Effect of pH on the rheological response of reconstituted gastric mucin
Efecto del pH en la respuesta reológica de mucina gástrica reconstituida
DOI:
https://doi.org/10.15446/ing.investig.v35n2.50019Keywords:
Mucin, rheology, z-potential, creep, elasticity (en)Mucina, reología, potencial zeta, fluencia lenta, elasticidad. (es)
The rheological response of reconstituted gastric mucin was studied by time dependent rheological experiments as function of pH. Mucin concentrations of 5% and 10% were prepared in buffer dispersions at pH of 1.15, 2.00, 2.55, 4.00 and 7.67. The isoelectric point was identified by z-potential between pH 2.00 to 2.55. Dynamic light scattering showed that as pH reduced, a second population of larger mucin aggregates was formed indicating the presence of new structures. Steady shear rheological measurements reflected the pseudoplastic behavior of mucin dispersions and the effect of concentration on viscosity. Creep-recovery measurements were performed on the regenerated mucus at different levels of pH. By creep, it was possible to determine the values of zero-shear-viscosity of mucin suspensions, with higher precision and in a lower experimental time than steady shear measurements. Additionally, it was found that at pH ~1.15, the viscosity of the mucus increased to high values, which is an indicative of a gel-like structure. By recovery experiments, it was possible to find that even the very low viscosities the mucin suspensions at pH ~1.15 possessed a defined elastic character. By the use of a four-element mechanistic viscoelastic model, it was concluded that this elasticity underwent retardation due to the combined effect of viscous and elastic responses.
Se estudió la respuesta reológica de mucina gástrica reconstituida como función del pH mediante experimentos reológicos dependientes del tiempo. Se usaron concentraciones de mucina del 5% y 10% en soluciones buffer a valores de pH de 1.15, 2.00, 2.55, 4.00 y 7.67. Mediante potencial zeta se identifico que el punto isoeléctrico se ubicaba entre pH de 2.00 a 2.55. Los estudios de dispersión de luz dinámica mostraron que a mediada que el pH se reduce, una segunda población de agregados de mucina se forma, indicando el estructuramiento dentro del fluido. Las mediciones reológicas en estado estable, mostraron el comportamiento seudoplástico y el efecto de la concentración en las soluciones de mucina. Se llevaron a cabo determinaciones de fluencia lenta-recuperación sobre las suspensiones de mucina a diferentes valores de pH. Mediante fluencia lenta, se determinaron los valores de viscosidad a cero corte con mayor precisión y menor tiempo experimental que las mediciones de estado estable. Adicionalmente, se encontró que para pH ~1.15 la viscosidad del mucus incrementó a valores altos, lo cual es una característica de las estructuras tipo gel. Los experimentos de recuperación mostraron que aun a bajas viscosidades las suspensiones de mucina a pH ~1.15 poseían un carácter elástico definido. Mediante el uso de un modelo viscoelástico de cuatro elementos, se concluyó que esta elasticidad presentaba respuesta retardada debido al efecto combinado de los efectos viscoso y elástico.
DOI: https://doi.org/10.15446/ing.investig.v35n2.50019
Effect of pH on the rheological response
of reconstituted gastric mucin
Efecto del pH en la respuesta reológica
de mucina gástrica reconstituida
J. Caicedo1, and J. E. Perilla2
1 Jonathan Argenis Caicedo: Chemical Engineer, Universidad Nacional de Colombia, Bogotá, Colombia. Affiliation: Universidad Nacional de Colombia-Sede Bogotá- Department of Chemical and Environmental Engineering, Bogotá D.C. Colombia. E-mail: jacaicedol@unal.edu.co.
2 Jairo E. Perilla: Chemical Engineer, Magister in Chemical Engineering, Universidad Nacional de Colombia, Bogotá, Colombia. PhD in Polymer Engineering, The University of Akron, Akron, OH, USA. Affiliation: Universidad Nacional de Colombia-Sede Bogotá - Department of Chemical and Environmental Engineering, Bogotá D.C. Colombia. E-mail:jeperillap@unal.edu.co.
How to cite: Caicedo, J., & Perilla, J. E. (2015). Effect of pH on the rheological response of reconstituted gastric mucin. Ingeniería e Investigación, 35(2), 43-48.
DOI:https://doi.org/10.15446/ing.investig.v35n2.50019
ABSTRACT
The rheolo gical response of reconstituted gastric mucin was studied by time dependent rheological experiments as function of pH. Mucin concentrations of 5 % and 10 % were prepared in buffer dispersions at pH of 1.15, 2.00, 2.55, 4.00 and 7.67. The isoelectric point was identified by z-potential between pH 2.00 to 2.55. Dynamic light scattering showed that as pH reduced, a second population of larger mucin aggregates was formed indicating the presence of new structures. Steady shear rheological measurements reflected the pseudoplastic behavior of mucin dispersions and the effect of concentration on viscosity. Creep-recovery measurements were performed on the regenerated mucus at different levels of pH. By creep, it was possible to determine the values of zero-shear-viscosity of mucin suspensions, with higher precision and in a lower experimental time than steady shear measurements. Additionally, it was found that at pH~1.15, the viscosity of the mucus increased to high values, which is an indicative of a gel-like structure. By recovery experiments, it was possible to find that even the very low viscosities the mucin suspensions at pH~1.15 possessed a defined elastic character. By the use of a four-element mechanistic viscoelastic model, it was concluded that this elasticity underwent retardation due to the combined effect of viscous and elastic responses.
