Absceso pélvico que se confunde con tumor maligno de ovario en una mujer posmenopáusica. Reporte de caso
Pelvic abscess mistaken for malignant ovarian tumor in a postmenopausal woman. Case report
DOI:
https://doi.org/10.15446/cr.v7n1.87199Palabras clave:
Absceso, Neoplasias Ováricas, Fibroma, Posmenopausia, Informes de Caso (es)Abscess, Fibroma, Ovarian Neoplasms, Postmenopausal, Case Reports (en)
Descargas
Introducción. Los abscesos tubo ováricos son poco frecuentes en la posmenopausia y se asocian con patologías ginecológicas benignas como endometriosis, pólipo endometrial o leiomioma uterino, y con patologías malignas como adenocarcinoma de endometrio, tumores malignos epiteliales y no epiteliales de ovario, carcinoma escamocelular de cérvix y adenocarcinoma de colon. Su presentación representa un reto diagnóstico y terapéutico para el médico.
Presentación del caso. Paciente femenina de 72 años, quien consultó por cuadro clínico de tres días de evolución consistente en dolor y distensión abdominal asociados a fiebre y síntomas urinarios irritativos y retención urinaria. Los exámenes de ingreso mostraron leucocitosis con neutrofilia y CA-125 en 222 U/mL. El ultrasonido y la resonancia magnética evidenciaron una masa retrouterina solido-quística de 15 cm. Ante sospecha de tumor versus absceso tubo-ovárico, se realizó biopsia dirigida por tomografía mediante la cual se encontró material purulento fétido. Se practicó laparotomía que confirmó masa pélvica retrouterina sólido quística con contenido purulento, adherencias y compromiso inflamatorio de las trompas uterinas. El resultado de patología informó fibroma ovárico y absceso tubo ovárico. La paciente evolucionó satisfactoriamente en el posoperatorio y en los controles posteriores.
Conclusiones. El caso reportado ilustra cómo, en ocasiones, un posible diagnóstico de carcinomatosis por cáncer de ovario puede ser realmente una patología benigna (absceso tubo ovárico) que responde bien a un tratamiento médico-quirúrgico. Las imágenes diagnósticas y los marcadores tumorales son de gran ayuda para diferenciar una patología ovárica maligna de un proceso benigno.
Introduction: Tubo-ovarian abscesses are rare in postmenopausal women. They have been associated with benign gynecological conditions such as endometriosis, uterine polyp or leiomyoma, and malignant diseases such as endometrial adenocarcinoma, epithelial and non-epithelial malignant ovarian tumors, squamous cell carcinoma of the cervix, and adenocarcinoma of the colon. Their presentation represents a diagnostic and therapeutic challenge for clinicians.
Case report: A 72-year-old female patient was admitted with a 3-day history of abdominal pain and distension, fever, irritative urinary symptoms and urinary retention. Lab tests on admission showed elevated white blood cells and neutrophils count, and CA-125 at 222 U/mL. Ultrasound and magnetic resonance imaging revealed a solid retrouterine cystic mass of 15 cm. Suspecting tumor versus tubo-ovarian abscess, a tomography-directed biopsy was performed, finding foul-smelling purulent material. An exploratory laparotomy was performed with intraoperative findings of solid-cystic retrouterine pelvic mass with purulent content, adhesions, and inflammatory involvement of the uterine tubes. Pathology reported ovarian fibroma and tubo-ovarian abscess. The patient evolved satisfactorily in the postoperative period and in the subsequent follow-up appointments.
Conclusions: The reported case illustrates how a possibility of ovarian cancer with peritoneal carcinomatosis can actually be a benign condition (tubo-ovarian abscess) that responds well to medical-surgical treatment. Diagnostic imaging and tumor markers are helpful in differentiating a malignant ovarian disease from a benign process.
https://doi.org/10.15446/cr.v7n1.87199
Pelvic abscess mistaken for malignant ovarian tumor in a postmenopausal woman. Case report
Keywords: Abscess; Fibroma; Ovarian Neoplasms; Postmenopausal; Case Reports.
Palabras clave: Absceso; Neoplasias ováricas; Fibroma; Posmenopausia; informes de caso.
Edgar Páez-Castellanos
Ariel Iván Ruiz-Parra
Edith Ángel-Müller
Universidad Nacional de Colombia -
Bogotá Campus - Faculty of Medicine -
Department of Obstetrics and Gynecology -
Bogotá, D.C. - Colombia.
Iván David Ruiz-Ángel
Universidad Nacional de Colombia -
Bogotá Campus - Faculty of Medicine -
Department of Radiology and diagnostic images -
Bogotá, D.C. - Colombia.
Corresponding author
Edith Ángel-Müller. Departamento de Obstetricia y Ginecología, Facultad de Medicina,
Universidad Nacional de Colombia.
Bogotá D.C. - Colombia.
E-mail: eangelm@unal.edu.co.
Received: 11/05/2020 Accepted: 05/08/2020
Resumen
Introducción. Los abscesos tubo-ováricos son poco frecuentes en la posmenopausia y se asocian con patologías ginecológicas benignas como endometriosis, pólipo endometrial o leiomioma uterino, y con patologías malignas como adenocarcinoma de endometrio, tumores malignos epiteliales y no epiteliales de ovario, carcinoma escamocelular de cérvix y adenocarcinoma de colon. Su presentación representa un reto diagnóstico y terapéutico para el médico.
