Synthesis and characterization of mixed-ligand silver(I) saccharinate complex containing (2-(2-pyridyl)benzimidazole
Síntesis y caracterización del complejo mixto sacarinato de plata (I) con (2-(2-piridil)bencimidazol)
Síntese e caracterização do complexo misto sacarinato de prata (I) com (2-(2-piridil)benzimidazol)
DOI:
https://doi.org/10.15446/rev.colomb.quim.v47n2.68225Palabras clave:
saccharinate, silver, 2-(2-pyridyl)benzimidazole, XPS (en)sacarinato, plata, 2-(2-piridil)bencimidazol, XPS (es)
sacarina, prata, 2-(2-piridil)benzimidazol, XPS (pt)
Descargas
A new silver coordination compound [Ag(sac)(pbi)] was synthesized by reaction of silver(I) saccharinate with 2-(2-pyridyl)benzimidazole (pbi) in 64% yield. The characterization was performed by elemental analysis, IR, UV-Visible, XPS, 1H-NMR, and 13C-NMR spectroscopy. According to the results, silver is coordinating through three nitrogen atoms: one from saccharinate and the others from 2-(2-pyridyl)benzimidazole forming with this ligand a five-membered chelate ring.
Se sintetizó un nuevo compuesto de coordinación de plata, [Ag(sac)(pbi)], por reacción de sacarinato de plata(I) con 2-(2-piridil)bencimidazol (pbi) con un rendimiento de 64%. La caracterización se realizó por análisis elemental, espectroscopia IR, UV-Visible, XPS, 1H-RMN y 13C-RMN. De acuerdo con los resultados obtenidos la plata está coordinada a través de tres átomos de nitrógeno, uno del sacarinato y los dos restantes del 2-(2-piridil)-bencimidazol formando con este ligando un anillo quelato de cinco miembros.
O presente trabalho compreende a síntese do composto de coordenação de prata, [Ag(sac)(pbi)], por reação de sacarinato de prata(I) com 2-(2-piridil)benzimidazol (pbi) com 64% de rendimento. A caracterização foi realizada por análise elementar, espectroscopia IR, UV-Visível, XPS, 1H-RMN e 13C-RMN. De acordo com os resultados obtidos, a prata é coordenada através de três átomos de nitrogênio, um do sacarinato e os outros dois do 2-(2-piridil)benzimidazol que formam com este ligando um anel de quelato de cinco membros.

Fisicoquímica e Inorgánica
Synthesis and characterization of mixed-ligand silver(I) saccharinate complex containing (2-(2-pyridyl)benzimidazole
Síntesis y caracterización del complejo de plata(I) con ligandos mixtos sacarinato y 2-(2-piridil)bezimidazol
Síntese e caracterização do complexo de prata(I) com ligandos mistos sacarinato e 2-(2-piridil)benzimidazol
Synthesis and characterization of mixed-ligand silver(I) saccharinate complex containing (2-(2-pyridyl)benzimidazole
Revista Colombiana de Química, vol. 47, no. 2, 2018
Universidad Nacional de Colombia
Abstract: A new silver coordination compound [Ag(sac)(pbi)] was synthesized by reaction of silver(I) saccharinate with 2-(2-pyridyl)benzimidazole (pbi) in 64% yield. The characterization was performed by elemental analysis, IR, UV-Visible, XPS, 1H-NMR, and 13C-NMR spectroscopy. According to the results, silver is coordinating through three nitrogen atoms: one from saccharinate and the others from 2-(2-pyridyl)benzimidazole forming with this ligand a five-membered chelate ring.
Keywords: saccharinate, silver, 2-(2-pyridyl)benzimidazole, XPS.
Resumen: Se sintetizó un nuevo compuesto de coordinación de plata, [Ag(sac)(pbi)], por reacción de sacarinato de plata(I) con 2-(2-piridil)bencimidazol (pbi) con un rendimiento de 64%. La caracterización se realizó por análisis elemental, espectroscopia IR, UV-Visible, XPS, 1H-RMN y 13C-RMN. De acuerdo con los resultados obtenidos la plata está coordinada a través de tres átomos de nitrógeno, uno del sacarinato y los dos restantes del 2-(2-piridil)-bencimidazol formando con este ligando un anillo quelato de cinco miembros.
Palabras clave: sacarinato, plata, 2-(2-piridil)bencimidazol, XPS.
Resumo: O presente trabalho compreende a síntese de um novo composto de coordenação de prata, [Ag(sac)(pbi)], por reação de sacarinato de prata(I) com 2-(2-piridil)benzimidazol (pbi) com 64% de rendimento. A caracterização foi realizada por análise elementar, espectroscopia IV, UV-Visível, XPS, 1H-RMN e 13C-RMN. De acordo com os resultados obtidos, a prata é coordenada através de três átomos de nitrogênio, um do sacarinato e os outros dois do 2-(2-piridil)benzimidazol que formam com este ligando um anel de quelato de cinco membros.
Palavras-chave: sacarinato, prata, 2-(2-piridil)benzimidazol, XPS.
Introduction
Saccharin (Hsac) is a non-caloric sweetener, relatively insoluble in water but soluble in solvents such as ethanol, acetone, and methanol ( 1 ). The pKa value (1.60) for saccharine in aqueous solution indicates that this compound give up its proton (NH group) giving rise to saccharinate (sac) ( 2 ).
The first studies of saccharin complexes were carried out with the formation of aquo-complexes, due to the ability to coordinate with metals through the nitrogen atom. However, its polyfunctionality allowed to increase the number of possible complexes and the study of new chemical properties. The different functional groups, in combination with a series of metal centers, allow a series of coordination modes and chemical structures that will be discussed later. Baran and Yilmaz ( 2 , 3 ) developed some structural characteristics of complexes models based on the different types saccharinate bonds.
The first homoleptic silver(I) saccharinate reported by R. Weber ( 4 ) was prepared by adding AgNO3 to aqueous Na(sac) solution, forming a white precipitate which was recrystallized from ammonia solution. The second homoleptic silver(I) saccharinate was obtained by Veysel T. Yilmaz ( 5 ) by the reaction of Na(sac) and AgNO3 in the presence of 2-pyridinepropanol and water.
There are several silver(I) saccharinate heteroleptic compounds in which silver is coordinated with other ligands and important interactions have been reported, such as those of the type Ag···Ag that contribute to the structural formation and have different properties ( 3 ). Other nearby interactions have also been reported, such as Carom···Ag, Ag··· π and C-H···Ag ( 6 ). The geometries founded for AgI complexes type Agy[(sac) (L)]x are generally trigonal plane, T-shaped, tetrahedral, and trigonal pyramidal ( 7 ), as well as monomeric and dimeric units. There are many types of coordination for silver(I) saccharinate heteroleptic complexes ( 6 ).
Various silver(I) saccharinate compounds have been reported and they can be classified according to the chemical structure of the additional ligand as shown in Table 1.