Keywords: Mucin, rheology, z-potential, creep, elasticity.
RESUMEN
Se estudió la respuesta reológica de mucina gástrica reconstituida como función del pH mediante experimentos reológicos dependientes del tiempo. Se usaron concentraciones de mucina del 5 % y 10 % en soluciones buffer a valores de pH de 1.15, 2.00, 2.55, 4.00 y 7.67. Mediante potencial zeta se identifico que el punto isoeléctrico se ubicaba entre pH de 2.00 a 2.55. Los estudios de dispersión de luz dinámica mostraron que a mediada que el pH se reduce, una segunda población de agregados de mucina se forma, indicando el estructuramiento dentro del fluido. Las mediciones reológicas en estado estable, mostraron el comportamiento seudoplástico y el efecto de la concentración en las soluciones de mucina. Se llevaron a cabo determinaciones de fluencia lenta-recuperación sobre las suspensiones de mucina a diferentes valores de pH. Mediante fluencia lenta, se determinaron los valores de viscosidad a cero corte con mayor precisión y menor tiempo experimental que las mediciones de estado estable. Adicionalmente, se encontró que para pH~1.15 la viscosidad del mucus incrementó a valores altos, lo cual es una característica de las estructuras tipo gel. Los experimentos de recuperación mostraron que aun a bajas viscosidades las suspensiones de mucina a pH~1.15 poseían un carácter elástico definido. Mediante el uso de un modelo viscoelástico de cuatro elementos, se concluyó que esta elasticidad presentaba respuesta retardada debido al efecto combinado de los efectos viscoso y elástico.
Palabras clave: Mucina, reología, potencial zeta, fluencia lenta, elasticidad.
Received: April 6th 2015
Accepted: June 16th 2015
Introduction
Mucus is a viscous layer that covers the internal walls of the human cavities, which otherwise would be exposed to the environment with no protection (Ashida et al., 2012). This includes the respiratory, gastrointestinal, reproductive, nasal and ocular surfaces (Hong et al., 2005). The main functions of the mucus are protection and lubrication. In fact, mucus is an efficient barrier to pathogens and other substances, but allows the passage of nutrients and the exchange of gases with the epithelium (Harding, 2006).
The study of the interaction between mucus and polymers has deserved considerable attention in the last years, due to the development of mucoadhesive formulations for controlled release of pharmaceuticals. In this way, the residence time of the dosage form can be appreciably increased optimizing the delivery of an active substance. Mucoadhesion is a complex phenomenon that comprises the humectation of the polymer in contact with the mucus, and also specific interactions between mucoadhesive polymers and mucin. Excellent reviews can be found elsewhere (Boegh & Nielsen, 2015; Alexander, 2011; Harding, 2006; Smart, 2005).
Mucus is composed of water (95 %), mucins (5 %), and other components in much lower amounts (Alexander, 2011; Hong et al., 2005). Mucins are glycoproteins with a high degree of glycolization that can be treated as high polymers (Smart, 2005). Therefore, as in polymer dispersions, the conformation of mucins in dispersion defines the viscoelastic character as well as other properties of the mucus (Celli et al., 2005). The exact composition, thickness and structure of mucus are mainly governed by the nature of the tissue that is being protected, and the amount of salts and other minor components. In the case of gastric mucus, it has been studied that in acid media, mucins change their conformation forming a high viscous gel (Boegh & Nielsen, 2015; Hong et al., 2005). This gel becomes an efficient layer that protects the stomach from being ingested by its own gastric acid during digestion.
Gelation of gastric mucus has been studied by several experimental techniques (Bansil et al., 2013; Hong et al., 2005). It has been concluded that conformational changes in mucin are developed as the pH is reduced to pH < 2. Z-potential has also demonstrated the existence of an isoelectric point (IEP) at pH between 2 and 3, indicating a change in the charge of the molecule as pH decreases (Bansil & Turner, 2006). However, the mechanism of mucin aggregation/gelation and the sol-gel transition in gastric mucin at low pH is not fully understood. Bansin and coworkers (2013) have proposed the formation of gel as the result of a complex interaction between hydrophilic and hydrophobic groups. It is believed that the formation of large linear glycoproteins take place via disulfide (S-S) linkages in the cysteine terminals of the molecule. As the pH is reduced, hydrophobic associations in the non-glycosilated domains induce the formation of non-covalent crosslinks. Thus, a physical crosslinked network is consolidated which is able to stabilize water molecules by the hydrophilic groups forming a protective gel.