Presentación del caso. Paciente femenina de 72 años, quien consultó por cuadro clínico de tres días de evolución consistente en dolor y distensión abdominal asociados a fiebre y síntomas urinarios irritativos y retención urinaria. Los exámenes de ingreso mostraron leucocitosis con neutrofilia y CA-125 en 222 U/mL. El ultrasonido y la resonancia magnética evidenciaron una masa retrouterina solido-quística de 15 cm. Ante sospecha de tumor versus absceso tubo-ovárico, se realizó biopsia dirigida por tomografía mediante la cual se encontró material purulento fétido. Se practicó laparotomía que confirmó masa pélvica retrouterina sólido-quística con contenido purulento, adherencias y compromiso inflamatorio de las trompas uterinas. El resultado de patología informó fibroma ovárico y absceso tubo-ovárico. La paciente evolucionó de satisfactoriamente en el posoperatorio y en los controles posteriores.
Conclusiones. El caso reportado ilustra cómo, en ocasiones, un posible diagnóstico de carcinomatosis por cáncer de ovario puede ser realmente una patología benigna (absceso tubo-ovárico) que responde bien a un tratamiento médico-quirúrgico. Las imágenes diagnósticas y los marcadores tumorales son de gran ayuda para diferenciar una patología ovárica maligna de un proceso benigno.
Abstract
Introduction: Tubo-ovarian abscesses are rare in postmenopausal women. They have been associated with benign gynecological conditions such as endometriosis, uterine polyp or leiomyoma, and malignant diseases such as endometrial adenocarcinoma, epithelial and non-epithelial malignant ovarian tumors, squamous cell carcinoma of the cervix, and adenocarcinoma of the colon. Their presentation represents a diagnostic and therapeutic challenge for clinicians.
Case report: A 72-year-old female patient was admitted with a 3-day history of abdominal pain and distension, fever, irritative urinary symptoms and urinary retention. Lab tests on admission showed elevated white blood cells and neutrophils count, and CA-125 at 222 U/mL. Ultrasound and magnetic resonance imaging revealed a solid retrouterine cystic mass of 15 cm. Suspecting tumor versus tubo-ovarian abscess, a tomography-directed biopsy was performed, finding foul-smelling purulent material. An exploratory laparotomy was performed with intraoperative findings of solid-cystic retrouterine pelvic mass with purulent content, adhesions, and inflammatory involvement of the uterine tubes. Pathology reported ovarian fibroma and tubo-ovarian abscess. The patient evolved satisfactorily in the postoperative period and in the subsequent follow-up appointments.
Conclusions: The reported case illustrates how a possibility of ovarian cancer with peritoneal carcinomatosis can actually be a benign condition (tubo-ovarian abscess) that responds well to medical-surgical treatment. Diagnostic imaging and tumor markers are helpful in differentiating a malignant ovarian disease from a benign process.
Introduction
Tubo-ovarian abscess (TOA) is a complication of pelvic inflammatory disease (PID), which consists of the formation of a purulent collection and distortion of the normal structure of the fallopian tubes and ovary (1). TOA may be accompanied by disabling complications such as pelvic pain, ectopic pregnancy, and rupture of the abscess or intestinal obstruction (2). This type of abscess accounts for 1-2% of admissions to gynecology services and usually occurs in women of reproductive age after exposure to sexually transmitted infections (3), although it has also been observed without preceding sexual activity.
Risk factors for developing a TOA include demographic variables such as low socioeconomic status, risky sexual behavior (multiple sexual partners, previous episodes of PID and early onset of unprotected sex), and recent application of intrauterine devices (3). Age is also a relevant risk factor since it has been found that the younger the age, the higher the risk of PID.
Other factors that may contribute to the development of this phenomenon are cervicovaginal microbiota, cervical ectropion with a larger transformation zone that favors the exposure of columnar epithelium to sexually transmitted infections, and the higher frequency of risky sexual behavior in young patients (4).
Only 1.7% of TOA occurs in postmenopausal women (5). It should be noted that this complication is associated both with benign gynecological conditions (stage III and IV endometriosis, endometrioma, endometrial polyp, uterine leiomyoma) (6,7) and with malignant gynecological and non-gynecological diseases (endometrial adenocarcinoma, malignant epithelial and non-epithelial ovarian tumors, squamous cell carcinoma of the cervix and adenocarcinoma of the colon) (1,8-10).
Case presentation
A 72-year-old female patient from Bogotá, Colombia, Hispanic, housewife and affiliated to the public health scheme, attended the gynecology service of a quaternary care university hospital due to abdominal distention associated with intermittent urinary retention, urinary irritative symptoms, and 3 days unquantified fever.
The patient had consulted another health care institution two weeks earlier for similar symptoms and was diagnosed with upper urinary tract infection by Escherichia coli resistant to quinolones and ampicillin. She received hospital treatment with a third-generation cephalosporin for 10 days.
Relevant medical history included high blood pressure and stage 3A chronic kidney disease. She had also undergone cystopexy with mesh reinforcement 10 years ago and had 5 pregnancies, 4 deliveries and 1 abortion. At the time of consultation, she had no active sex life and had no postmenopausal bleeding. No data were obtained from the last cytology.
On admission examination, the patient was found with normal vital signs, distended abdomen with diffuse pain, no signs of peritoneal irritation, and positive bilateral fist percussion. No mass was palpated in the abdomen. The gynecological examination established that the external genitalia were atrophic. Bimanual palpation revealed elastic vagina with normal temperature and short, atrophic, and closed cervix displaced anteriorly by a painful, firm, fixed mass of about 12cm in diameter that occupied the bottom of the recto-uterine pouch and distended the posterior fornix. This mass prevented the individualization of the uterus and its adnexa and gave the clinical impression of a pelvic abscess, without being able to rule out an adnexal tumor. The results of the admission lab tests are shown in Table 1.