| Ligand | Silver(I) saccharinate compounds |
| Pyridine | · [Ag2(sac)2(pyet)2]) and [Ag4(sac)4(pypr)2] (pyet=2-pyridineethanol pypr=2-pyridinepropanol) ( 8 ). · [Ag(sac)(μ-mpy)]n and [Ag(μ-sac)(dmpy)]n (mpy=2-pyridylmethanol and dmpy=2,6-pyridinedimethanol) ( 9 ). · [Ag2(sac)2(bpeh)] and [Ag(sac)(bpma)]n (bpeh=1,2-Bis[1-(pyridine-2-yl)ethylene]hidrazine and bpma=N,N-bis(pyridine-2-ylmethyl)amine) ( 10 ). · [Ag(sac)(dpa)] (dpa=2,2´-dipyridylamine) ( 11 ). |
| Pyrazole and imidazole | · [Ag2(sac)2(pydz)2] and [Ag(sac)(pyz)]n (pydz=pyridazine and pyz=pyrazine) ( 3 ). · [Ag(sac)(mpr)]2 (mpr=2-methyl-1-pyrroline) ( 6 ). · [Ag2(sac)2(o-bix)2] (o-bix=1,2–bis(imidazole-1-ylmethyl)benzene) ( 11 ). · [Ag(sac)(pz)(H2O)]n and [Ag(sac)(im)].2H2O (pz=pyrazole e im=imidazole) ( 12 ). · [Ag2(sac)2(μ-pbix)2] and [Ag2(sac)2(μ-mbix)] (pbix=1,4-bis(imidazole-1-ylmethyl)benzene and mbix=1,3–bis(imidazole-1-ylmethyl)benzene) ( 13 ). |
| Piperazine | · [[Ag(μ-sac)2(μ-hep)2]n, (hep=N-2-Hidroxyethylpyperazine) ( 14 ). · [Ag4(sac)(mpyz)2(H2O)2] and [Ag2(sac)2(pyzca)2] (mpyz=2-methylpyrazine and pyzca=pyrazine-2-carboxamide) ( 15 ). |
| Aliphatic amines | · [Ag2(sac)2(pen)2] and [Ag2(sac)2(nmen)]n (pen=1,3-diaminepropane and nmen=N-methylethylenediamine)( 16 ). · [Ag2(μ3-sac)2(μ-nmpen)]n, (nmpen=N-methyl-1,3-propanediamine) ( 6 ). · Ag(sac)(edmen)] and [Ag(Sac)(teten)] (edmen=N–ethyl-N´,N´-dimethylethylenediamine and teten=N,N,N´,N´-tetrakis(2-hidroxyethyl) ethylenediamine) ( 11 ). |
| Sulphur | · [Ag(sac)(Ph2SNH)] (Ph2SNH=S,S–diphenylsulfimide) ( 17 ). |
| Phosphine | · [Ag(μ-sac)(PPh3)2]2, [Ag(μ-sac)(PPh2Cy)]2, [Ag(μ-sac)(PPhCy2)]2 and [Ag(μ-sac)(PCy3)]n, (PPh3=triphenylphosphane; PPh2Cy=cyclohexyldiphenylphosphane; PPhCy2=dicyclohexylphenylphosphane and PCy3=tricyclohexylphosphane) ( 18 ). |
| Others | · [Ag2(sac)2(MeCN)2]n ( 19 ) and [Ag4(sac)4(mpyz)2(H2O)2] and [Ag2(sac)2(pyzca)2] donde (mpyz=2-methylpyrazine, pyzca=pyrazine-2-carboxamide) ( 20 ). |
Saccharin, as well as some metal-saccharin complexes, possesses biological activity (2). For example, saccharinates of Cu (II), Zn (II), Ce (IV), and Hg (II) exhibit inhibitory activity of carbonic anhydrase and aqueous metal saccharinates are effective in the dismutation of the superoxide anion. In other cases, saccharin derivatives act as an inhibitor of enzymes that cause inflammatory diseases. Some metal saccharinates, especially those of Ag (I), Cu (II), and lanthanides, possess microbiological activity. Furhtermore, benzimidazole derivatives have great biological importance since its heteorcyclic system is present in antiparasitic compounds, anticonvulsants, analgesics, antihistaminics, antiulcer, antihypertensives, antiviral, anticancer, antifungals, anti-inflammatory agents, proton pump inhibitors and anticoagulants ( 21 ).
Likewise, some metal derivatives of 2-(2'-pyridyl)benzimidazole have visible-light photosensitizer properties for the isomerization of aromatic alkenes ( 22 ) and can be use as electroluminescent materials in light-emitting electrochemical cells ( 23 ). Because of the 2-(2'-pyridyl)benzimidazole reactivity, it is an area of chemical interest, particularly in medicine. Therefore, the present work aims to synthesize a new heteroleptic silver compound bonded to saccharin and 2- (2'-pyridyl)benzimidazole (pbi).
Materials and methods
All chemicals were of reagent grade and quality HPLC solvents of the brand Merck from Germany. Infrared Spectrophotometer with Fourier Transform Thermo Nicolet iS10 (Germany), UV-Visible Thermo Scientific Helios (Peru), Micro Leco Elemental Analyzer Truspec Micro (Spain), X-Ray Photoelectron Spectroscopy (Spain), K-Alpha, and Nuclear Magnetic Resonance (1H and 13C 200MHz) Varian Gemini 200 (Germany) were used.
Synthesis of [Ag(sac)(pbi)]
AgNO3 (0.05 g, 0.29 mmol) dissolved in a mixture of CH3COCH3, CH3CN, and CH3OH (6 mL, 1:1:1) was mixed with Na(sac)⋅2H2O (0.07 g, 0.29 mmol) dissolved in the same solvent mixture (6 mL). The mixture was stirred for 30 min and silver(I) saccharinate was formed. Then, 2-(2-pyridyl)benzimidazole (pbi) (0.06 g, 0.29 mmol) dissolved in the above solvent mixture (5 mL) was added and stirred for 2 h. The entire process (Figure 1) was performed at room temperature and isolated from light. Then, white precipitate was vacuum filtered, washed with cold CH3CN (3 x 5 mL) and dried for 1 h at 50°C. Finally, the remaining solid was recrystallized from acetonitrile.
The resulting white acicular in appearance product weighed 0.09 g (64% yield) and was soluble in CHCl3, DMF, DMSO and warm CH3CN. Decomposition point 272-274 °C.
![Figure
1. Sequence of chemical reactions to synthesize
[Ag(sac)(pbi)].](https://revistas.unal.edu.co/index.php/rcolquim/article/download/68225/version/54127/67468/396828/309055565009_gf2.png)
Figure 1. Sequence of chemical reactions to synthesize [Ag(sac)(pbi)].
Elemental analysis for C19H13SO3N4Ag calculated: C 47.03%, H 2.70%, N 11.55%, S 6.61%. Found: C 47.00%, H 2.89%, N 11.88%, S 6.13%.
FT-IR (cm-1): 3260 m (NHimidazol),3063 m (CHarom), 1628 s (C=O), 1551 s (C=Carom), 1447 m (C=Carom), 1404 m (Cimidazol-N), 1269 s (SO2), 1254 sh (SO2), 1153 s (SO2), 1053 w (CHarom), 1146 vs (SO2), 968 m (CNS), 745 m (CCC), 676 w (CNC), 605 w (CNC).
UV-Vis λmax(DMF)/nm: 263 and 305.
XPS (eV): N 1s 398.70, 399.26, 400.36, 406.45; O 1s 531.95, 532.4, 531.41; S 2p 168.20, 169.38; C 1s 284.92, 286.90, 288.09; Ag 3d5/2 368.42, 3d3/2 374.44.
1H RMN (200 MHz, DMSO-d6, Me4Si) d: 7.40 (2 H m), 7.70 (1H, m), 7.75-7.79 (4H, m), 7.91 (1 H, m), 7.83 (1 H, d), 8.42 (1 H, d), 8.19 (1 H, m), 8.82 (1H d).
13C {1H} RMN (200 MHz, DMSO-d6, Me4Si) d: 167.00 (C3=O), 150.91 (C6’’H), 150.50 (C2’), 145.75 (C2’’), 143.78 (C9 and C4’), 139.28 (C4’’H), 133.35 (C6’H and C7’H), 133.22 (C6H and C7H)), 132.75 (C4 and C9’), 126.61 (C5’’H), 124.48 (C8H and C5’H)), 123.93 (C5H), 122.44 (C3’’H), 120.57 (C8’H).
Results and discussion
The elemental analysis of the compound obtained is consistent with the formula [Ag(sac)(pbi)]. Also, the presence of a silver center with +1 oxidation state was verified by XPS.
Two main and overlapping bands were determined by UV-visible spectroscopy, the highest intensity at 263 nm λmax corresponding to the saccharinate and one less intense with λmax at 305 nm of the pbi. The bands shown originate from the nàπ* and πàπ* of molecular orbitals from C=N, C=O and S=O bonds. There are no metal-ligand transitions between 400 nm and 700 nm because saccharinate and 2-(2-pyridyl)benzimidazole are low field ligands, whereby the absence of color in the complex is warranted.
The most useful infrared bands of 2-(2-pyridyl)benzimidazole and saccharinate could be found as follows: ν(streching N-H) a 3260 cm-1 from pbi, ν(streching CPh-H) aromatic at 3063 cm-1, ν(streching CPh-C) and ν(streching Cim=N) between 1447 cm-1 and 1651 cm-1 for both ligands, ν(streching C-N-S) at 1339 cm-1, ν(streching SO2) between 1269 and 1254 cm-1, ν(bending C-N-S) at 968 cm-1 and ν(bending C-N-C) between 605 cm-1 and 676 cm-1.