It is known that the formation of gels in gastric mucin at low pH develops also unique rheological features (Bansil et al., 2013; Celli et al., 2007; 2005). Gels are characterized by a viscoelastic response originated by the combined effect of both solid-like and liquid-like behavior. Oscillatory shear rheometry reveals that at pH > 4, the loss modulus (G’’) becomes higher than the storage modulus (G’) at low shear frequencies, as it is characteristic of the viscous flow of polymer dispersions (Macosko, 1994). On the other hand, in acidic media, the formation of the endless interconnected structure consolidates the elastic behavior of the gel characterized by G’> G’’. Oscillatory shear rheology has also been used to characterize muadhesion (Mayol et al., 2008), and other polymeric gels (Perilla et al., 2010). However, it has been found that the elastic character of the gels is not totally developed, probably because in most of the experimentations reconstituted gastric mucin was used. Apparently, the purification process to obtain mucin removes salts and other minor components that play a determinant role in the gel formation process (Celli et al., 2007; 2005).
Creep-recovery is a time dependent rheological experiment, which is particularly useful in gels where long relaxation times need to be characterized. Figure 1 sketches the principle of creep-recovery. At t = 0, a constant shear stress, τ0, is applied and the shear strain, y(t), is recorded as a function of time. At the so-called creep time, τr , the stress is removed and the shear recovery, , is measured during the recovery time . From creep-recovery data, creep compliance, and recoverable creep compliance may defined by:
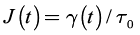 (1)
(1)
 (2)
(2)
Creep-recovery allows characterizing the terminal region of the linear viscoelastic spectra in a much lower experimental time than in dynamic rheology. In this report, the rheological behavior of reconstituted gastric mucin was investigated as function of pH by creep-recovery. The advantages of creep recovery experiments were evident to characterize this process, and the results were compared with steady state rheology, dynamic light scattering and zeta potential.
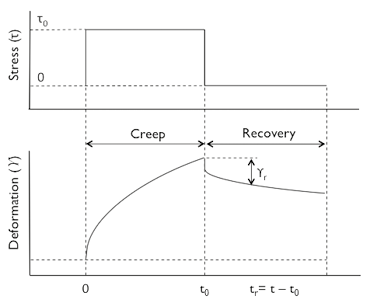
Figure 1. Schematic of a creep-recovery experiment.
Materials and methods
Materials
Partially purified mucin from porcine stomach type II was purchased from SIGMA and maintained at T~ 4ºC. Buffer dispersions were prepared using distilled water and acid, and bases of analytical grade. Mucin dispersions were mixed in sterilized flasks, at 5 and 10 % weight to volume in a laminar flow chamber. Five values of pH were investigated in this report, pH~1.15, 2.00, 2.55, 4.00 and 7.67. After prepared, dispersions were kept at T~ 4ºC, and tested within 24 to 72 hours after preparation.
Characterizations
Scattering and z-potential: The size of mucin aggregates was measured by dynamic light scattering (DLS). DLS and z-potential were determined in a ZetasizerNano ZS (Malvern Instruments, U.K.). Samples were prepared at a concentration of 0.1 mg/ml at pH 1.15, 2.00, 2.55, 4.00 and 7.67. Prior to analysis, the samples were stirred at room temperature and then, centrifuged at 20000 X g for 20 minutes in a Hermle-Z233 M-2. After centrifugation, samples from each supernatant were used for size analysis and zeta potential. Measurements were run at T~ 25ºC, with a time of stabilization of 120 s.
Steady rheological measurements: Rheological characterization of mucin dispersions was carried out in a rotational rheometer Malvern Bohlin-Instruments CVOR rotational rheometer. For the steady state measurements, a 40 mm cone-and-plate setup was used with a cone angle of 2°. A controlled strain sweep was carried out varying the shear rates between ~ 0.001-10 s-1. Shear viscosity, η, was recorded as function of
~ 0.001-10 s-1. Shear viscosity, η, was recorded as function of . Testing temperature of T ~ 25ºC was controlled by a Peltier plate.
. Testing temperature of T ~ 25ºC was controlled by a Peltier plate.
Constant stress rheological measurements: In order to study the viscoelastic nature of the regenerated mucus, creep-recovery measurements were carried out on the samples using a Malvern Bohlin-Instruments CVOR rotational rheometer with a 40 mm cone-and-plate arrangement with a cone angle of 2°. An experimental temperature of T~ 25ºC was controlled with a Peltier plate. At t = 0, a constant shear stress of τ0 ~ 0.006 Pa, was applied to the samples, and the shear strain was recorded up to a creep time of τo ~ 100 s. Then, the stress was removed and the shear recovery was recorded for τr ~ 100 s. In order to account the effect of the residual torque during recovery, the true recovery,![]() , was computed from the measured recovery,
, was computed from the measured recovery, 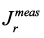 as indicated in Equation (3) (Münstedt, 2014).
as indicated in Equation (3) (Münstedt, 2014).