Table 1. Lab test results
|
Date |
Laboratory |
Results |
|
5/12/2019 (admission) |
Hemoglobin |
14 g/dL |
|
Leukocytes |
21.880/mm3 |
|
|
Neutrophils |
19.290/mm3 |
|
|
Platelets |
425.000/mm3 |
|
|
Creatinine |
0.6 mg/dL |
|
|
BUN |
12.8 mg/dL |
|
|
Electrolytes |
Normal |
|
|
Arterial gases |
Normal |
|
|
7/12/2019 |
Lactate dehydrogenase |
223 U/L |
|
7/12/2019 |
BhCG: 0.14 mlU/mL |
0.14 mlU/mL |
|
10/12/2019 |
CA-125 |
222 U/L |
|
10/12/2019 |
Carcino-embryonic antigen |
1.40 ng/mL |
|
10/12/2017 |
Alpha-fetoprotein |
1 UI/mL |
Source: Own elaboration.
The initial patient’s diagnosis was a recurrent complicated urinary tract infection and, given the risk of a multidrug-resistant bacteria, antibiotic treatment was initiated with meropenem (1g every 8 hours intravenously (IV)). A transabdominal and transvaginal ultrasound was subsequently performed, showing a solid-cystic lesion of 115x62x139mm located in the recto-uterine pouch and with low-resistance Doppler flow at the center of the lesion. The mass pushed the uterus forward (Figure 1).

Figure 1. Transabdominal ultrasound obtained using Toshiba Xario 100 with 6mHz convex transducer. A) Uterus (*) displaced by a solid (arrowhead) and cystic (arrow) lesion; B) Doppler of the lesion with low-resistance flow in the solid component.
Source: Document obtained during the course of the study.
A contrast-enhanced computed tomography (CT) of the abdomen and pelvis showed that the mass had the appearance of a pelvic abscess with liquid density lesions with multiple septa in both adnexa. These lesions were accompanied by edema of the surrounding fat, reactive-looking lymph nodes in retroperitoneum, and scarce free fluid in the cavity. It should be noted that the solid component of the mass was not evident with this diagnostic method (Figure 2).
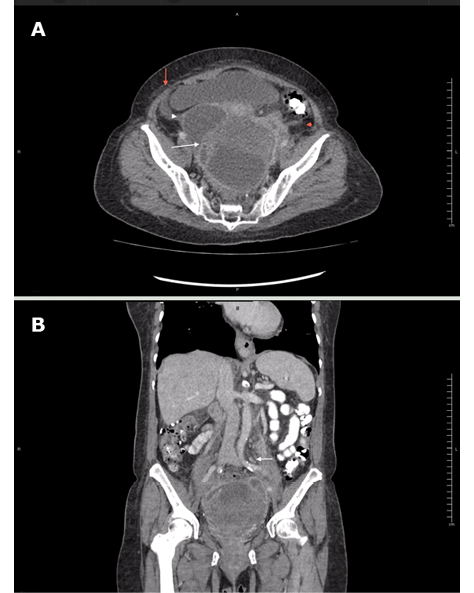
Figure 2. Contrast-enhanced CT scan obtained during arterial phase using an 80-slice multidetector system by Toshiba. A) axial plane: left ovarian lesion with multiple septa, hypodense center, marginal enhancement (white arrow), adjacent fat stranding (red arrowhead) and scarce free fluid (red arrow), and cystic-looking lesion in right adnexa (white arrowhead); B) coronal plane: multiple, retroperitoneal lymph nodes (white arrow).
Source: Document obtained during the course of the study.
The patient was evaluated by the gynecologic oncology service, which considered the possibility of ovarian carcinoma with a low probability of abscess. Contrasted magnetic resonance imaging (MRI) was requested, finding right adnexal mass compatible with simple cyst and left adnexal mass with solid and cystic component of 152x120x103mm; the solid zone had low signal on T2, without enhancement or restriction, and the cystic component showed significant restricted diffusion and low apparent diffusion coefficient (ADC). This technique also showed that the mass pushed the uterus, bladder, and rectum, and confirmed the presence of scarce fluid in the cavity (Figure 3).
Given the findings, a complex adnexal mass M3 was considered. The risk of malignancy index was IRM-1: 1998 and IOTA - ADNEX model: 8 (84% malignancy) (Table 1).
Upper digestive tract endoscopy was performed, finding grade B erosive esophagitis and chronic gastritis, and a colonoscopy showed grade II internal hemorrhoids and diverticulosis; a colon biopsy was taken. The patient was again assessed by gynecologic oncology, which considered the possibility of ovarian carcinoma with a low probability of abscess.
A CT-guided biopsy was performed for histological study, staging and evaluation of surgical benefit, finding abundant purulent foul-smelling material. In view of the diagnostic doubt of malignant tumor versus TOA, an exploratory laparotomy was performed using a midline laparotomy infraumbilical incision.
Laparotomy showed a double-lobed retrouterine pelvic mass of 10x7x5cm and solid-cystic appearance. It was attached to the pelvic walls, the posterior side of the uterus, the pouch of Douglas, and the anterior surface of the sigmoid rectum. One of the locules of the mass had a smooth, purplish, renitent surface with liquid content. The mass had a 6cm light brown solid component in one of its poles, thick walls, and it was firmly adhered to neighboring structures. It was also full of foul-smelling purulent material (Figure 4).