The 1H-NMR and 13C-NMR spectra of [Ag(sac)(pbi)] are very complex. The presence of protons in similar chemical environments generated displacements and undefined signals, usually overlapping multiplets. However, with the help of 13C-DEPT-NMR, two-dimensional 1H{1H}-NMR/COSY 45º, 1H{13C}-NMR/HMBC, and 1H{13C}-NMR/HSQC spectra were possible to complete assignments of all the carbon and hydrogen atoms for the compound synthesized as shown in Figure 2.
![Figure 2. Numbering H and C atoms for [Ag(sac)(pbi)]
related to NMR spectra assignment.](https://revistas.unal.edu.co/index.php/rcolquim/article/download/68225/version/54127/67468/396829/309055565009_gf3.png)
Figure 2. Numbering H and C atoms for [Ag(sac)(pbi)] related to NMR spectra assignment.
1H-NMR data for [Ag(sac)(pbi)] (Table 2) shows four protons of saccharinate: 7.83 ppm (H5), between 7.32 and 7.42 (H8) and the range 7.75-7.79 ppm (H6 and H7). Protons at higher fields are protected by the benzene ring and are further from the electronically dense C=O and SO2 groups.
The protons for pbi in [Ag(sac)(pbi)] are displaced in different regions of the 1H-NMR spectrum as shown in Table 2, those are at higher fields related to the benzene ring: 7.40 ppm for the H5' and H8' positions and the interval 7.75-7.79 ppm for the protons at positions H6 'and H7', closely aligned with the H6 and H7 protons of the saccharinate. The pyridine protons that resonate to high fields 8.82 ppm (H6''), 8.42 ppm (H3''), and 8.19 ppm (H4'') are out of phase due to the inductive effect of imidazole nitrogen atoms.
The 1H-NMR signal of H6” was reported by He ( 24 ) for [Cl2(pbi)Pt] at 9.47 ppm, far from 8.73 ppm for the pbi compound. That significant downfield shift is not observed for H6” in [Ag(sac)(pbi)] as a result of a variation in molecular electron distribution between those compounds. The value of the spin-spin (J) coupling constant between the multiple peaks of the spectrum lies in the range of 2-4 Hz, related to the aromatic sequence of atoms for benzimidazole ( 25 ). The amino (N-H) proton signal for pbi is not observed as for [PtCl2(pbi)] who does not report it either ( 24 ).
| Assignment | ppm | N° of H, multiplicities |
| 5 | 7.83 | 1, m |
| 6, 7, 6´,7´ | between 7.75 and 7.79 | 4, m |
| 8, 5´ | between 7.38 and 7.42 | 2, m |
| 8´ | 7.91 | 1, m |
| 3´´ | 8.42 | 1, d |
| 4´´ | 8.19 | 1, m |
| 5´´ | 7.70 | 1, m |
| 6´´ | 8.82 | 1, d |
The 13C-NMR analysis (Table 3) shows chemical shifts to lower fields corresponding to the carbon atoms near the deprotecting electronegative atoms: C=O at 167 ppm, C = Nimidazole at 150.50 ppm (C2'), C = Npy at 150.91 ppm (C6''), and C-Npy at 145.75 ppm (C2''). The aromatic bridging carbon atoms of the benzothiazol and benzimidazole rings have similar chemical environments, so their signals overlap and are not differentiable in the spectrum: C4-C9' at 132.75 ppm and C9-C4' at 143.78 ppm.
Resonance plays an important role in the determination of the chemical environment, so its effect also influences the chemical shift found. The benzene CH carbon atoms are positioned in different regions of the spectrum 133.35 ppm (C7, C6'), 133.22 ppm (C6, C7'), and 124.48 ppm (C5', C8') depending on their location with respect to the electronegative groups and overlapping in the presence of similar chemical environments. Significant differences are observed if we compare the 13C-NMR spectrum of free pbi ligand ( 26 ) to [Ag(PBI)(sac)] complex. Therefore, the coordination to the metal bond can be confirmed.
The 2D-NMR correlation spectroscopy (1H{1H}NMR/COSY 45) performed indicates which hydrogens are coupled to other hydrogens within the molecule, whereas 1H(13C) –NMR/HMBC and 1H{13C}NMR/HSQC provides the correlation between the protons and carbons which are directly linked to each other and separated by two or more bonds, respectively. Both analyses allowed to define the chemical environment and the atomic relations for sac and pbi coordinated to AgI ion.
| Assignment | Bond C-H (Análisis DEPT) | Shift 13C-RMN (ppm) | Relation H-H (HETCOR /COSY) |
| 3 | C=O | 167.00 | 5 |
| 4, 9´ | C | 132.75 | 6, 7, 6´, 7´ |
| 5 | CH | 123.93 | 6, 7 |
| 6, 7 | CH | 133.22 | 5, 8 |
| 8, 5´ | CH | 124.48 | 6´, 7´ |
| 9, 4´ | C | 143.78 | 5, 6, 7, 6´, 7´ |
| 2´ | C | 150.50 | 3´´ |
| 6´,7´ | CH | 133.35 | 5´, 8´ |
| 8´ | CH | 120.57 | 6´, 7´ |
| 2´´ | C | 145,75 | 3´´, 4´´, 6´´ |
| 3´´ | CH | 122.44 | 4´´, 5´´ |
| 4´´ | CH | 139.28 | 3´´, 5´´, 6´´ |
| 5´´ | CH | 126.61 | 3´´, 4´´, 6´´ |
| 6´´ | CH | 150.91 | 4´´, 5´´ |
The oxidation state of the metallic center was confirmed by the XPS Ag3d5/2 spectrum that presents a signal at 368.42 eV. This signal is consistent with the values reported in the literature for silver(I) that fluctuates between 368.2-368.5eV according to Haiyan et al. ( 27 ) and 369.0 eV according to Angulo et al. ( 28 ). The XPS spectrum results of nitrogen N1s show three different signals for nitrogen. There are two signals of equal intensity (400.36 eV and 398.70 eV) and the third highest intensity signal at 399.26 eV. The NH group observed at 400.6 eV is consistent with that reported by other authors (400.0 and 400.6 eV) for similar compounds ( 28 , 29 , 30 ).
The presence of a metal-N bond is observed in the XPS N1s spectrum in two different signals. The signal of lower intensity corresponds to the bond (N-Ag, 398.7 eV) of saccharinate with silver(I), while the signal (399.26 eV) of greater intensity corresponds to two nitrogen atoms (=N-Ag) of the ligand 2-(2-pyridyl)benzimidazole forming a chelate ring (Figure 3).
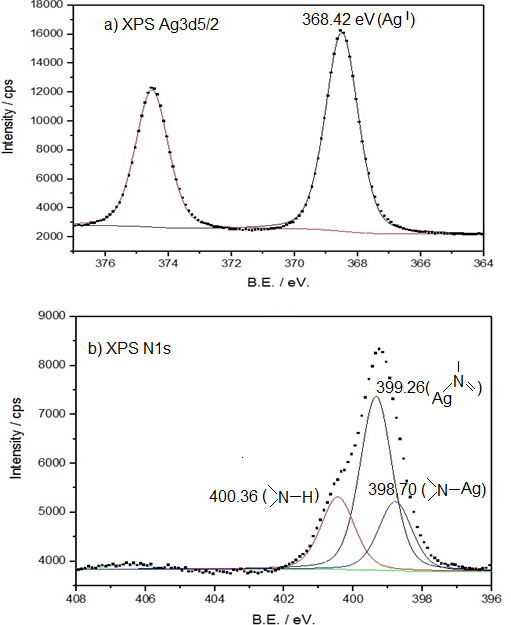
Figure 3. XPS signals a) XPS AgI 3d5/2 and b) XPS N1s
Conclusions
A new heteroleptic silver(I) saccharinate compound was synthesized by reacting 2-(2-pyridyl)benzimidazole with silver(I) saccharinate in a mixture of CH3COCH3, CH3CN, and CH3OH. This compound was characterized by the appropriate spectroscopic techniques due to the complexity of its ligand. The silver(I) has a coordination number of three. Ag-Nsac, Ag-Npbi(im), and Ag-Npbi(py) bonds present in [Ag(sac)(pbi)] were established.