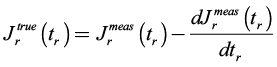 (3)
(3)
Results and discussion
It has been recognized that as the pH of the media is reduced, conformational changes take place in gastric mucin inducing the formation of a stable gel in the mucus. These changes are reflected in Figure 2, where the z-potential is represented as a function of the pH. In Figure 2 it can be seen that the z-potential of the dispersion increases drastically when the pH of the mucin dispersion decreases from pH ~7.67 to 4.00. As the mucin dispersion becomes more acidic (pH < 4.00), the zeta potential increases moderately, and the change from negative to positive z-potential values lies roughly between pH values of 2.00 and 2.55. This point is known as the isoelectric point (IEP), and indicates that the interaction between the mucin and the media changes as the pH is reduced. Thus, as the environment becomes acidic, the chain conformation may change inducing a mucin-mucin interaction stronger than mucin-buffer, resulting in a physical network capable to form gels.
Figure 3 presents the size of the mucin aggregates with respect to the pH, measured by DLS. At pH ~7.67, which is the more basic buffer used in this study, DLS shows that mucins form a monodisperse dispersion. When the pH is reduced, the amount of mucins forming larger aggregates increases, evidenced by the bimodal dispersion for pH ≤4.00. A closer look of Figure 3 reveals that in the vicinity of the IEP (pH ~2.00-2.55), the second peak becomes much stronger. For the most acidic dispersion studied (pH ~1.15), the population of larger aggregates is reduced and the shape of the particle size distribution resembles the one detected at pH ~7.67. The tendency of mucins to form aggregates has been proved by simulation studies using dissipative particle dynamics (Moreno et al., 2015).
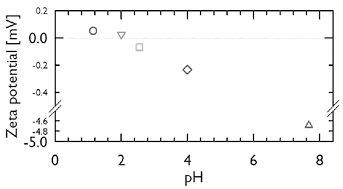
Figure 2. Evolution of zeta potential [mV] with pH for mucin dispersions at 25ºC.
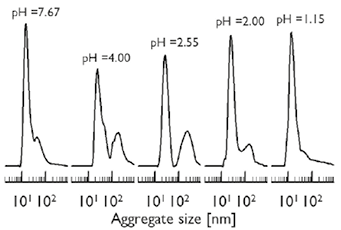
Figure 3. Particle size distribution of mucin dispersions for the investigated levels of pH at 25ºC.
From the results of DLS and zeta potential, one may conclude that mucins interact with the media differently as the pH is reduced, and, that the conformational changes in the mucin are responsible for the formation of gel structure. As it is known, a sol-gel transition is characterized by an abrupt increase in the viscosity. In order to investigate this effect, the rheological behavior of mucin dispersions for pH values between 1.15 and 7.67 was determined in a cone-and-plate rotational rheometer. Figures 4(a) and 4(b) present the η vs.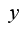 plots for mucin dispersions with concentrations of 5 % and 10 % respectively. As it may be anticipated a shear, thinning behavior is observed for the mucin dispersions and the increase in viscosity with concentration is reflected. However, the effects of pH are not clearly seen and the data is very scattered to associate with the structural changes studied in Figures 2 and 3. Therefore, for the shear rates studied in this research, the levels of viscosity are still very low to be adequately detected by steady shear rheometry. It is necessary to emphasize at this time, that in this research partially purified gastric mucin was used, and that as result of the purification processes and the absence of the minor components present in the physiological mucus, the possibilities of gelification may be strongly reduced. Additionally, the strength of the physical crosslinks in the gel may be so weak that they could be easily degraded at the shear rates investigated.
plots for mucin dispersions with concentrations of 5 % and 10 % respectively. As it may be anticipated a shear, thinning behavior is observed for the mucin dispersions and the increase in viscosity with concentration is reflected. However, the effects of pH are not clearly seen and the data is very scattered to associate with the structural changes studied in Figures 2 and 3. Therefore, for the shear rates studied in this research, the levels of viscosity are still very low to be adequately detected by steady shear rheometry. It is necessary to emphasize at this time, that in this research partially purified gastric mucin was used, and that as result of the purification processes and the absence of the minor components present in the physiological mucus, the possibilities of gelification may be strongly reduced. Additionally, the strength of the physical crosslinks in the gel may be so weak that they could be easily degraded at the shear rates investigated.
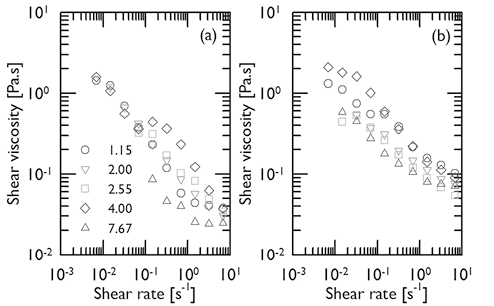
Figure 4. Shear viscosity vs. shear rate for mucin dispersions at T ∼25ºC, different pH (series) and mucin concentrations of 5 % (a) and 10 % (b).
