
Cell cultures and viral strains
African green monkey kidney cells (Vero cells) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco™) containing 4.5 g/L glucose, 2 g/L sodium bicarbonate, 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin (Gibco™) and 2.5 µg/mL anphotericine B supplemented with fetal bovine serum (FBS, Sigma). Vero cells were maintained at 37 °C under 5% CO2 atmosphere. In order to prepare virus stocks, Vero cells were infected with HSV-1 susceptible to Acyclovir® (KOS strain) at a multiplicity of infection (MOI) of 0.1 (28). After 24 hours of postinfection (p.i.), cells were lysed by three cycles of freezing and thawing, centrifuged at 400 x g at 4 °C for 20 min. The viral titration was made by the plaque reduction assay ( 29 ).
Evaluation of antiviral and cytotoxic activity
Monolayers of Vero cells placed in 24-well plates (2 x 105 per well) were infected with HSV-1 at MOI of 1 for 1 h at 37 °C under 5% CO2 atmosphere. After this period, the cells were treated with different concentrations of the compounds. After incubation for 24 h, cells were lysed by three cycles of freezing and thawing for viral titration. Cytotoxicity was evaluated by the MTT method and the results were analyzed comparing the absorbance of treated wells with that of non-treated controls ( 30 ).
Results and discussion
To select the most promising soft corals in terms of dolabellane content, we previously ran TLC plates of crude extracts obtained from a large collection of soft corals, available in our group, collected at various sites of the Colombian Caribbean. Based on TLC profiles, the most promising crude extracts were fractionated through a SPE Diol cartridge and fractions were further analyzed by gas chromatography electronic impact mass spectrometry (GC-EIMS). GCMS analyses showed a major chromatographic peak (RT: 44.7 min, KI: 2271 and m/z: 286) in two of the extracts. These signals were attributed to the previously known compound dolabellatrienone (2) due to the high degree of coincidence in the Kovats index and EIMS spectra. The promising extracts were obtained from the soft corals Eunicea laciniata and E. asperula from Santa Marta. Therefore, extracts from both organisms were submitted to several steps of purification by column chromatography yielding six compounds in total that are shown in Figure 1
Figure 1:
Compound 1 was isolated as a yellowish oil and was the major component in fraction FL1 obtained from E. laciniata. GC-MS analysis indicated that compound 1 was pure enough and was identified without further purification steps. The analysis showed a major peak with a molecular ion at m/z = 272 corresponding to [M]+ and consistent with a C20H32 molecular formula. Compound 1 1H-NMR spectrum had signals in δH 5.25 (1H, dd, J = 11.0, 4.8 Hz, H-3) and 4.90 (1H, br d, J = 10.4 Hz, H-7) corresponding to olefinic protons and five additional signals were observed in δH 1.65 (3H, s, H-17), 1.63 (3H, s, H-19), 1.60 (3H, s, H-20), 1.45 (3H, s, H-16) and 1.13 (3H, s, H-15) corresponding to methyl groups.
On the other hand, the APT spectrum of 1 showed twenty signals, which were assigned as five methyls, seven methylenes (all sp3), three methines (one sp3 and two sp2), and five quaternary carbons (one sp3 and four sp2). All this information suggested that compound 1 was a dolabellane diterpene with two trisubstituted and one tetrasubstituted double bonds. Comparison of spectral data were consistent with those reported for the known dolabellane β-araneosene obtained by total synthesis ( 26 ) and allowed us to identify compound 1. The stereochemistry at the ring fusion was defined as 1S, 11R based on the similar value of optical rotation comparable to that obtained by total synthesis.
Compound 2 was isolated as a yellow oil. 1H-NMR and APT spectra were quite similar to those obtained for compound 1. However, minimal differences suggested the presence of additional functional groups. 1H-NMR spectrum had five methyl signals but two of them were in δH 2.20 (3H, br s, H-20) and 1.81 (3H, s, H-19) while the APT spectrum showed a signal in δC 207.2 (C-13) together with a deshielded methylene in δC 54.8 (CH2-14). These observations were consistent with the presence of a conjugated ketone carbonyl and justified the observed signals. The spectroscopic properties and experimental optical rotation ( +15.0 (c 0.9 CHCl3)) were consistent with those reported in dolabellatrienone (2) ( +32.0 (c 0.14 CHCl3)), a dolabellane previously isolated from several Eunicea species and also obtained by total synthesis ( 27 ).