Acknowledgments
The authors thank Innóvate Perú for contract No 364-PNICP-PIBA-2014 and Dr. Lothar Hennig from Leipzig University, Germany, for the NMR spectra.
References
1. Roth, K.; Lück, E. Die Saccharin-Saga. Chem. Unserer Zeit. 2011, 45, 406-423. DOI: https://doi.org/10.1002/ciuz.201100574
2. Baran, J. E.; Yilmaz, V.T. Metal complexes of saccharin. Coord. Chem. Rev. 2006, 250, 1980-1999. DOI: https://doi.org/10.1016/j.ccr.2005.11.021.
3. Yilmaz, V.T.; Hamamci, S.; Gumus, S. Photoluminescence of two silver(I) saccharinate complexes with pyrazine and pyridazine: Experiment and DFT calculations. Chem. Phys. Lett. 2006, 425, 361-366. DOI: https://doi.org/10.1016/j.cplett.2006.05.052.
4. Weber, R.; Gilles, M.; Bergerhoff, G. Crystal structure of 1,2-benzisothiazol-3(2H)-one-1,1-dioxide (saccharin) silver salt, Ag(C7H4NO3S). Z. Kristallogr. - Cryst. Mater. 1993, 206, 273-274. DOI: https://doi.org/10.1524/zkri.1993.206.12.273.
5. Yilmaz, V.T.; Hamamci, S.; Thöne, C. Synthesis, Crystal Structure, IR Spectra and Thermal Analysis of Na[Ag(sac)2] (sac=saccharinate). Z. Anorg. Allg. Chem. 2004, 630, 1641- 1644. DOI: https://doi.org/10.1002/zaac.200400216.
6. Yesilel, O.Z.; Guneay. G.; Buyukgungor, O. Novel silver(I)–saccharinate complexes exhibiting Ag···π and C–H···Ag close interactions with a new coordination mode of saccharinate. Polyhedron, 2011, 30, 364-371. DOI: https://doi.org/10.1016/j.poly.2010.11.001.
7. Real, J.A.; Andres, E.; Muñoz, M.C.; Julve, M.; Granier, T.; Bousseksou, A.; Varret, F. Spin Crossover in a Catenane Supramolecular System. Science, 1995, 268, 265-267. DOI: https://doi.org/10.1126/science.268.5208.265.
8. Yilmaz, V.T.; Hamamci, S.; Harrison, W.T.A.; Thone C. Di- and tetranuclear silver (I)-saccharinate complexes with 2-pyridineethanol and 2-pyridinepropanol: Syntheses, crystal structures, spectroscopic and thermal analyses of [Ag2(sac)2(pyet)2] and [Ag4(sac)4(pypr)2]. Polyhedron. 2005, 24, 693-699. DOI: https://doi.org/10.1016/j.poly.2005.01.006.
9. Yilmaz, V.T.; Hamamci, S.; William, T.A.; Harrison, C. T. Silver(I)-saccharin coordination polymers with 2-pyridylmethanol and 2,6 pyridinedimethanol. Synthetic, spectroscopic, thermal and structural studies of Ag(sac)(μ-mpy)]n and [Ag(μ-sac)(dmpy)]n. Solid State Sci. 2005, 7, 423-429. DOI: https://doi.org/10.1016/j.solidstatesciences.2005.01.004.
10. Guney, E.; Yilmaz, V.T.; Buyukgungor, O. Dimeric and polymeric silver(I) saccharinato complexes of two bis(pyridine) ligands: Synthesis, structural, spectroscopic, fluorescent and thermal properties. Polyhedron, 2010, 29, 1437-1442. DOI: https://doi.org/10.1016/j.poly.2010.01.019.
11. Yesilel, O. Z.; Karamahmut B.; Semerci, F.; Yesiloz, Y. Synthesis, crystal structures, spectroscopic and thermal properties of silver(I) saccharinate complexes with N-donor ligands. Polyhedron, 2012, 42, 307-314. DOI: https://doi.org/10.1016/j.poly.2012.05.034
12. Yilmaz, V.T.; Hamamci, S.; Buyukgungor, O. Synthesis and Characterization of silver(I) Saccharinate Complexes with Pyrazole and Imidazole Ligands: [Ag(sac)(pz)(H2O)]n and [Ag(sac)(im)]·2H2O. Z. Naturforsch. 2006, 61b, 189-193.
13. Erer, H.; Karacam, S.; Arici, M.; Yesilel, O.Z.; Celik, O. Hydrothermal synthesis and characterization of Zn(II), Cd(II) and Ag(I)-saccharinate complexes containing bis(imidazol) derivatives. Polyhedron, 2015, 98, 180-189. DOI: https://doi.org/10.1016/j.poly.2015.06.011.
14. Hamamci, S.; Yilmaz, V.T.; Harrison, W.T.A. Synthesis, IR spectra, thermal analysis and crystal structure of a one-dimensional coordination polymer containing both three- and four- coordinate silver(I) centers bridged by both saccharinate and N-(2-hydroxyethyl)piperazine ligands. J. Mol. Struct. 2005, 734, 191-195. DOI: https://doi.org/10.1016/j.molstruc.2004.09.023.
15. Yilmaz, V.T.; Senel, E.; Guney, E.; Kazak, C. Two fluorescent silver(I)–saccharinate complexes of 2-methylpyrazine and pyrazine-2-carboxamide with Ag⋯Ag interactions. Inorg. Chem. Commun. 2008, 11, 1330-1333. DOI: https://doi.org/10.1016/j.inoche.2008.08.014.
16. Ilker, I.; Yesilel, O.Z.; Gunay, G.; Buyukgungor, O. Dinuclear and polynuclear silver(I) saccharinate complexes with 1,3-diaminopropane and N-methylethylenediamine constructed from Ag⋯C interactions. J. Organomet. Chem. 2009, 694, 4178-4184. DOI: https://doi.org/10.1016/j.jorganchem.2009.09.009.
17. Gumus, S.; Hamamci, S.; Yilmaz, V.T.; Kazak, C. A luminescent silver-saccharinate complex with S, S-diphenylsulfimide: Synthesis, spectroscopic, thermal, structural and DFT computational studies. J. Mol. Struct. 2007, 828, 181-187. DOI: https://doi.org/10.1016/j.molstruc.2006.05.053.
18. Yilmaz, V.T.; Gocmen, E.; Icsel, C.; Gengiz, M.; Susluer, S.Y.; Buyukgungor, O. Synthesis, crystal structures, in vitro DNA binding, antibacterial and cytotoxic activities of new di – and polynuclear silver (I) saccharinate complexes with tertiary monophosphanes. J. Photochem. Photobiol. B. 2014, 131, 31-42. DOI: https://doi.org/10.1016/j.jphotobiol.2013.12.014.
19. Yilmaz, V.T.; Hamamci, S.; Kazak, C. A novel two-dimensional silver(I) saccharinate coordination polymer constructed from weak Ag⋯C interactions: Synthesis, IR spectra and X-ray structure. J. Organomet. Chem. 2008, 693, 3885-3888. DOI: https://doi.org/10.1016/j.jorganchem.2008.09.053.
20. Guney, E.; Yilmaz, V.T.; Buyukgungor, O. A three-dimensional silver(I) coordination polymer involving a new bridging mode of saccharinate. Inorg. Chem. Commun. 2010, 13, 563-567. DOI: https://doi.org/10.1016/j.inoche.2010.02.005.
21. Bansal, Y.; Silakari O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. DOI: https://doi.org/10.1016/j.bmc.2012.09.013.
22. Lee, J, J.; Yap, C. P.; Chwee, T. S.; Fan, W. Y. Highly-phosphorescent tungsten(0) carbonyl pyridyl-imidazole complexes as photosensitisers. Dalton Trans. 2017, 46(33), 11008-11012. DOI: https://doi.org/10.1039/C7DT02397A.
23. Martinez-Alonso, M.; Cerda, J.; Momblona, C.; Pertegas, A.; Junquera-Hernandez, J. M.; Heras, A.; Rodriguez, A. M.; Espino, G.; Bolink, H.; Orti, E. Highly Stable and Efficient Light-Emitting Electrochemical Cells Based on Cationic Iridium Complexes Bearing Arylazole Ancillary Ligands. Inorg. Chem. 2017, 56(17), 10298-10310. DOI: https://doi.org/10.1021/acs.inorgchem.7b01167.