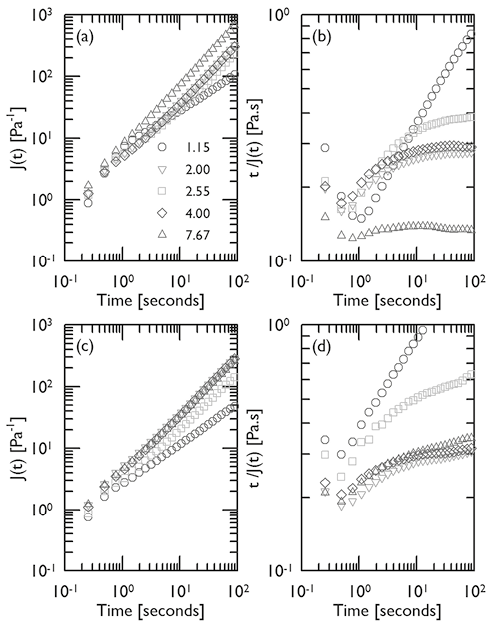
Figure 5. Logaritmic plots of J(t) (a and c) and t/J(t) (b and d) as functions of creep time and pH (series), for mucin dispersions at T ∼25ºC and mucin concentrations of 5 % (a and b) and 10 % (c and d).
The steady rheological measurements presented in Figure 4 indicate that in order to characterize the gel-like structure for the mucin dispersions, it would be necessary to carry out the rheological experiments at very low shear rates in steady rheology or very low frequencies in shear oscillatory experiments. This kind of experiments would become impractical for aqueous and biological dispersions due to the effects of evaporation and degradation at long experimental times. Thus, in order to overcome this effect, creep-recovery tests were performed on the samples. Figures 5(a) and (c) show the creep measurements for 5 % and 10 % mucin dispersions at T~25ºC respectively. For all traces, the effect of a finite rate of response of less than a second, and a very small value of J0~ 1.5 [Pa-1] could be detected. This small elastic response is too slight to indicate that a true and stable molecular network was developed. For pH~4.00 and 7.67, creep curves follows a linear tendency with a slope of the log J(t) vs. log t plot approaching to one. This is a feature of the linear viscoelastic behavior (Münstedt, 2014), which demonstrates that the t0 fixed in this research characterized the mucin dispersions at practically unattainable levels in steady or oscillatory shear rheometry. Figures 5(a) and (c) also reveal that for pH~2.55, the slope of the log J(t) vs. log t plot is slightly lower than one which may be the result of higher viscosities generated by the structures observed in Figure 3 at this pH. This effect is more notorious as mucin concentration increases. When the pH is further reduced (pH~2), the tendency of the plots resembles that of pH~4.00 and 7.67, with a slope at long creep times approaching to one. These changes in creep are attributed to the conformational changes characteristic of the IEP. Interestingly, at the lowest pH studied in this research, pH~1.15, the slope of the creep curve is clearly lower than one. Figures 5(b) and (d) present the plots of t / J(t) vs. t, for mucin dispersions at 5 % and 10 % respectively. It is known that at long creep times the following relationship is obtained (Münstedt, 2014):
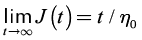 (4)
(4)
where η0 is the zero shear viscosity. Equation (4) indicates that the function t / J(t) plotted in Figures 5(b) and (d) attain a constant value as time increases, which corresponds to η0. Thus, Figures 5(b) and (d) clearly shows that there is a distinctive change in viscosity in the vicinity of the IEP attributed to the larger structures reflected in Figure 3. Just after IEP the viscosity decreases to values observed at higher pH. This behavior is characteristic of the IEP and has been reported for other protein systems (Ding Pacek, 2008; White et al., 2008). In this vein, Figures 5(b) and (d) demonstrate that creep measurements offer a reliable alternative for the determination of η0. However, t / J(t) vs. t plots for mucin concentrations of 5 % and 10 % show no constant value at long times for pH~1.15, but a continuous increase to a very large values. The tendency to high values of viscosity is recognized as a distinctive feature of the gel state, that was not detected in the steady shear rheometry at the levels of shear studied (Figure 4). Creep experiments are in fact a powerful technique to characterize biopolymer dispersions, even of very low concentrations where steady or oscillatory shear rheometry is impractical.
The recovery after creep is a proper characteristic of the elastic behavior (Figure 1). Figures 6(a) and (b) show that for mucin concentrations of 5 % and 10 % respectively, the corrected recovery compliance 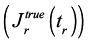 give negative values for all pH > 1.55. These results would indicate that no elastic behavior was registered in the rheometer, or the measured stress is much lower than the limit of detection of the instrument. Thus, it can be assumed that for those samples a pure viscous behavior was the rheological characteristic. Only positive values of recovery compliance were detected for the lowest pH studied in this research (pH~1.15), which is a clear indicative of the elastic character in the sample. This result is in line with what it was learned in Figure 5. Thus, it is concluded that, in an acidic medium of pH~1.15, the RGM self-assemble forming structures that induce a viscoelastic behavior associated with the formation of gels.
give negative values for all pH > 1.55. These results would indicate that no elastic behavior was registered in the rheometer, or the measured stress is much lower than the limit of detection of the instrument. Thus, it can be assumed that for those samples a pure viscous behavior was the rheological characteristic. Only positive values of recovery compliance were detected for the lowest pH studied in this research (pH~1.15), which is a clear indicative of the elastic character in the sample. This result is in line with what it was learned in Figure 5. Thus, it is concluded that, in an acidic medium of pH~1.15, the RGM self-assemble forming structures that induce a viscoelastic behavior associated with the formation of gels.