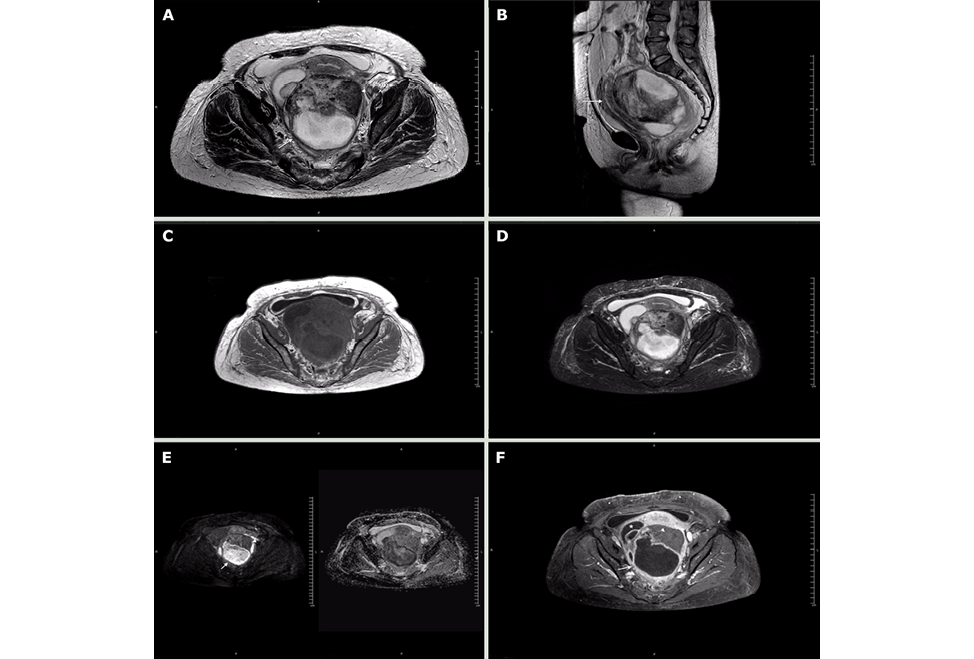
Figure 3. Magnetic resonance imaging obtained using a 1.5T Philips MRI machine. Axial T2-weighted slices. A) axial plane; B) sagittal plane; C) axial T1; D) axial STIR sequence; E) B500 and axial ADC map; F) axial T1 with fat suppression and contrast.
Note: The cystic component is marked in B) with the arrow and shows restricted diffusion in G); the solid component does not show restricted diffusion (G-arrowhead) or enhancement (H-arrowhead). The displacement of the uterus (arrow) and bladder (arrowhead) is evident in C). The asterisk in F) marks the location of the right ovarian cyst.
Source: Document obtained during the course of the study.

Figure 4. Surgical specimen of pelvic mass. A) ovarian fibroma (solid) on the left and abscess (cystic component) on the right;
B) thickened and fibrinopurulent walls of the abscess on the right side of the solid component.
Source: Own elaboration.
Laparotomy also established that the Cook 8 Fr multipurpose drainage catheter that had been placed by interventional radiology was well located in the abscess and that the fallopian tubes were edematous and involved in the inflammatory process. In addition, serous fluid was found on the subdiaphragmatic surface.
Due to the patient’s condition, purulent material culture, bilateral salpingo-oophorectomy, multipurpose catheter removal, drainage, and abdominopelvic cavity washing were performed. Jackson-Pratt drains were left in the pouch of Douglas and the abdominal wall was sutured in planes. On the fourth postoperative day, along with the infectious diseases service, the antibiotic treatment was switched to piperacillin-tazobactam (4.5g every 8 hours IV) after obtaining negative urine and blood cultures at 72 hours.
Pathology report revealed benign stromal ovarian fibroid tumor with areas of necrosis, neutrophil infiltrate, congestion, and edema, corresponding to a TOA. Subdiaphragmatic fluid samples with reactive mesothelial hyperplasia were negative for malignancy, as were colon biopsies obtained during colonoscopy. Enterococcus raffinosus of the usual pattern was isolated in the purulent fluid of the pelvic collection.
Following the surgical procedure, acute kidney injury KDIGO 3 was observed, which was associated with drug-induced nephrotoxicity (dipyrone and metoclopramide) and resolved upon discontinuation. The patient progressed satisfactorily, was discharged 5 days after surgery and completed 14 days of parenteral antibiotic treatment without adverse reactions. The patient was asymptomatic during follow-ups at one week and one month after surgery.
Discussion
TOA is a condition rarely observed after menopause. Gockley et al. (11), in a retrospective study with 61 postmenopausal women, found a wide range in the age of presentation of this disease —from 50 to 87 years—, which coincides with other retrospective studies and case reports (5,6,9,10).
TOA is usually polymicrobial with aerobic and anaerobic bacteria. Enterococcus fecalis, Escherichia coli, Bacteroides fragilis, Peptostreptococcus magnus, Steptococcus sp, Pseudomonas aeruginosa and Clostridium perfringens can be isolated (6).
Most patients with TOA present with abdominal pain as the first symptom (84%), followed by fever (34%), nausea or vomiting (28%), or vaginal bleeding (45%). This condition is also accompanied by comorbidities such as diverticulitis (34%), high blood pressure (25%), and diabetes (10%) (6,9).