Compound 3 was isolated as a colorless oil. 1H-NMR signals in δH 5.11 (1H, d, J = 9.1 Hz) and 2.72 (1H, t, J =3.4 Hz) corresponded with an olefinic proton and an oxygenated methine, respectively. Additionally, five methyl signals were also identified in δH 1.70 (3H, s), 1.22 (3H, s), 1.14 (1H, d, J = 7.0 Hz), 0.97 (1H, d, J = 7.0 Hz), 0.88 (3H, s). The APT spectrum showed signals for five methyls (δC 16.8, 17.4, 18.1, 21.3 and 22.8), six methylenes, (25.3, 26.3, 33.8, 38.1, 40.3, 58.6), five methines, one olefinic, one oxygenated (δC 27.9, 42.0, 60.2, 60.4 and 125.8), and four quaternary carbons, one oxygenated (δC 59.8), one olefinic (δC 135.1), and a ketone carbonyl (δC 219.2). We inferred that compound 3 was a dolabellane diterpene with a trisubstituted double bond and an epoxide group, and the structural elucidation process was conducted by 2D-NMR spectroscopy.
However, compound 3 decomposed during the experiments suggesting an unexpected lability and a low probability of application as an antiviral compound. Despite this, we proposed a planar structure for compound 3 based on information deduced from 1D-NMR spectra and comparison with other dolabellanes. We observed two doublet methyls with chemical shifts and coupling constants quite similar to a previous reported dolabellane without a double bound between positions C-12 and C-18 ( 31 ). Moreover, signals at δC 219.2 and 60.2 indicated the presence of a ketone with and an alfa methine, and they were also consistent with those reported in the known compound. The position of the double bond position was deduced taking advantage of the high degree of conservation in the chemical shifts for double bonds in dolabellanes. We have observed in many dolabellanes that the chemical shift for carbon atoms in a double bond between C-3 and C-4 differs from that showed by a double bond between C-7 and C-8 (17, 31). Compound 3 had the expected signals for a double bond between C-3 and C-4 while the epoxide group was located between C-7 and C-8. Figure 2 shows the planar structure of compound 3 and some related structures that were useful to our elucidation purposes.
Figure 2:
Compound 4 was isolated as a white solid. The EIMS spectrum showed a molecular ion corresponding to [M]+ in m/z = 302. 1H and 13C-NMR (100 MHz, CDCl3) spectra showed similarities with dolabellatrienone (2) but there were differences that indicated the presence of an additional functional group in 4. It was observed one signal at δH 2.90 (1H, br d, J = 8.7 Hz, H-7) together with signals at δC 65.8 (CH-7) and 60.6 (C-8) which corresponded to an epoxide functionality. As previously explained, the double bond was located between C-3 and C-4 due to the observed chemical shifts for the trisubstituted double bond in δC 124.8 (CH-3) and 137.6 (C-4). The experimental optical rotation and spectroscopic properties were consistent with those reported for the compound (1R,3E,7R,8R,11S)-7,8-epoxy-13-keto-dolabella-3,12(18)-diene and allowed us identifying compound 4 ( 32 ).
Compound 5 exhibited 1H-NMR, APT, and EIMS spectral data almost identical to compound 4, with minimal differences that indicated they were structural isomers. The observed signals at δH 2.97 (1H, dd, J = 11.0, 2.9 Hz, H-3), δC 64.0 (CH-3), 61.6 (C-4) allowed confirming the presence of an epoxide group while signals at δC 133.1 (C-8) and 128.4 (CH-7) located the double bond between C-7 and C-8. Compound 5 was identified by comparison with the spectroscopic properties and optical rotation values reported in the total synthesis of the dolabellane claenone ( 27 ).