24. He, X.-F.; Vogels, C.M.; Decken, A.;Westcott, S.A. Pyridyl benzimidazole, benzoxazole, and benzothiazole platinum complexes. Polyhedron. 2004, 23, 155-160. DOI: https://doi.org/10.1016/j.poly.2003.09.020.
25. Wade, L.G. Química Orgánica, 5th ed. Pearson Prentice Hill: Barcelona, España, 2008; pp 1205.
26. National Institute of Advanced Industrial Science and Technology (AIST). Spectral Database for Organic Compounds, SDBS. 1,2-benzisothiazol-3(2H)-one 1,1-dioxide spectrum. Retrieved from: http://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (Actualized to 2017).
27. Haiyan, A.; Yangguang L.; Enbo, W.; Dongrong, X.; Chunyan S.; Lin, X. Self-Assembly of a Series of Extended Architectures Based on Polyoxometalate Clusters and Silver Coordination Complexes. Inorg. Chem. 2005, 44(17), 6062-6070. DOI: https://doi.org/10.1021/ic050636x.
28. Angulo-Cornejo, J.; Lino-Pacheco, M.; Richter, R.; Hennig, L.; Hallmaier, K-H.; Beyer, L. Metal chelates of N-benzothiazol-2-yl-, N-benzoxazol-2-yl- and N-(1H-benzimidazol-2-yl)-benzamide. Inorg. Chim. Acta. 2000, 305, 38-45. DOI: https://doi.org/10.1016/S0020-1693(00)00109-2.
29. Beyer, L.; Richter, R.; Wolf, R.; Zaumseil, J.; Lino-Pacheco, M.; Angulo-Cornejo, J. Synthesis and molecular structure of bis (2-benzoylimino-benzimidazolinato) copper (II)-dimethylformamide- and metal containing guanidine derivative. Inorg. Chem. Commun. 1999, 2, 184-187. DOI: https://doi.org/10.1016/S1387-7003(99)00043-X.
30. Angulo-Cornejo, J.; Ayala, K.; Richter, R.; Böhlig, H.; Hennig, L.; Beyer, L. Hydrogen bonds in 1,1-bis(2-hydroxyethyl)-3-benzoylthiourea and its nickel (II)- and copper(II)-chelate complexes. Z. Anorg. Allg. Chem. 2005, 631, 3040-3045. DOI: https://doi.org/10.1002/zaac.200500266.
Author notes
mceronig@unmsm.edu.pe
Abstract
A new silver coordination compound [Ag(sac)(pbi)] was synthesized by reaction of silver(I) saccharinate with 2-(2-pyridyl)benzimidazole (pbi) in 64% yield. The characterization was performed by elemental analysis, IR, UV-Visible, XPS, 1H-NMR, and 13C-NMR spectroscopy. According to the results, silver is coordinating through three nitrogen atoms: one from saccharinate and the others from 2-(2-pyridyl)benzimidazole forming with this ligand a five-membered chelate ring.
Keywords
saccharinate, silver, 2-(2-pyridyl)benzimidazole, XPS.Resumen
Se sintetizó un nuevo compuesto de coordinación de plata, [Ag(sac)(pbi)], por reacción de sacarinato de plata(I) con 2-(2-piridil)bencimidazol (pbi) con un rendimiento de 64%. La caracterización se realizó por análisis elemental, espectroscopia IR, UV-Visible, XPS, 1H-RMN y 13C-RMN. De acuerdo con los resultados obtenidos la plata está coordinada a través de tres átomos de nitrógeno, uno del sacarinato y los dos restantes del 2-(2-piridil)-bencimidazol formando con este ligando un anillo quelato de cinco miembros.
Palabras clave
sacarinato, plata, 2-(2-piridil)bencimidazol, XPS.Resumo
O presente trabalho compreende a síntese de um novo composto de coordenação de prata, [Ag(sac)(pbi)], por reação de sacarinato de prata(I) com 2-(2-piridil)benzimidazol (pbi) com 64% de rendimento. A caracterização foi realizada por análise elementar, espectroscopia IV, UV-Visível, XPS, 1H-RMN e 13C-RMN. De acordo com os resultados obtidos, a prata é coordenada através de três átomos de nitrogênio, um do sacarinato e os outros dois do 2-(2-piridil)benzimidazol que formam com este ligando um anel de quelato de cinco membros.
Palavras-chave
sacarinato, prata, 2-(2-piridil)benzimidazol, XPS.Introduction
Saccharin (Hsac) is a non-caloric sweetener, relatively insoluble in water but soluble in solvents such as ethanol, acetone, and methanol ( 1 ). The pKa value (1.60) for saccharine in aqueous solution indicates that this compound give up its proton (NH group) giving rise to saccharinate (sac) ( 2 ).
The first studies of saccharin complexes were carried out with the formation of aquo-complexes, due to the ability to coordinate with metals through the nitrogen atom. However, its polyfunctionality allowed to increase the number of possible complexes and the study of new chemical properties. The different functional groups, in combination with a series of metal centers, allow a series of coordination modes and chemical structures that will be discussed later. Baran and Yilmaz ( 2 , 3 ) developed some structural characteristics of complexes models based on the different types saccharinate bonds.
The first homoleptic silver(I) saccharinate reported by R. Weber ( 4 ) was prepared by adding AgNO3 to aqueous Na(sac) solution, forming a white precipitate which was recrystallized from ammonia solution. The second homoleptic silver(I) saccharinate was obtained by Veysel T. Yilmaz ( 5 ) by the reaction of Na(sac) and AgNO3 in the presence of 2-pyridinepropanol and water.
There are several silver(I) saccharinate heteroleptic compounds in which silver is coordinated with other ligands and important interactions have been reported, such as those of the type Ag···Ag that contribute to the structural formation and have different properties ( 3 ). Other nearby interactions have also been reported, such as Carom···Ag, Ag··· π and C-H···Ag ( 6 ). The geometries founded for AgI complexes type Agy[(sac) (L)]x are generally trigonal plane, T-shaped, tetrahedral, and trigonal pyramidal ( 7 ), as well as monomeric and dimeric units. There are many types of coordination for silver(I) saccharinate heteroleptic complexes ( 6 ).
Various silver(I) saccharinate compounds have been reported and they can be classified according to the chemical structure of the additional ligand as shown in Table 1.
Table 1. Silver(I) saccharinate compounds.
Ligand
Silver(I) saccharinate compounds
Pyridine
·
[Ag2(sac)2(pyet)2])
and [Ag4(sac)4(pypr)2] (pyet=2-pyridineethanol pypr=2-pyridinepropanol) (
8
).
·
[Ag(sac)(μ-mpy)]n
and [Ag(μ-sac)(dmpy)]n (mpy=2-pyridylmethanol and dmpy=2,6-pyridinedimethanol)
(
9
).
·
[Ag2(sac)2(bpeh)]
and [Ag(sac)(bpma)]n (bpeh=1,2-Bis[1-(pyridine-2-yl)ethylene]hidrazine
and bpma=N,N-bis(pyridine-2-ylmethyl)amine) (
10
).
·
[Ag(sac)(dpa)] (dpa=2,2´-dipyridylamine) (
11
).
Pyrazole and imidazole
· [Ag2(sac)2(pydz)2]
and [Ag(sac)(pyz)]n (pydz=pyridazine and pyz=pyrazine) (
3
). · [Ag(sac)(mpr)]2
(mpr=2-methyl-1-pyrroline) (
6
). · [Ag2(sac)2(o-bix)2]
(o-bix=1,2–bis(imidazole-1-ylmethyl)benzene) (
11
). · [Ag(sac)(pz)(H2O)]n
and [Ag(sac)(im)].2H2O (pz=pyrazole e im=imidazole) (
12
). · [Ag2(sac)2(μ-pbix)2]
and [Ag2(sac)2(μ-mbix)]
(pbix=1,4-bis(imidazole-1-ylmethyl)benzene and mbix=1,3–bis(imidazole-1-ylmethyl)benzene)
(
13
).
Piperazine
·
[[Ag(μ-sac)2(μ-hep)2]n,
(hep=N-2-Hidroxyethylpyperazine) (
14
).
·
[Ag4(sac)(mpyz)2(H2O)2]
and [Ag2(sac)2(pyzca)2]
(mpyz=2-methylpyrazine and pyzca=pyrazine-2-carboxamide) (
15
).