37% of women with TOA have history of a recent pelvic surgery. On physical examination, a palpable mass can be found in only 55% of cases; signs of peritoneal irritation are not common (6). In laboratory studies, the levels of leukocytes (mean 13.700/mm3) and CA-125 are usually high, the latter in 77% of patients (mean 101 U/mL), as was the case of our patient. The size of the abscess varies with a mean of 6.0cm (range 1-15cm), and there seems to be no preference for laterality, and at least 10% of cases are bilateral (11).
The patient in the present case was admitted with symptoms similar to those reported in the literature and irritative urinary symptoms that, together with the recent history of urinary tract infection, initially led to suspect the recurrence or persistence of a urinary tract infection. However, based on her clinical evolution and the findings of the abdominal examination, imaging studies were requested; those results, together with elevated levels of CA-125, led to suspect ovarian carcinoma, pelvic abscess-TOA, and abscessed tumor.
The main purpose of the evaluation of an adnexal mass is to rule out malignancy, but since there are no non-invasive techniques to diagnose ovarian cancer, surgical exploration is required, which in many women results in the extraction of benign masses (12).
The treatment of TOA in post-menopause consists of the rapid initiation of broad-spectrum antibiotics, parenteral clindamycin 600mg IV every 6 hours plus gentamicin 3-5 mg/kg IV every 24 hours, cefotetan 2g IV every 12 hours plus doxycycline 100mg IV every 12 hours, or ampicillin/sulbactam 3g IV every 6 hours plus doxycycline 100mg IV every 12 hours (13). IV antibiotic therapy may be switched to oral therapy after 24 hours of clinical improvement, and the recommendation is to complete 14 days with doxycycline. If TOA is preceded or associated with a recent gynecological procedure, extended coverage with metronidazole or clindamycin should be added for anaerobes (14,15).
It should be noted that the decision to combine antibiotic therapy with surgical drainage depends on the patient’s clinical condition and the size of the abscess (14). When the patient has clinical deterioration, signs of sepsis, or suspected abscess rupture, surgical management should be performed. If the patient is stable, the decision to perform surgery will be made taking into account the size of the abscess (even though there is no consensus, some authors have proposed that surgery should be performed when the abscess is between 5 and 8cm in diameter) (14-16).
Some authors have found that women in post-menopause are more likely to develop malignant tumors, both gynecological and non-gynecological, compared to pre-menopause women (12,17). For example, Gockley et al. (11) found in their study that 13.1% of patients with TOA had cancer; four of them had endometrial adenocarcinoma, one had mucinous borderline tumor, two had uterine malignancies, and one had colon adenocarcinoma. Lipscomb & Ling (6) also showed a higher incidence (30%) of malignancy, including endometrial adenocarcinoma and serous malignant ovarian tumor.
Given the high frequency of co-existing cancer with TOA, it has been proposed that surgical management in post-menopause should be individualized and dependent on risk factors for malignancy and differential diagnoses, because in this age group, intraoperative and post-surgical complications are more frequent and include sepsis, infection of the operative site, intestinal injury, and rectovaginal fistulas (11,14).
On the other hand, some case reports and retrospective cohorts have found coexistence between TOA and benign lesions in postmenopausal women, including endometrial polyps (10%), uterine fibroids (3%), and benign serous cystadenomas (2%), usually diagnosed incidentally (6,7,10,11,17). In the present case, the diagnosis was made based on a pelvic mass approach, in which, due to the age group, imaging findings and tumor markers led to suspect a malignant ovarian tumor. However, the CT guided biopsy allowed finding purulent material, so surgical management was decided, revealing the co-existence of TAO with ovarian fibroma.
Ovarian fibroma is a benign ovarian sex cord-stromal tumor, which is made up of collagen-producing fibroblasts, accounting for 4% of ovarian neoplasms. It is the most common pure ovarian stromal tumor (18) and is most common in adolescents and young women. At least 1% of these tumors may be associated with ascites and hydrothorax (Meigs syndrome), and 11.3%, with increased CA-125 levels (range of 36-1848 U/mL), which are higher in large tumors (>10cm in diameter).
Due to its clinical, imaging, and serological characteristics, ovarian fibroma is often mistaken for malignant epithelial tumors in postmenopausal women, so these patients are usually taken to surgical procedures (oophorectomy or unilateral salpingo-oophorectomy). In general, the prognosis of this type of fibroma is good and the recurrence rate is very low (19,20).
The literature review conducted for this report yielded no record of concomitant TAO and ovarian fibroma in postmenopausal patients; however, as mentioned above, some other benign ovarian tumors may co-exist with TOA.
In the present case, TOA explains the leukocytosis with neutrophilia presented by the patient, as well as the unquantified fever, since no persistent or recurrent urinary tract infection was demonstrated. CA-125 can be elevated in malignant epithelial ovarian tumors, but also in physiological conditions such as menstruation and pregnancy and in benign processes such as endometriosis and inflammatory diseases of the peritoneum (21-23), such as the patient in the present case.
Several malignancy indexes have been developed using biomarkers to identify which patients with adnexal masses should be referred to gynecologic oncology, including OVA1 and ROMA (12,24) and MIA2G (overa ®); the latter uses the following biomarker results: apolipoprotein A-1, CA-125, human epididymis protein 4, follicle-stimulating hormone, and transferrin. Fredericks et al. (25) found that the combination of a positive MIA2G test result with an ultrasound had greater sensitivity (93.5%) and specificity (85%) than either of the two individual tests.
From the point of view of diagnostic imaging, in general, complex ovarian or pelvic masses in postmenopausal patients should make the clinician consider malignancies, especially when accompanied by one or more positive tumor markers; however, some characteristics may help discriminate between benign and malignant lesions. In this sense, radiologists take clinical and laboratory characteristics as pretest odds to calculate the final risk of malignancy.