Compound 6 showed an EIMS with a molecular ion at m/z = 318, which was consistent with a C20H30O3 molecular formula. The 1H-NMR spectrum showed signals at 3.05 (1H, dd, J = 11.1, 2.7 Hz, H-3) and 2.96 (1H, d, J = 7.8 Hz, H-7) while the APT spectrum showed four signals for oxygenated carbons at δC 63.7 (C-7), 63.0 (CH-3), 60.6 (CH-8) and 60.3 (C-4). This information confirmed the presence of two epoxide groups and indicated that compound 6 was a diepoxide analogue of 4 and 5. The remaining signals and experimental optical rotation allowed us to identify compound 6 as (1R,3R,4R,7R,8R,11S)-di-3,4:7,8-epoxy-13-keto-dolabell-12(18)-ene, a dolabellane previously isolated from an unidentified Eunicea species and whose absolute stereochemistry was determined previously ( 17 ).
Compounds 1-6 were isolated from Eunicea laciniata whereas only compounds 2, 4, and 5 were isolated from E. asperula, where 4 was the major compound, and 2 and 5 were minor components. Previous works on the chemistry of this particular species, showed that E. asperula collected in Tobago yielded cembrane and asperketal diterpenes ( 33 ). Although variations in chemical profiles is not unusual between organisms collected at different places, the diterpene core usually remains the same ( 34 , 35 ). Interestingly, our sample yielded dolabellane diterpenes suggesting it might be a different chemotype, assuming, in both studies, that taxonomic identity is correct.
Previous studies with E. laciniata have shown the high dolabellane content of this species and the considerable number of compounds with variable oxygenation grade. Samples collected in Puerto Rico, Bahamas, Tobago, Honduras, and Colombia have reported the isolation of dolabellanes β-araneosene (1) and dolabellatrienone (2) ( 14 , 24 ). In this work we report the isolation of compounds 3-6 from E. laciniata which were not previously described from this species. Nevertheless, it was found that following purification, compound 2 experiments an oxidation process to yield a mixture of compounds 4 and 5. Figure 3a shows a GC-EIMS analysis for compound 2 (initially pure) and after several weeks of storage. The chromatogram shows a major peak corresponding to compound 2 and two minor peaks with Kovats index and EIMS coincident with 4 and 5.
Figure 3:
Interestingly, compound 5 has been reported as a natural product only from a Clavularia soft coral collected in Japan ( 36 ). Considering this, the isolation of compounds 4 and 5 in this study suggested that these could be artifacts produced during storage of the extract and fractions. Alternatively, the presence of those compounds in a particular sample could reflect a different chemotype. In order to establish if compounds 4 and 5 were storage artifacts, a new GC-EIMS analysis was conducted after extraction and fractionation of a fresh specimen. As an additional precaution, fractions were kept under argon atmosphere and frozen at temperature below zero, dissolving them just before the chromatographic analysis. In E. laciniata, compounds 2, 4 and 5 were identified in the fraction that eluted with hex:EtOAc 70:30 (Figure 3c). Compound 6 was not found in this fraction nor in those of greater polarity. We conclude that although minimal quantities of compounds 4 and 5 are present in the extracts from E. laciniata, the absence of compound 6 suggests that both monoepoxides are mainly oxidation products of (2) in this soft coral. On the other hand, compound 2 and the major compound 4 were found in E. asperula but compound 5 was not (Figure 3b), suggesting that compound 5 is the only oxidation product of (2) in E. asperula.
Vero cells have been used to evaluate anti-HSV-1 activity of a wide range of natural products, including dolastane and dolabellane diterpenes ( 20 ). Cytotoxic activity in Vero cells was determined for compounds 2, 4, 5, 6 with Acyclovir® as a positive control. We did not test compound 1 since it was not pure enough to run the assays. Compounds 2 (CC50 = 146 µM) and 5 (CC50 = 467 µM) showed a greater cytotoxic effect as compared to Acyclovir® (CC50 = 960 µM) while compounds 4 (CC50 = 526 µM) and 6 (CC50 = 959 µM) had comparable values.
An important feature for any antiviral compound is that it must not be toxic and must be selective to infected cells. For this reason, we decided to evaluate antiviral activity only for compounds 4 and 6 in an HSV-1 inhibition assay using concentration of 50 µM for each compound. Compound 4 showed a 42.6% of inhibition while compound 6 exhibited a 73.7% of inhibition. Considering that 6 also has a remarkable in vitro anti-HIV-1 activity ( 25 ), it is a potential candidate to perform further studies to assess its full potential as an antiviral agent as well as understanding its mechanism of action against viruses including HSV-1.