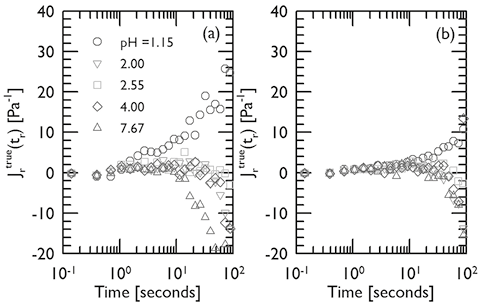
Figure 6. Recovery measurements for mucin dispersions at different pH, T ∼25ºC and mucin concentrations of 5 % (a) and 10 % (b). Corrections to the measured values according with Equation (3).
It is important to note in Figure 6, that the level of recovery for the 5 % mucin dispersion is higher than the recovery for the concentration of 10 %. This apparently unexpected result, may obey to the combined viscous and elastic components of the structures reflected in the rheological experiments. Probably, the simplest mechanistic model that qualitatively captures the viscoelastic response of polymers is presented in Figure 7 (Joye, 1993).
This four-element model could reproduce the instantaneous elastic response corresponding to J0 in creep by the spring-1. As it was studied in Figure 5, no precise value of J0 was detected during creep for all the samples. In a gel, this element would be associated to change of molecular conformations during deformation as it may occur in polymer networks. In gastric mucus, the formation of an elastic network would depend on the minor components of the mucus that are lost during the purification process of the mucin, and that is why it is seen neither in this research nor in previous reports.
Dashpot 1 corresponds to the irrecoverable viscous flow, whose features appear in Figures 5(b) and 5(d), where no recovery is seen for 2.00 < pH < 7.67. The elastic effect comes from the simultaneous response of dashpot-2 and spring-2. As these two elements are in parallel, they work simultaneously and the elastic response is not instantaneous but retarded. It was learned from Figure 4, that an increase in viscosity is observed when the mucin concentration increases. Thus, the higher viscosity of dashpot-2 for the 10 % mucin dispersion produces a much higher retardation effect on the elasticity than in the dispersion with 5 % mucin, which is reflected in Figure 6 as a lower recovery.
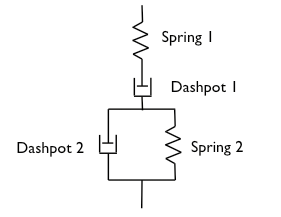
Figure 7. Four-element model.
Conclusions
Gastric mucin dispersions undergo conformational changes as function of pH. At the lowest pH investigated (pH~1.15), mucins self-assemble forming gel-like structures characterized by very high viscosities and the development of elasticity as it was evidenced by creep-recovery measurements. The elasticity of these dispersions is defined by the simultaneous action of viscous and elastic forces. In the vicinity of the IEP (pH~2.00 and pH~2.55), characteristic changes in viscosity were detected by creep measurements. Contrary to previous reports, it was possible to determine the existence of gel-like structures for dispersions with reconstituted gastric mucin. However, the changes in mucin originated during the purification process produce dispersions of very low viscosities whose structures are not feasible to be characterized by steady shear rheometry. This research demonstrated that time dependent rheological experiments such as creep-recovery are a very efficient tool to characterize rheological changes in the region of slow relaxations. DLS also demonstrated the existence of larger mucin aggregates as pH decreased.
Acknowledgement
Financial support was provided by COLCIENCIAS through the grant number 1101-521-28526, and Universidad Nacional de Colombia through the grants 15371, 16356 and 18481.
References
Alexander, A. (2011). Mechanism responsible for mucoadhesion of mucoadhesive drug delivery system: a review. International Journal of Applied Biology and Pharmaceutical Technology, 2(1), 434-445.
Ashida, H., Ogawa, M., Kim, M., Mimuro, H., & Sasakawa, C. (2012). Bacteria and host interactions in the gut epithelial barrier. Nature Chemical Biology, 8(1), 36-45.
DOI: 10.1038/nchembio.741.
Bansil, R., Celli, J. P., Hardcastle, J. M., & Turner, B. S. (2013). The influence of mucus microstructure and rheology in Helicobacter pylori infection. Frontiers in Immunology, 4 (310), 1-12.