According to the literature, there are more than 80 predictors of ovarian malignancy, among which ADNEX stands out. It is based on the imaging criteria described by the IOTA group (International Ovarian Tumor Analysis) and has an area under the ROC curve of 0.943 (95%CI: 0.934-0.952) to differentiate between benign and malignant lesions (26-29). Additionally, 0-RADS is an ovarian-adnexal imaging-reporting-data system that provides a risk stratification designed to make consistent interpretations, assign a risk of malignancy, and give a treatment recommendation (30).
According to the IOTA group consensus and the studies that support it, malignancy predictors in ultrasounds include the presence of solid and cystic components, papillary projections, multiple septa, thick septa, and Doppler flow in the solid component; the total size of the lesion and the size of the solid component are also considered (22,23).
Characterization of indeterminate masses is not easy, but complementary imaging studies perform as follows:
- Doppler: sensitivity 84% (95%CI: 81-97) and specificity 82% (95%CI: 79-85)
- CT scan: sensitivity 81% (95%CI: 73-85%) and specificity 87% (95%CI: 81-94)
- Simple MRI: sensitivity 76% (95%CI: 70-82) and specificity 97% (95%CI: 95-98)
- Contrast-enhanced MRI: sensitivity 81% (95%CI: 77-84) and specificity 98% (95%CI: 97-99)
Finally, it should be noted that currently one of the best predictors of ovarian malignancy, along with contrast, is the ADC value. A low value in a solid component suggests a malignant lesion, while a low value in a liquid component suggests an abscess. In the present case, a low ADC was found in the liquid component, which is in favor of abscess, and a high ADC in the solid component, suggesting a benign tumor lesion (32).
Conclusions
The reported case illustrates how, sometimes, a possible diagnosis of ovarian cancer carcinomatosis may actually be a benign condition, such as TOA, which responds well to medical-surgical treatment. Diagnostic imaging and tumor markers are of great help in differentiating a malignant ovarian condition from a benign process.
Ethical considerations
The patient provided her informed consent for this case report.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
None stated by the authors.
References
1.Kim SH, Kim SH, Yang DM, Kim KA. Unusual causes of tubo-ovarian abscess: CT and MR imaging findings. Radiographics. 2004;24(6):1575-89. https://doi.org/dqxgzx.
2.Osborne NG. Tubo-ovarian abscess: Pathogenesis and management. J Natl Med Assoc. 1986;78
(10):937-51.
3.Krival TC, Cooksey C, Propst AM. Tubo-Ovarian Abscess: diagnosis, medical and surgical management. Comp Ther. 2004;30(2):93-100. https://doi.org/bc5swp.
4.Lareau SM, Beigi RH. Pelvic Inflammatory Disease and Tubo-ovarian Abscess. Infect Dis Clin North Am. 2008;22(4):693-708. https://doi.org/dcvqww.
5.Blumenfeld Z, Toledano C, Eitan A, Barzilai A, Brandes JM. Tubo-ovarian Abscess in the Postmenopausal Woman. World J Surg. 1982;6(5):634-6. https://doi.org/d8h6fd.
6.Lipscomb GH, Ling FW. Tubo-Ovarian abscess in postmenopausal patients. South Med J. 1992;85(7):696-9. https://doi.org/dfg4bm.
7.Chen MJ, Yang JH, Yang YS, Ho HN. Increased occurrence of tubo-ovarian abscesses in women with stage III and IV endometriosis. Fertil Steril. 2004;82(2):498-9. https://doi.org/fr92fq.
8.Yoshida M, Katsuragawa H, Miyamoto U, Ohnishi S, Katsura Y. Estrogen-producing ovarian adenocarcinoma with large abscess formation. Gynecol Obstet Invest. 1993;35(4):245-8. https://doi.org/bjbphb.
9.Khan NA, Maajeeni EH. Tubo-ovarian abscess in a postmenopausal woman with underlying ovarian carcinoma. Saudi Med J. 2005;26(6):1010-1.
10.Wetchler SJ, Dunn LJ. Ovarian abscess. Report of a case and reviwe of the literature. Obstet Gynecol Surv. 1985;40(7):476-85. https://doi.org/fs8zh9.
11.Gockley AA, Manning-Geist BL, Boatin AA, Gu X, Cohen S. Tubo-ovarian abscesses in postmenopausal women: Clinical presentation and outcomes. Maturitas. 2019;125:20-6. https://doi.org/ftmd.
12.Muto MG. Management of an adnexal mass. UpToDate. 2020 [cited 2020 Aug 20]. Available from: https://bit.ly/2N0ggDu.
13.Centers for Disease Control and Prevention (CDC). 2015 Sexually Transmitted Diseases Treatment Guidelines. Atlanta: CDC; 2015 [cited 2020 Jun 1] Available from: https://bit.ly/3k0YRGZ.
14.Chappell CA, Wiesenfeld HC. Pathogenesis, diagnosis, and management of severe pelvic inflammatory disease and tuboovarian abscess. Clin Obstet Gynecol. 2012;55(4):893-903. https://doi.org/ftmf.
15.Rosen M, Breitkopf D, Waud K. Tubo-Ovarian Abscess Management options for women who desire fertility. Obstet Gynecol Surv. 2009;64(10):681-9. https://doi.org/bdqm93.
16.Farid H, Lau TC, Karmon AE, Styer AK. Clinical Characteristics Associated with Antibiotic Treatment Failure for Tuboovarian Abscesses. Infect Dis Obstet Gynecol. 2016;2016:5120293. https://doi.org/ftmg.