Conclusions
Six dolabellane diterpenes from the soft corals Eunicea laciniata and E. asperula, collected in Santa Marta, Colombian Caribbean Sea, were isolated in this work. Compounds 1, 2, 4-6 were identified by spectroscopic means and compared with previously reported dolabellanes. Compound 3 was not fully characterized due to its decomposition, suggesting it had a high degree of reactivity. Dolabellatrienone (2) and (1R,3E,7R,8R,11S)-7,8-epoxy-13-keto-dolabella-3,12(18)-diene (4) were the major compounds isolated from E. laciniata and E. asperula, respectively. To the best of our knowledge, this is the first study of E. asperula collected in the Colombian Caribbean and dolabellanes were found as the major diterpenes. If is considered that variations in the family of isolated diterpenes do not depend on location, either this work studied a different chemotype or there were problems with taxonomical characterization. However, further studies should be developed in order to confirm this hypothesis.
GC-EIMS analyses proved that prolonged storage of pure dolabellatrienone (2) induces a spontaneous oxidation to yield compounds 4 and 5. This finding evidences a need to improve the manipulation and storage conditions of extracts and fractions obtained from those organisms in order to minimize the artifact formation and increase the reproducibility of chemical studies.
Biological assays showed that compound 6 inhibited HSV-1 replication at a concentration of 50 µM and was not cytotoxic. Although the remaining compounds were more toxic than Acyclovir®, their antiviral activity was determined to calculate selectivity indexes and establish their potential as antivirals. These results encourage us to continue the study of dolabellanes of marine origin and evaluating their antiviral activity.
Aknowledgments
Financial support was provided by Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación Francisco José de Caldas through the “Programa jóvenes investigadores” as well as the DIB–Universidad Nacional de Colombia and Fundación para la promoción de la investigación y la tecnología (FPIT) Banco de la Republica. Maria Leonisa Sanchez Nuñez, Izabel Christina Nunes de Palmer Paixão and Valeria Laneuville Teixeira are grateful to CNPq (Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq) and FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro) for financial support. The Ministerio de Ambiente, Vivienda y Desarrollo Territorial granted permission for research on marine organisms (Contratos de Acceso a Recurso Genético No 99, 100 and 109).
Marine natural products.
Referencias
Blunt, J. W.; Copp, B. R.; Keyzers, R. A.; Munro, M. H. G.; Prinsep, M. R.; Faulkner, D. J. Marine natural products. Nat. Prod. Rep. 2016, 33 (3), 382–431. DOI: http://dx.doi.org/10.1039/C5NP00156K.
Zhou, X.; Liu, J.; Yang, X. W.; Liu, Y. Marine Natural Products with Anti-HIV Activities in the Last Decade. Curr. Med. Chem. 2013, 20 (7), 953-973. DOI: http://dx.doi.org/10.2174/0929867311320070009.
De Clercq, E. Antivirals: past, present and future. Biochem. Pharmacol. 2013, 85 (6), 727-744. DOI: http://dx.doi.org/10.1016/j.bcp.2012.12.011.
Looney, D.; Ma, A.; Johns, S. HIV therapy-the state of art. Curr. Top. Microbiol. Immunol. 2015, 389, 1-29. DOI: http://dx.doi.org/10.1007/82_2015_440.
Kinch, M. S.; Patridge, E. An analysis of FDA-approved drugs for infectious disease: HIV/AIDS drugs. Drug. Discov. Today. 2014, 19 (10), 1510-1513. DOI:
http://dx.doi.org/10.1016/j.drudis.2014.05.012.
James, S. H.; Prichard, M. N. Current and future therapies for herpes simplex virus infections: mechanism of action and drug resistance. Curr. Opin. Virol. 2014, 8, 54-61. DOI: http://dx.doi.org/10.1016/j.coviro.2014.06.003.
Hawkins, T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Res. 2010, 85 (1), 201-209. DOI: http://dx.doi.org/10.1016/j.antiviral.2009.10.016.
Margolis, A. M.; Heverling, H.; Pham, P. A.; Stolbach, A. A review of the toxicity of HIV medications. J. Med. Toxicol. 2014, 10 (1), 26-39. DOI: http://dx.doi.org/10.1007/s13181-013-0325-8.