Bansil, R., & Turner, B. S. (2006). Mucin structure, aggregation, physiological functions and biomedical applications. Current Opinion in Colloid & Interface Science, 11, 164-170. DOI: 10.1016/j.cocis.2005.11.001
Boegh, M., & Nielsen, H.M. (2015) Mucus as a barrier to drug delivery-understanding and mimicking the barrier properties. Basic & Clinical Pharmacology & Toxicology. 116, 179-186. DOI: 10.1111/bcpt.12342.
Celli, J.P., Turner, B.S., Afdhal, N.H., Ewoldt, R.H., McKinley, G.H., Bansil, R., & Erramilli, S. (2007). Rheology of gastric mucin exhibits a pH-Dependent sol-gel transition. Biomacromolecules, 8, 1580-1586. DOI: 10.1021/bm0609691.
Celli, J., Gregor, B., Turner, B., Afdhal, N.H., Bansil, R., & Erramilli, S. (2005). Viscoelastic Properties and Dynamics of Porcine Gastric Mucin. Biomacromolecules, 6, 1329-1333. DOI: 10.1021/bm0493990.
Ding, P., & Pacek, A. (2008). Effect of pH on deagglomeration and rheology/morphology of aqueous suspensions of goethite nanopowder. Journal of Colloid and Interface Science, 325, 165-172. DOI: 10.1016/j.jcis.2008.04.077.
Harding, S. E. (2006). Trends in mucoadhesive analysis. Trends in Food Science & Technology, 17, 255-262.
DOI: 10.1016/j.tifs.2005.12.007.
Hong, Z., Chasan, B., Bansil, R., Turner, B. S., Bhaskar, K. R., & Afdhal, N. H. (2005). Atomic force microscopy reveals aggregation of gastric mucin at low pH. Biomacromolecules, 6, 3458-3466. DOI: 10.1021/bm0505843.
Joye, D. D. (1993). Stress–relaxation in three-element and four-element mechanical models of viscoelastic materials. Journal of Applied Polymer Science, 47 (2), 345-350.
DOI: 10.1002/app.1993.070470214.
Macosko, C. W. (1994). Rheology. Principles, measurements and applications. New York: Wiley-VCH.
Mayol, L., Quaglia, F., Borzacchiello, A., Ambrosio, L., & La Rotonda, M. I. (2008). A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. European Journal of Pharmaceutics and Biopharmaceutics, 70, 199–206. DOI: 10.1016/j.ejpb.2008.04.025.
Moreno, N., Perilla, J. E., Colina, C. M., & Lísal, M. (2015). Mucin aggregation from a rod-like meso-scale model. Molecular Physisc, 113 (9-10), 898-909.
DOI: 10.1080/00268976.2015.1023750.
Münstedt, H. (2014). Rheological experiments at constant stress as efficient method to characterize polymeric materials. Journal of Rheology , 58 (3), 565-587.
DOI: 10.1122/1.4866049.
Perilla, J. E., Lee, B.-J., & Jana, S. C. (2010). Rheological investigation of interactions between sorbitol and polyhedral oligomeric silsesquioxane in development of nanocomposites of isotactic polypropylene. Journal of Rheology, 54 (4), 761-779. DOI: 10.1122/1.3439695.
Smart, J. D. (2005). The basics and underlying mechanisms of mucoadhesion. Advanced Drug Delivery Reviews, 57, 1556-1568. DOI: 10.1016/j.addr.2005.07.001.
White, D., Fisk, I., Mitchell, J., Wolf, B., Hill, S., & Gray, D. (2008). Sunflower-seed oil body emulsions: Rheology and stability as-sessment of a natural emulsion. Food Hydrocolloids, 22, 1224–1232.
DOI: 10.1016/j.foodhyd.2007.07.004.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Mohammad Firoznezhad, Rita Abi-Rached, Federica Fulgheri, Matteo Aroffu, Francisco-Javier Leyva-Jiménez, María de la Luz Cádiz Gurrea, Maria Cristina Meloni, Francesco Corrias, Elvira Escribano-Ferrer, Josè Esteban Peris, Maria Letizia Manca, Maria Manconi. (2023). Design and in vitro effectiveness evaluation of Echium amoenum extract loaded in bioadhesive phospholipid vesicles tailored for mucosal delivery. International Journal of Pharmaceutics, 634, p.122650. https://doi.org/10.1016/j.ijpharm.2023.122650.
2. Gustavo Ruiz-Pulido, Dora I. Medina. (2021). An overview of gastrointestinal mucus rheology under different pH conditions and introduction to pH-dependent rheological interactions with PLGA and chitosan nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics, 159, p.123. https://doi.org/10.1016/j.ejpb.2020.12.013.
3. H. Teixeira, A.C. Branco, I. Rodrigues, D. Silva, S. Cardoso, R. Colaço, A.P. Serro, C.G. Figueiredo-Pina. (2021). Effect of albumin, urea, lysozyme and mucin on the triboactivity of Ti6Al4V/zirconia pair used in dental implants. Journal of the Mechanical Behavior of Biomedical Materials, 118, p.104451. https://doi.org/10.1016/j.jmbbm.2021.104451.