Aliphatic amines
·
[Ag2(sac)2(pen)2]
and [Ag2(sac)2(nmen)]n
(pen=1,3-diaminepropane and nmen=N-methylethylenediamine)(
16
).
·
[Ag2(μ3-sac)2(μ-nmpen)]n,
(nmpen=N-methyl-1,3-propanediamine) (
6
).
·
Ag(sac)(edmen)] and [Ag(Sac)(teten)]
(edmen=N–ethyl-N´,N´-dimethylethylenediamine and teten=N,N,N´,N´-tetrakis(2-hidroxyethyl)
ethylenediamine) (
11
).
Sulphur
· [Ag(sac)(Ph2SNH)]
(Ph2SNH=S,S–diphenylsulfimide)
(
17
).
Phosphine
· [Ag(μ-sac)(PPh3)2]2,
[Ag(μ-sac)(PPh2Cy)]2, [Ag(μ-sac)(PPhCy2)]2
and [Ag(μ-sac)(PCy3)]n, (PPh3=triphenylphosphane;
PPh2Cy=cyclohexyldiphenylphosphane; PPhCy2=dicyclohexylphenylphosphane
and PCy3=tricyclohexylphosphane) (
18
).
Others
· [Ag2(sac)2(MeCN)2]n
(
19
) and [Ag4(sac)4(mpyz)2(H2O)2] and [Ag2(sac)2(pyzca)2] donde (mpyz=2-methylpyrazine, pyzca=pyrazine-2-carboxamide)
(
20
).
Saccharin, as well as some metal-saccharin complexes, possesses biological activity (2). For example, saccharinates of Cu (II), Zn (II), Ce (IV), and Hg (II) exhibit inhibitory activity of carbonic anhydrase and aqueous metal saccharinates are effective in the dismutation of the superoxide anion. In other cases, saccharin derivatives act as an inhibitor of enzymes that cause inflammatory diseases. Some metal saccharinates, especially those of Ag (I), Cu (II), and lanthanides, possess microbiological activity. Furhtermore, benzimidazole derivatives have great biological importance since its heteorcyclic system is present in antiparasitic compounds, anticonvulsants, analgesics, antihistaminics, antiulcer, antihypertensives, antiviral, anticancer, antifungals, anti-inflammatory agents, proton pump inhibitors and anticoagulants ( 21 ).
Likewise, some metal derivatives of 2-(2'-pyridyl)benzimidazole have visible-light photosensitizer properties for the isomerization of aromatic alkenes ( 22 ) and can be use as electroluminescent materials in light-emitting electrochemical cells ( 23 ). Because of the 2-(2'-pyridyl)benzimidazole reactivity, it is an area of chemical interest, particularly in medicine. Therefore, the present work aims to synthesize a new heteroleptic silver compound bonded to saccharin and 2- (2'-pyridyl)benzimidazole (pbi).
Materials and methods
All chemicals were of reagent grade and quality HPLC solvents of the brand Merck from Germany. Infrared Spectrophotometer with Fourier Transform Thermo Nicolet iS10 (Germany), UV-Visible Thermo Scientific Helios (Peru), Micro Leco Elemental Analyzer Truspec Micro (Spain), X-Ray Photoelectron Spectroscopy (Spain), K-Alpha, and Nuclear Magnetic Resonance (1H and 13C 200MHz) Varian Gemini 200 (Germany) were used.
Synthesis of [Ag(sac)(pbi)]
AgNO3 (0.05 g, 0.29 mmol) dissolved in a mixture of CH3COCH3, CH3CN, and CH3OH (6 mL, 1:1:1) was mixed with Na(sac)⋅2H2O (0.07 g, 0.29 mmol) dissolved in the same solvent mixture (6 mL). The mixture was stirred for 30 min and silver(I) saccharinate was formed. Then, 2-(2-pyridyl)benzimidazole (pbi) (0.06 g, 0.29 mmol) dissolved in the above solvent mixture (5 mL) was added and stirred for 2 h. The entire process (Figure 1) was performed at room temperature and isolated from light. Then, white precipitate was vacuum filtered, washed with cold CH3CN (3 x 5 mL) and dried for 1 h at 50°C. Finally, the remaining solid was recrystallized from acetonitrile.
The resulting white acicular in appearance product weighed 0.09 g (64% yield) and was soluble in CHCl3, DMF, DMSO and warm CH3CN. Decomposition point 272-274 °C.
Figure
1. Sequence of chemical reactions to synthesize
[Ag(sac)(pbi)].
Elemental analysis for C19H13SO3N4Ag calculated: C 47.03%, H 2.70%, N 11.55%, S 6.61%. Found: C 47.00%, H 2.89%, N 11.88%, S 6.13%.
FT-IR (cm-1): 3260 m (NHimidazol),3063 m (CHarom), 1628 s (C=O), 1551 s (C=Carom), 1447 m (C=Carom), 1404 m (Cimidazol-N), 1269 s (SO2), 1254 sh (SO2), 1153 s (SO2), 1053 w (CHarom), 1146 vs (SO2), 968 m (CNS), 745 m (CCC), 676 w (CNC), 605 w (CNC).
UV-Vis λmax(DMF)/nm: 263 and 305.
XPS (eV): N 1s 398.70, 399.26, 400.36, 406.45; O 1s 531.95, 532.4, 531.41; S 2p 168.20, 169.38; C 1s 284.92, 286.90, 288.09; Ag 3d5/2 368.42, 3d3/2 374.44.
1H RMN (200 MHz, DMSO-d6, Me4Si) d: 7.40 (2 H m), 7.70 (1H, m), 7.75-7.79 (4H, m), 7.91 (1 H, m), 7.83 (1 H, d), 8.42 (1 H, d), 8.19 (1 H, m), 8.82 (1H d).
13C {1H} RMN (200 MHz, DMSO-d6, Me4Si) d: 167.00 (C3=O), 150.91 (C6’’H), 150.50 (C2’), 145.75 (C2’’), 143.78 (C9 and C4’), 139.28 (C4’’H), 133.35 (C6’H and C7’H), 133.22 (C6H and C7H)), 132.75 (C4 and C9’), 126.61 (C5’’H), 124.48 (C8H and C5’H)), 123.93 (C5H), 122.44 (C3’’H), 120.57 (C8’H).
Results and discussion
The elemental analysis of the compound obtained is consistent with the formula [Ag(sac)(pbi)]. Also, the presence of a silver center with +1 oxidation state was verified by XPS.
Two main and overlapping bands were determined by UV-visible spectroscopy, the highest intensity at 263 nm λmax corresponding to the saccharinate and one less intense with λmax at 305 nm of the pbi. The bands shown originate from the nàπ* and πàπ* of molecular orbitals from C=N, C=O and S=O bonds. There are no metal-ligand transitions between 400 nm and 700 nm because saccharinate and 2-(2-pyridyl)benzimidazole are low field ligands, whereby the absence of color in the complex is warranted.
The most useful infrared bands of 2-(2-pyridyl)benzimidazole and saccharinate could be found as follows: ν(streching N-H) a 3260 cm-1 from pbi, ν(streching CPh-H) aromatic at 3063 cm-1, ν(streching CPh-C) and ν(streching Cim=N) between 1447 cm-1 and 1651 cm-1 for both ligands, ν(streching C-N-S) at 1339 cm-1, ν(streching SO2) between 1269 and 1254 cm-1, ν(bending C-N-S) at 968 cm-1 and ν(bending C-N-C) between 605 cm-1 and 676 cm-1.
The 1H-NMR and 13C-NMR spectra of [Ag(sac)(pbi)] are very complex. The presence of protons in similar chemical environments generated displacements and undefined signals, usually overlapping multiplets. However, with the help of 13C-DEPT-NMR, two-dimensional 1H{1H}-NMR/COSY 45º, 1H{13C}-NMR/HMBC, and 1H{13C}-NMR/HSQC spectra were possible to complete assignments of all the carbon and hydrogen atoms for the compound synthesized as shown in Figure 2.
Figure 2. Numbering H and C atoms for [Ag(sac)(pbi)]
related to NMR spectra assignment.
1H-NMR data for [Ag(sac)(pbi)] (Table 2) shows four protons of saccharinate: 7.83 ppm (H5), between 7.32 and 7.42 (H8) and the range 7.75-7.79 ppm (H6 and H7). Protons at higher fields are protected by the benzene ring and are further from the electronically dense C=O and SO2 groups.