17.Protopapas AG, Diakomanolis ES, Milingos SD, Rodolakis AJ, Markaki SN, Vlachos GD, et al. Tubo-ovarian abscesses in postmenopausal women: Gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114(2):203-9. https://doi.org/drsbh2.
18.Krurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of Tumours of Female Reproductive Organs. 4th ed. Geneva: World Health Organization; 2014.
19.Shen Y, Liang Y, Cheng X, Lu W, Xie X, Wan X. Ovarian fibroma/fibrothecoma with elevated serum CA125 level: A cohort of 66 cases. Medicine (Baltimore). 2018;97(34):e11926. https://doi.org/ftv5.
20.Cho YJ, Lee HS, Kim JM, Lee SY, Song T, Seong SJ, et al. Ovarian-sparing local mass excision for ovarian fibroma/fibrothecoma in premenopausal women. Eur J Obstet Gynecol Reprod Biol. 2015;185:78-82. https://doi.org/f62rqv.
21.Buamah P. Benign Conditions Associated With Raised Serum CA-125 Concentration. J Surg Oncol. 2000;75(4):264-5. https://doi.org/b84v7q.
22.Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/ggdxs6.
23.Khoiwal K, Bahadur A, Kumari R, Bhattacharya N, Rao S, Chaturvedi J. Assessment of Diagnostic Value of Serum Ca-125 and Risk of Malignancy Index Scoring in the Evaluation of Adnexal Masses. J Midlife Health. 2019;10(4):192-6. https://doi.org/ftv6.
24.Kumar V, Rajan S, Gupta S, Akhtar N, Sharma S, Sinha P, et al. Diagnostic Value of Risk of Malignancy Algorithm (ROMA) in Adnexal Masses. J Obstet Gynaecol India. 2020;70(3):214-9. https://doi.org/ftv7.
25.Fredericks TI, Goodrich ST, Ueland FR, Smith A, Bulloock RG, Bonato V, et al. Combining a second generation multivariate index assay with ovarian imaging improves the preoperative assessment of an adnexal mass. J Surg Oncol. 2019;2(3):2-9. https://doi.org/ftv8.
26.Van Calster B, Van Hoorde K, Froyman W, Kaijser L, Wynants L, Landolfo C, et al. Practical guidance for applying the ADNEX model from the IOTA group to discriminate between different subtypes of adnexal tumors. Facts Views Vis Obgyn. 2015;7(1):32-41.
27.Timmerman D, Valentin L, Bourne TH, Collins WP, Verrelst H, Vergote I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol. 2000;16(5):500-5. https://doi.org/cd86x9.
28.Froyman W, Timmerman D. Methods of Assessing Ovarian Masses: International Ovarian Tumor Analysis Approach. Obstet Gynecol Clin North Am. 2019;46(4):625-41. https://doi.org/ftv9.
29.Van Calster B, Valentin L, Froyman W, Landolfo C, Ceusters J, Testa AC, et al. Validation of models to diagnose ovarian cancer in patients managed surgically or conservatively: multicentre cohort study. BMJ. 2020;370:m2614. https://doi.org/ftwc.
30.Andreotti RF, Timmerman D, Strachowski LM, Froyman W, Benacerraf BR, Bennett GL, et al. O-RADS US Risk Stratification and Management System: A Consensus Guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology. 2020;294(1):168-85. https://doi.org/ftwd.
31.Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194(2):311-21. https://doi.org/fm9zrz.
32.Rockall AG. Diffusion weighted MRI in ovarian cancer. Curr Opin Oncol. 2014;26(5):529-35. https://doi.org/f6dhbr.
Referencias
Kim SH, Kim SH, Yang DM, Kim KA. Unusual causes of tubo-ovarian abscess: CT and MR imaging findings. Radiographics. 2004;24(6):1575-89. https://doi.org/dqxgzx
Osborne NG. Tubo-ovarian abscess: Pathogenesis and management. J Natl Med Assoc. 1986;78(10):937-51.
Krival TC, Cooksey C, Propst AM. Tubo-Ovarian Abscess: diagnosis, medical and surgical management. Comp Ther. 2004;30(2):93-100. https://doi.org/bc5swp
Lareau SM, Beigi RH. Pelvic Inflammatory Disease and Tubo-ovarian Abscess. Infect Dis Clin North Am. 2008;22(4):693-708. https://doi.org/dcvqww
Blumenfeld Z, Toledano C, Eitan A, Barzilai A, Brandes JM. Tubo-ovarian Abscess in the Postmenopausal Woman. World J Surg. 1982;6(5):634-6. https://doi.org/d8h6fd
Lipscomb GH, Ling FW. Tubo-Ovarian abscess in postmenopausal patients. South Med J. 1992;85(7):696-9. https://doi.org/dfg4bm
Chen MJ, Yang JH, Yang YS, Ho HN. Increased occurrence of tubo-ovarian abscesses in women with stage III and IV endometriosis. Fertil Steril. 2004;82(2):498-9. https://doi.org/fr92fq
Yoshida M, Katsuragawa H, Miyamoto U, Ohnishi S, Katsura Y. Estrogen-producing ovarian adenocarcinoma with large abscess formation. Gynecol Obstet Invest. 1993;35(4):245-8. https://doi.org/bjbphb
Khan NA, Maajeeni EH. Tubo-ovarian abscess in a postmenopausal woman with underlying ovarian carcinoma. Saudi Med J. 2005;26(6):1010-1.