Yodsaoue, O.; Sonprasit, J.; Karalai, C.; Ponglimanont, C.; Tewtrakul, S.; Chantrapromma, S. Diterpenoids and triterpenoids with potential anti-inflammatory activity from the leaves of Aglaia odorata. Phytochemistry 2012, 76, 83-91. DOI: http://dx.doi.org/10.1016/j.phytochem.2012.01.015.
Aboushoer, M. I.; Fathy, H. M.; Abdel-Kader, M. S.; Goetz, G.; Omar, A. A. Terpenes and flavonoids from an Egyptian collection of Cleome droserifolia. Nat. Prod. Res. 2010, 24 (7), 687-696. DOI: http://dx.doi.org/10.1080/14786410903292433.
Cai, X. H.; Wang, Y. Y.; Zhao, P. J.; Li, Y.; Luo, X. D. Dolabellane diterpenoids from Aglaia odorata. Phytochemistry 2010, 71 (8-9), 1020-1024. DOI: http://dx.doi.org/10.1016/j.phytochem.2010.03.005.
Yoshimitsu, H.; Miyashita, H.; Nishida, M.; Mineno, T.; Nohara, T. Dolabellane Diterpene and Three Cycloartane Glycosides from Thalictrum squarrosum. Chem. Pharm. Bull. 2010, 58 (8), 1043-1046. DOI: http://dx.doi.org/10.1248/cpb.58.1043.
Hinkley, S. F.; Mazzola, E. P.; Fettinger, J. C.; Lam, Y.-F.; Jarvis, B. B. Atranones A–G, from the toxigenic mold Stachybotrys chartarum. Phytochemistry 2000, 55 (6), 663-673. DOI: http://dx.doi.org/10.1016/S0031-9422(00)00231-4.
Rodríguez, A. D.; González, E.; Ramírez, C. The structural chemistry, reactivity, and total synthesis of dolabellane diterpenes. Tetrahedron, 1998, 54 (39), 11683-11729. DOI: http://dx.doi.org/10.1016/S0040-4020(98)83033-0.
Wang, S.-K.; Huang, M.-J.; Duh, C.-Y. Cytotoxic constituents from the formosan soft coral Clavularia inflata var. luzoniana. J. Nat. Prod. 2006, 69 (10), 1411-1416. DOI: http://dx.doi.org/10.1021/np0601253.
Duh, C.-Y.; Chia, M.-C.; Wang, S.-K.; Chen, H.-J.; El-Gamal, A. A. H.; Dai, C.-F. Cytotoxic dolabellane diterpenes from the Formosan soft coral Clavularia inflata. J. Nat. Prod. 2001, 64 (8), 1028-1031. DOI: http://dx.doi.org/10.1021/np010106n.
ei odr gue , A. D.; Baran, P.; Raptis, R. G. Dolabellane-Type Diterpenoids with Antiprotozoan Activity from a Southwestern Caribbean Gorgonian Octocoral of the Genus Eunicea. J. Nat. Prod. 2010, 73 (5), 925-934. DOI: http://dx.doi.org/10.1021/np100074r.
Barbosa, J. P.; Pereira, R. C.; Abrantes, J. L.; Cirne dos Santos, C.C.; Rebello, M. A.; Frugulhetti, I. C. et al. In vitro Antiviral Diterpenes from the Brazilian Brown Alga Dictyota pfaffii. Planta Med. 2004, 70 (09), 856-860. DOI: http://dx.doi.org/10.1055/s-2004-827235
Pardo-Vargas, A.; de Barcelos Oliveira, I.; Stephens, P.R.S.; Cirne-Santos, C.C.; de Palmer Paixão, I.C.N.; Ramos, F.A. et al. Dolabelladienols A–C, New Diterpenes Isolated from Brazilian Brown Alga Dictyota pfaffii. Mar. Drugs, 2014, 12, 4247-4259. DOI: http://dx.doi.org/10.3390/md12074247.
Abrantes, J. L.; Barbosa, J.; Cavalcanti, D.; Pereira, R. C.; Frederico Fontes, C. L.; Teixeira, V. L. et al. The effects of the diterpenes isolated from the Brazilian brown algae Dictyota pfaffii and Dictyota menstrualis against the herpes simplex type-1 replicative cycle. Planta Med. 2010, 76 (4), 339-44. DOI: http://dx.doi.org/10.1055/s-0029-1186144.