The protons for pbi in [Ag(sac)(pbi)] are displaced in different regions of the 1H-NMR spectrum as shown in Table 2, those are at higher fields related to the benzene ring: 7.40 ppm for the H5' and H8' positions and the interval 7.75-7.79 ppm for the protons at positions H6 'and H7', closely aligned with the H6 and H7 protons of the saccharinate. The pyridine protons that resonate to high fields 8.82 ppm (H6''), 8.42 ppm (H3''), and 8.19 ppm (H4'') are out of phase due to the inductive effect of imidazole nitrogen atoms.
The 1H-NMR signal of H6” was reported by He ( 24 ) for [Cl2(pbi)Pt] at 9.47 ppm, far from 8.73 ppm for the pbi compound. That significant downfield shift is not observed for H6” in [Ag(sac)(pbi)] as a result of a variation in molecular electron distribution between those compounds. The value of the spin-spin (J) coupling constant between the multiple peaks of the spectrum lies in the range of 2-4 Hz, related to the aromatic sequence of atoms for benzimidazole ( 25 ). The amino (N-H) proton signal for pbi is not observed as for [PtCl2(pbi)] who does not report it either ( 24 ).
Table 2. 1H-RMN
chemical shifts for [Ag(sac)(pbi)] (DMSO-d6, 25 °C, 200 MHz).
Assignment
ppm
N° of H, multiplicities
5
7.83
1,
m
6,
7, 6´,7´
between
7.75 and 7.79
4,
m
8,
5´
between
7.38 and 7.42
2,
m
8´
7.91
1,
m
3´´
8.42
1,
d
4´´
8.19
1,
m
5´´
7.70
1,
m
6´´
8.82
1,
d
The 13C-NMR analysis (Table 3) shows chemical shifts to lower fields corresponding to the carbon atoms near the deprotecting electronegative atoms: C=O at 167 ppm, C = Nimidazole at 150.50 ppm (C2'), C = Npy at 150.91 ppm (C6''), and C-Npy at 145.75 ppm (C2''). The aromatic bridging carbon atoms of the benzothiazol and benzimidazole rings have similar chemical environments, so their signals overlap and are not differentiable in the spectrum: C4-C9' at 132.75 ppm and C9-C4' at 143.78 ppm.
Resonance plays an important role in the determination of the chemical environment, so its effect also influences the chemical shift found. The benzene CH carbon atoms are positioned in different regions of the spectrum 133.35 ppm (C7, C6'), 133.22 ppm (C6, C7'), and 124.48 ppm (C5', C8') depending on their location with respect to the electronegative groups and overlapping in the presence of similar chemical environments. Significant differences are observed if we compare the 13C-NMR spectrum of free pbi ligand ( 26 ) to [Ag(PBI)(sac)] complex. Therefore, the coordination to the metal bond can be confirmed.
The 2D-NMR correlation spectroscopy (1H{1H}NMR/COSY 45) performed indicates which hydrogens are coupled to other hydrogens within the molecule, whereas 1H(13C) –NMR/HMBC and 1H{13C}NMR/HSQC provides the correlation between the protons and carbons which are directly linked to each other and separated by two or more bonds, respectively. Both analyses allowed to define the chemical environment and the atomic relations for sac and pbi coordinated to AgI ion.
Table 3. 13C-NMR
chemical shifts for [Ag(sac)(pbi)] (DMSO-d6, 25°C, 200MHz).
Assignment
Bond C-H (Análisis DEPT)
Shift 13C-RMN (ppm)
Relation H-H
(HETCOR /COSY)
3
C=O
167.00
5
4, 9´
C
132.75
6, 7, 6´, 7´
5
CH
123.93
6, 7
6, 7
CH
133.22
5, 8
8, 5´
CH
124.48
6´, 7´
9, 4´
C
143.78
5, 6, 7, 6´, 7´
2´
C
150.50
3´´
6´,7´
CH
133.35
5´, 8´
8´
CH
120.57
6´, 7´
2´´
C
145,75
3´´, 4´´, 6´´
3´´
CH
122.44
4´´, 5´´
4´´
CH
139.28
3´´, 5´´, 6´´
5´´
CH
126.61
3´´, 4´´, 6´´
6´´
CH
150.91
4´´, 5´´
The oxidation state of the metallic center was confirmed by the XPS Ag3d5/2 spectrum that presents a signal at 368.42 eV. This signal is consistent with the values reported in the literature for silver(I) that fluctuates between 368.2-368.5eV according to Haiyan et al. ( 27 ) and 369.0 eV according to Angulo et al. ( 28 ). The XPS spectrum results of nitrogen N1s show three different signals for nitrogen. There are two signals of equal intensity (400.36 eV and 398.70 eV) and the third highest intensity signal at 399.26 eV. The NH group observed at 400.6 eV is consistent with that reported by other authors (400.0 and 400.6 eV) for similar compounds ( 28 , 29 , 30 ).
The presence of a metal-N bond is observed in the XPS N1s spectrum in two different signals. The signal of lower intensity corresponds to the bond (N-Ag, 398.7 eV) of saccharinate with silver(I), while the signal (399.26 eV) of greater intensity corresponds to two nitrogen atoms (=N-Ag) of the ligand 2-(2-pyridyl)benzimidazole forming a chelate ring (Figure 3).
Figure 3.
XPS signals a) XPS AgI 3d5/2 and b) XPS N1s
Conclusions
A new heteroleptic silver(I) saccharinate compound was synthesized by reacting 2-(2-pyridyl)benzimidazole with silver(I) saccharinate in a mixture of CH3COCH3, CH3CN, and CH3OH. This compound was characterized by the appropriate spectroscopic techniques due to the complexity of its ligand. The silver(I) has a coordination number of three. Ag-Nsac, Ag-Npbi(im), and Ag-Npbi(py) bonds present in [Ag(sac)(pbi)] were established.
Acknowledgments
The authors thank Innóvate Perú for contract No 364-PNICP-PIBA-2014 and Dr. Lothar Hennig from Leipzig University, Germany, for the NMR spectra.
References
Referencias
Roth, K.; Lück, E. Die Saccharin-Saga. Chem. Unserer Zeit. 2011, 45, 406-423. DOI: https://doi.org/10.1002/ciuz.201100574.
Baran, J. E.; Yilmaz, V.T. Metal complexes of saccharin. Coord. Chem. Rev. 2006, 250, 1980-1999. DOI: https://doi.org/10.1016/j.ccr.2005.11.021.
Yilmaz, V.T.; Hamamci, S.; Gumus, S. Photoluminescence of two silver(I) saccharinate complexes with pyrazine and pyridazine: Experiment and DFT calculations. Chem. Phys. Lett. 2006, 425, 361-366. DOI: https://doi.org/10.1016/j.cplett.2006.05.052.
Weber, R.; Gilles, M.; Bergerhoff, G. Crystal structure of 1,2-benzisothiazol-3(2H)-one-1,1-dioxide (saccharin) silver salt, Ag(C7H4NO3S). Z. Kristallogr. - Cryst. Mater. 1993, 206, 273-274. DOI: https://doi.org/10.1524/zkri.1993.206.12.273.
Yilmaz, V.T.; Hamamci, S.; Thöne, C. Synthesis, Crystal Structure, IR Spectra and Thermal Analysis of Na[Ag(sac)2] (sac=saccharinate). Z. Anorg. Allg. Chem. 2004, 630, 1641- 1644. DOI: https://doi.org/10.1002/zaac.200400216.
Yesilel, O.Z.; Guneay. G.; Buyukgungor, O. Novel silver(I)–saccharinate complexes exhibiting Ag···π and C–H···Ag close interactions with a new coordination mode of saccharinate. Polyhedron, 2011, 30, 364-371. DOI: https://doi.org/10.1016/j.poly.2010.11.001.
Real, J.A.; Andres, E.; Muñoz, M.C.; Julve, M.; Granier, T.; Bousseksou, A.; Varret, F. Spin Crossover in a Catenane Supramolecular System. Science, 1995, 268, 265-267. DOI: https://doi.org/10.1126/science.268.5208.265.