Wetchler SJ, Dunn LJ. Ovarian abscess. Report of a case and review of the literature. Obstet Gynecol Surv. 1985;40(7):476-85. https://doi.org/fs8zh9
Gockley AA, Manning-Geist BL, Boatin AA, Gu X, Cohen S. Tubo-ovarian abscesses in postmenopausal women: Clinical presentation and outcomes. Maturitas. 2019;125:20-6. https://doi.org/ftmd
Muto MG. Management of an adnexal mass. UpToDate. 2020 [cited 2020 Aug 20]. Available from: https://bit.ly/2N0ggDu.
Centers for Disease Control and Prevention (CDC). 2015 Sexually Transmitted Diseases Treatment Guidelines. Atlanta: CDC; 2015 [cited 2020 Jun 1] Available from: https://bit.ly/3k0YRGZ
Chappell CA, Wiesenfeld HC. Pathogenesis, diagnosis, and management of severe pelvic inflammatory disease and tuboovarian abscess. Clin Obstet Gynecol. 2012;55(4):893-903. https://doi.org/ftmf
Rosen M, Breitkopf D, Waud K. Tubo-Ovarian Abscess Management options for women who desire fertility. Obstet Gynecol Surv. 2009;64(10):681-9. https://doi.org/bdqm93
Farid H, Lau TC, Karmon AE, Styer AK. Clinical Characteristics Associated with Antibiotic Treatment Failure for Tuboovarian Abscesses. Infect Dis Obstet Gynecol. 2016;2016:5120293. https://doi.org/ftmg
Protopapas AG, Diakomanolis ES, Milingos SD, Rodolakis AJ, Markaki SN, Vlachos GD, et al. Tubo-ovarian abscesses in postmenopausal women: Gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114(2):203-9. https://doi.org/drsbh2
Krurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of Tumours of Female Reproductive Organs. 4 th ed. Geneva: World Health Organization; 2014.
Shen Y, Liang Y, Cheng X, Lu W, Xie X, Wan X. Ovarian fibroma/fibrothecoma with elevated serum CA125 level: A cohort of 66 cases. Medicine (Baltimore). 2018;97(34):e11926. https://doi.org/ftv5
Cho YJ, Lee HS, Kim JM, Lee SY, Song T, Seong SJ, et al. Ovarian-sparing local mass excision for ovarian fibroma/fibrothecoma in premenopausal women. Eur J Obstet Gynecol Reprod Biol. 2015;185:78-82. https://doi.org/f62rqv
Buamah P. Benign Conditions Associated With Raised Serum CA-125 Concentration. J Surg Oncol. 2000;75(4):264-5. https://doi.org/b84v7q
Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/ggdxs6
Khoiwal K, Bahadur A, Kumari R, Bhattacharya N, Rao S, Chaturvedi J. Assessment of Diagnostic Value of Serum Ca-125 and Risk of Malignancy Index Scoring in the Evaluation of Adnexal Masses. J Midlife Health. 2019;10(4):192-6. https://doi.org/ftv6
Kumar V, Rajan S, Gupta S, Akhtar N, Sharma S, Sinha P, et al. Diagnostic Value of Risk of Malignancy Algorithm (ROMA) in Adnexal Masses. J Obstet Gynaecol India. 2020;70(3):214-9. https://doi.org/ftv7
Fredericks TI, Goodrich ST, Ueland FR, Smith A, Bulloock RG, Bonato V, et al. Combining a second generation multivariate index assay with ovarian imaging improves the preoperative assessment of an adnexal mass. J Surg Oncol. 2019;2(3):2-9. https://doi.org/ftv8
Van Calster B, Van Hoorde K, Froyman W, Kaijser L, Wynants L, Landolfo C, et al. Practical guidance for applying the ADNEX model from the IOTA group to discriminate between different subtypes of adnexal tumors. Facts Views Vis Obgyn. 2015;7(1):32-41.
Timmerman D, Valentin L, Bourne TH, Collins WP, Verrelst H, Vergote I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol. 2000;16(5):500-5. https://doi.org/cd86x9
Froyman W, Timmerman D. Methods of Assessing Ovarian Masses: International Ovarian Tumor Analysis Approach. Obstet Gynecol Clin North Am. 2019;46(4):625-41. https://doi.org/ftv9
Van Calster B, Valentin L, Froyman W, Landolfo C, Ceusters J, Testa AC, et al. Validation of models to diagnose ovarian cancer in patients managed surgically or conservatively: multicentre cohort study. BMJ. 2020;370:m2614. https://doi.org/ftwc
Andreotti RF, Timmerman D, Strachowski LM, Froyman W, Benacerraf BR, Bennett GL, et al. O-RADS US Risk Stratification and Management System: A Consensus Guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology. 2020;294(1):168-85. https://doi.org/ftwd
Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194(2):311-21. https://doi.org/fm9zrz
Rockall AG. Diffusion weighted MRI in ovarian cancer. Curr Opin Oncol. 2014;26(5):529-35. https://doi.org/f6dhbr
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2021 Case reports

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Los autores al someter sus manuscritos conservarán sus derechos de autor. La revista tiene el derecho del uso, reproducción, transmisión, distribución y publicación en cualquier forma o medio. Los autores no podrán permitir o autorizar el uso de la contribución sin el consentimiento escrito de la revista.
El Formulario de Divulgación Uniforme para posibles Conflictos de Interés y los oficios de cesión de derechos y de responsabilidad deben ser entregados junto con el original.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons 4.0 que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).


