4. Francesca Cuomo, Martina Cofelice, Francesco Venditti, Andrea Ceglie, Maria Miguel, Björn Lindman, Francesco Lopez. (2018). In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids and Surfaces B: Biointerfaces, 168, p.29. https://doi.org/10.1016/j.colsurfb.2017.11.047.
5. Ezzeddin M.A. Hejjaji, Alan M. Smith, Gordon A. Morris. (2018). Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. International Journal of Biological Macromolecules, 120, p.1610. https://doi.org/10.1016/j.ijbiomac.2018.09.185.
6. C.E. Wagner, K.M. Wheeler, K. Ribbeck. (2018). Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annual Review of Cell and Developmental Biology, 34(1), p.189. https://doi.org/10.1146/annurev-cellbio-100617-062818.
7. Younas Dadmohammadi, Hooman Torabi, Seyed Mohammad Davachi, Mackenzie Childs, Victoria Cao, Alireza Abbaspourrad. (2022). Physicochemical interactions between mucin and low-calorie sweeteners: Real-time characterization and rheological analyses. LWT, 159, p.113252. https://doi.org/10.1016/j.lwt.2022.113252.
8. Gustavo Ruiz-Pulido, David Quintanar-Guerrero, Luis Eduardo Serrano-Mora, Dora I. Medina. (2022). Triborheological Analysis of Reconstituted Gastrointestinal Mucus/Chitosan:TPP Nanoparticles System to Study Mucoadhesion Phenomenon under Different pH Conditions. Polymers, 14(22), p.4978. https://doi.org/10.3390/polym14224978.
9. Jie Hou, Chao Hu, Yanlong Wang, Jianying Zhang, Jason C. White, Kun Yang, Daohui Lin. (2022). Nano-bio interfacial interactions determined the contact toxicity of nTiO2 to nematodes in various soils. Science of The Total Environment, 835, p.155456. https://doi.org/10.1016/j.scitotenv.2022.155456.
10. Sumit Sunil Kumar, Travis Leadbetter, J. Brandon McClimon, Manuel A. Lema, Farhana M. Khan, Prashant K. Purohit, Adam B. Braunschweig, Robert W. Carpick. (2025). Interfacial Yield Stress Response in Synthetic Mucin Solutions. Advanced Materials Interfaces, 12(12) https://doi.org/10.1002/admi.202500066.
11. Jacob A. Erstling, Nirmalya Bag, Thomas C. Gardinier, Ferdinand F. E. Kohle, Naedum DomNwachukwu, Scott D. Butler, Teresa Kao, Kai Ma, Melik Z. Turker, Grant B. Feuer, Rachel Lee, Nada Naguib, James F. Tallman, Henry F. Malarkey, Lieihn Tsaur, William L. Moore, Dana V. Chapman, Tangi Aubert, Saurabh Mehta, Richard A. Cerione, Robert S. Weiss, Barbara A. Baird, Ulrich B. Wiesner. (2024). Overcoming Barriers Associated with Oral Delivery of Differently Sized Fluorescent Core‐Shell Silica Nanoparticles. Advanced Materials, 36(1) https://doi.org/10.1002/adma.202305937.
12. Cosmin Butnarasu, Nadia Barbero, Claudia Barolo, Sonja Visentin. (2020). Squaraine dyes as fluorescent turn-on sensors for the detection of porcine gastric mucin: A spectroscopic and kinetic study. Journal of Photochemistry and Photobiology B: Biology, 205, p.111838. https://doi.org/10.1016/j.jphotobiol.2020.111838.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2015 Jonathan Argenis Caicedo, Jairo Ernesto Perilla

This work is licensed under a Creative Commons Attribution 4.0 International License.
The authors or holders of the copyright for each article hereby confer exclusive, limited and free authorization on the Universidad Nacional de Colombia's journal Ingeniería e Investigación concerning the aforementioned article which, once it has been evaluated and approved, will be submitted for publication, in line with the following items:
1. The version which has been corrected according to the evaluators' suggestions will be remitted and it will be made clear whether the aforementioned article is an unedited document regarding which the rights to be authorized are held and total responsibility will be assumed by the authors for the content of the work being submitted to Ingeniería e Investigación, the Universidad Nacional de Colombia and third-parties;
2. The authorization conferred on the journal will come into force from the date on which it is included in the respective volume and issue of Ingeniería e Investigación in the Open Journal Systems and on the journal's main page (https://revistas.unal.edu.co/index.php/ingeinv), as well as in different databases and indices in which the publication is indexed;
3. The authors authorize the Universidad Nacional de Colombia's journal Ingeniería e Investigación to publish the document in whatever required format (printed, digital, electronic or whatsoever known or yet to be discovered form) and authorize Ingeniería e Investigación to include the work in any indices and/or search engines deemed necessary for promoting its diffusion;
4. The authors accept that such authorization is given free of charge and they, therefore, waive any right to receive remuneration from the publication, distribution, public communication and any use whatsoever referred to in the terms of this authorization.



