Cirne-Santos, C. C.; Souza, T. M.; Teixeira, V. L.; Fontes, C. F.; Rebello, M. A.; Castello-Branco, L. R. et al. The dolabellane diterpene Dolabelladienetriol is a typical noncompetitive inhibitor of HIV-1 reverse transcriptase enzyme. Antiviral Res. 2008, 77 (1), 64-71. DOI: http://dx.doi.org/10.1016/j.antiviral.2007.08.006.
Coll, J. C. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 1992, 92 (4), 613–631. DOI: http://dx.doi.org/10.1021/cr00012a006.
Sánchez, J. A. Systematics of the candelabrum gorgonian corals (Eunicea Lamouroux; Plexauridae; Octocorallia; Cnidaria). Zool. J. Linn. Soc. 2009, 157 (2), 237-263. DOI: http://dx.doi.org/10.1111/j.1096-3642.2008.00515.x.
Cuadrado, C.; Castellanos, L.; Osorno, O. E.; Ramos, F. A.; Duque, C.; Puyana, M. Estudio Químico y evaluación de la actividad antifouling del octocoral caribeño Eunicea laciniata. Quim. Nova. 2010, 33 (3), 656-661. DOI: http://dx.doi.org/10.1590/S0100-0422010000300033.
Pardo-Vargas, A.; Ramos, F. A.; Cirne-Santos, C. C.; Stephens, P. R.; Paixão, I. C. P.; Teixeira, V. L. et al. Semi-synthesis of oxygenated dolabellane diterpenes with highly in vitro anti-HIV-1 activity. Bioorg. Med. Chem. Lett., 2014, 24 (18), 4381-4383. DOI: http://dx.doi.org/10.1016/j.bmcl.2014.08.019.
Kingsbury, J. S.; Corey, E. J. Enantioselective Total Synthesis of Isoedunol and β-Araneosene Featuring Unconventional Strategy and Methodology. J. Am. Chem. Soc. 2005, 127 (40), 13813-13815. DOI: http://dx.doi.org/10.1021/ja055137+.
Miyaoka, H.; Isaji, Y.; Mitome, H.; Yamada, Y. Total synthesis of the dolabellane marine diterpenoids, claenone, palominol and dolabellatrienone. Tetrahedron, 2003, 59 (1), 61-75. DOI: http://dx.doi.org/10.1016/S0040-4020(02)01474-6.
Esquenazi, D.; Wigg, M. D.; Miranda, M. M. F. S.; Rodrigues, H. M.; Tostes, J. B. F.; Rozental, S. et al. Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn. (Palmae) husk fiber extract. Res. Microbiol. 2002, 153 (10), 647-652. DOI: http://dx.doi.org/10.1016/S0923-2508(02)01377-3.
Kuo, Y.-C.; Chen, C.-C.; Tsai, W.-J.; Ho, Y.-H. Regulation of herpes simplex virus type 1 replication in Vero cells by Psychotria serpens: relationship to gene expression, DNA replication, and protein synthesis. Antiviral Res. 2001, 51 (2), 95-109. DOI: http://dx.doi.org/10.1016/S0166-3542(01)00141-3
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65 (1), 55-63. DOI: http://dx.doi.org/10.1016/0022-1759(83)90303-4
Shin, J.; Fenical, W. Structures and reactivities of new dolabellane diterpenoids from the Caribbean gorgonian Eunicea laciniata. J. Org. Chem. 1991, 56 (10), 3392-3398. DOI: http://dx.doi.org/10.1021/jo00010a039.
Look, S. A.; Fenical, W., New bicyclic diterpenoids from the Caribbean gorgonian octocoral Eunicea calyculata. J. Org. Chem. 1982, 47 (21), 4129-4134. DOI: http://dx.doi.org/10.1021/jo00142a024.
Shin, J.; Fenical, W. Asperketals A-F, new diterpenoids of the dilophol class from the Caribbean gorgonian Eunicea asperula. J. Org. Chem. 1988, 53 (14), 3271-3276. DOI: http://dx.doi.org/10.1021/jo00249a024.
Puyana, M.; Narvaez, G.; Paz, A.; Osorno, O.; Duque, C. Pseudopterosin Content Variability of the Purple Sea Whip Pseudopterogorgia elisabethae at the Islands of San Andres and Providencia (Sw Caribbean). J. Chem. Ecol. 2004, 30 (6), 1183-1201. DOI: http://dx.doi.org/10.1023/B:JOEC.0000030271.73629.26.
Reina, E.; Puentes, C.; Rojas, J.; Garcia, J.; Ramos, F. A.; Castellanos, L. et al. Fuscoside E: a strong anti-inflammatory diterpene from Caribbean octocoral Eunicea fusca. Bioorg. Med. Chem. Lett. 2011, 21 (19), 5888-91. DOI: http://dx.doi.org/10.1016/j.bmcl.2011.07.092.
Mori, K.; Iguchi, K.; Yamada, N.; Yamada, Y.; Inouye, Y. Bioactive marine diterpenoids from Japanese soft coral of Clavularia sp. Chem. Pharm. Bull. 1988, 36 (8), 2840-2852. DOI: http://dx.doi.org/10.1248/cpb.36.2840.
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Andika Pramudya Wardana, Nanik Siti Aminah, Mila Rosyda, Muhammad Ikhlas Abdjan, Alfinda Novi Kristanti, Khun Nay Win Tun, Muhammad Iqbal Choudhary, Yoshiaki Takaya. (2021). Potential of diterpene compounds as antivirals, a review. Heliyon, 7(8), p.e07777. https://doi.org/10.1016/j.heliyon.2021.e07777.
2. Lipselotte de Jesús Infante Rivera, Miriam Vilca Arana, Ruth Katherine Mendivel Geronimo, David Raúl Hurtado Tiza, Edwin Huamán Gómez. (2025). Integración de la química verde en el currículo educativo: un enfoque sostenible. Revista Colombiana de Química, , p.3. https://doi.org/10.15446/rev.colomb.quim.v53n1.114986.
3. Fabián Amaya García, Claudio Cirne-Santos, Caroline de Souza Barros, Ana Maria Pinto, Maria Leonisa Sanchez Nunez, Valeria Laneuville Teixeira, Jackson A. L. C. Resende, Freddy A. Ramos, Izabel C. N. P. Paixão, Leonardo Castellanos. (2021). Semisynthesis of Dolabellane Diterpenes: Oxygenated Analogues with Increased Activity against Zika and Chikungunya Viruses. Journal of Natural Products, 84(4), p.1373. https://doi.org/10.1021/acs.jnatprod.1c00199.
4. Liliana Santacruz, Diana X. Hurtado, Roisin Doohan, Olivier P. Thomas, Mónica Puyana, Edisson Tello. (2020). Metabolomic study of soft corals from the Colombian Caribbean: PSYCHE and 1H-NMR comparative analysis. Scientific Reports, 10(1) https://doi.org/10.1038/s41598-020-62413-0.
5. O. I. Yarovaya, S. S. Laev, N. F. Salakhutdinov. (2023). Antiviral properties of diterpenes and their derivatives. Russian Chemical Reviews, 92(1), p.RCR5056. https://doi.org/10.57634/RCR5056.
6. Sandra L. Molina, Abel M. Forero, Farja I. Ayala, Mónica Puyana, Sven Zea, Leonardo Castellanos, Diego Muñoz, Gonzalo Arboleda, Adrián G. Sandoval-Hernández, Freddy A. Ramos. (2019). Metabolic Profiling of the Soft Coral Erythropodium caribaeorum (Alcyonacea: Anthothelidae) from the Colombian Caribbean Reveals Different Chemotypes. Marine Drugs, 18(1), p.4. https://doi.org/10.3390/md18010004.
7. Sasadhar Majhi, Sivakumar Manickam. (2024). Semisynthesis of Bioactive Compounds and their Biological Activities. , p.439. https://doi.org/10.1016/B978-0-443-15269-6.00005-5.
8. Abel M. Forero, Leonardo Castellanos, Adrián G. Sandoval‐Hernández, Alvicler Magalhães, Luzineide W. Tinoco, Fabian Lopez‐Vallejo, Freddy A. Ramos. (2021). Integration of NMR studies, computational predictions, and in vitro assays in the search of marine diterpenes with antitumor activity. Chemical Biology & Drug Design, 98(4), p.507. https://doi.org/10.1111/cbdd.13907.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons (CC. Atribución 4.0) que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).