Yilmaz, V.T.; Hamamci, S.; Harrison, W.T.A.; Thone C. Di- and tetranuclear silver (I)-saccharinate complexes with 2-pyridineethanol and 2-pyridinepropanol: Syntheses, crystal structures, spectroscopic and thermal analyses of [Ag2(sac)2(pyet)2] and [Ag4(sac)4(pypr)2]. Polyhedron. 2005, 24, 693-699. DOI: https://doi.org/10.1016/j.poly.2005.01.006.
Yilmaz, V.T.; Hamamci, S.; William, T.A.; Harrison, C. T. Silver(I)-saccharin coordination polymers with 2-pyridylmethanol and 2,6 pyridinedimethanol. Synthetic, spectroscopic, thermal and structural studies of Ag(sac)(μ-mpy)]n and [Ag(μ-sac)(dmpy)]n. Solid State Sci. 2005, 7, 423-429. DOI: https://doi.org/10.1016/j.solidstatesciences.2005.01.004.
Guney, E.; Yilmaz, V.T.; Buyukgungor, O. Dimeric and polymeric silver(I) saccharinato complexes of two bis(pyridine) ligands: Synthesis, structural, spectroscopic, fluorescent and thermal properties. Polyhedron, 2010, 29, 1437-1442. DOI: https://doi.org/10.1016/j.poly.2010.01.019.
Yesilel, O. Z.; Karamahmut B.; Semerci, F.; Yesiloz, Y. Synthesis, crystal structures, spectroscopic and thermal properties of silver(I) saccharinate complexes with N-donor ligands. Polyhedron, 2012, 42, 307-314. DOI: https://doi.org/10.1016/j.poly.2012.05.034
Yilmaz, V.T.; Hamamci, S.; Buyukgungor, O. Synthesis and Characterization of silver(I) Saccharinate Complexes with Pyrazole and Imidazole Ligands: [Ag(sac)(pz)(H2O)]n and [Ag(sac)(im)]·2H2O. Z. Naturforsch. 2006, 61b, 189-193.
Erer, H.; Karacam, S.; Arici, M.; Yesilel, O.Z.; Celik, O. Hydrothermal synthesis and characterization of Zn(II), Cd(II) and Ag(I)-saccharinate complexes containing bis(imidazol) derivatives. Polyhedron, 2015, 98, 180-189. DOI: https://doi.org/10.1016/j.poly.2015.06.011.
Hamamci, S.; Yilmaz, V.T.; Harrison, W.T.A. Synthesis, IR spectra, thermal analysis and crystal structure of a one-dimensional coordination polymer containing both three- and four- coordinate silver(I) centers bridged by both saccharinate and N-(2-hydroxyethyl)piperazine ligands. J. Mol. Struct. 2005, 734, 191-195. DOI: https://doi.org/10.1016/j.molstruc.2004.09.023.
Yilmaz, V.T.; Senel, E.; Guney, E.; Kazak, C. Two fluorescent silver(I)–saccharinate complexes of 2-methylpyrazine and pyrazine-2-carboxamide with Ag⋯Ag interactions. Inorg. Chem. Commun. 2008, 11, 1330-1333. DOI: https://doi.org/10.1016/j.inoche.2008.08.014.
Ilker, I.; Yesilel, O.Z.; Gunay, G.; Buyukgungor, O. Dinuclear and polynuclear silver(I) saccharinate complexes with 1,3-diaminopropane and N-methylethylenediamine constructed from Ag⋯C interactions. J. Organomet. Chem. 2009, 694, 4178-4184. DOI: https://doi.org/10.1016/j.jorganchem.2009.09.009.
Gumus, S.; Hamamci, S.; Yilmaz, V.T.; Kazak, C. A luminescent silver-saccharinate complex with S, S-diphenylsulfimide: Synthesis, spectroscopic, thermal, structural and DFT computational studies. J. Mol. Struct. 2007, 828, 181-187. DOI: https://doi.org/10.1016/j.molstruc.2006.05.053.
Yilmaz, V.T.; Gocmen, E.; Icsel, C.; Gengiz, M.; Susluer, S.Y.; Buyukgungor, O. Synthesis, crystal structures, in vitro DNA binding, antibacterial and cytotoxic activities of new di – and polynuclear silver (I) saccharinate complexes with tertiary monophosphanes. J. Photochem. Photobiol. B. 2014, 131, 31-42. DOI: https://doi.org/10.1016/j.jphotobiol.2013.12.014.
Yilmaz, V.T.; Hamamci, S.; Kazak, C. A novel two-dimensional silver(I) saccharinate coordination polymer constructed from weak Ag⋯C interactions: Synthesis, IR spectra and X-ray structure. J. Organomet. Chem. 2008, 693, 3885-3888. DOI: https://doi.org/10.1016/j.jorganchem.2008.09.053.
Guney, E.; Yilmaz, V.T.; Buyukgungor, O. A three-dimensional silver(I) coordination polymer involving a new bridging mode of saccharinate. Inorg. Chem. Commun. 2010, 13, 563-567. DOI: https://doi.org/10.1016/j.inoche.2010.02.005.
Bansal, Y.; Silakari O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. DOI: https://doi.org/10.1016/j.bmc.2012.09.013.
Lee, J, J.; Yap, C. P.; Chwee, T. S.; Fan, W. Y. Highly-phosphorescent tungsten(0) carbonyl pyridyl-imidazole complexes as photosensitisers. Dalton Trans. 2017, 46(33), 11008-11012. DOI: https://doi.org/10.1039/C7DT02397A.
Martinez-Alonso, M.; Cerda, J.; Momblona, C.; Pertegas, A.; Junquera-Hernandez, J. M.; Heras, A.; Rodriguez, A. M.; Espino, G.; Bolink, H.; Orti, E. Highly Stable and Efficient Light-Emitting Electrochemical Cells Based on Cationic Iridium Complexes Bearing Arylazole Ancillary Ligands. Inorg. Chem. 2017, 56(17), 10298-10310. DOI: https://doi.org/10.1021/acs.inorgchem.7b01167.
He, X.-F.; Vogels, C.M.; Decken, A.;Westcott, S.A. Pyridyl benzimidazole, benzoxazole, and benzothiazole platinum complexes. Polyhedron. 2004, 23, 155-160. DOI: https://doi.org/10.1016/j.poly.2003.09.020.
Wade, L.G. Química Orgánica, 5th ed. Pearson Prentice Hill: Barcelona, España, 2008; pp 1205.
National Institute of Advanced Industrial Science and Technology (AIST). Spectral Database for Organic Compounds, SDBS. 1,2-benzisothiazol-3(2H)-one 1,1-dioxide spectrum. Retrieved from: http://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (Actualized to 2017).
Haiyan, A.; Yangguang L.; Enbo, W.; Dongrong, X.; Chunyan S.; Lin, X. Self-Assembly of a Series of Extended Architectures Based on Polyoxometalate Clusters and Silver Coordination Complexes. Inorg. Chem. 2005, 44(17), 6062-6070. DOI: https://doi.org/10.1021/ic050636x.
Angulo-Cornejo, J.; Lino-Pacheco, M.; Richter, R.; Hennig, L.; Hallmaier, K-H.; Beyer, L. Metal chelates of N-benzothiazol-2-yl-, N-benzoxazol-2-yl- and N-(1H-benzimidazol-2-yl)-benzamide. Inorg. Chim. Acta. 2000, 305, 38-45. DOI: https://doi.org/10.1016/S0020-1693(00)00109-2.
Beyer, L.; Richter, R.; Wolf, R.; Zaumseil, J.; Lino-Pacheco, M.; Angulo-Cornejo, J. Synthesis and molecular structure of bis (2-benzoylimino-benzimidazolinato) copper (II)-dimethylformamide- and metal containing guanidine derivative. Inorg. Chem. Commun. 1999, 2, 184-187. DOI: https://doi.org/10.1016/S1387-7003(99)00043-X.
Angulo-Cornejo, J.; Ayala, K.; Richter, R.; Böhlig, H.; Hennig, L.; Beyer, L. Hydrogen bonds in 1,1-bis(2-hydroxyethyl)-3-benzoylthiourea and its nickel (II)- and copper(II)-chelate complexes. Z. Anorg. Allg. Chem. 2005, 631, 3040-3045. DOI: https://doi.org/10.1002/zaac.200500266.
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Pei Lay Yap, Trong Tuan Anh Tran, Le Yu, Thanh Tung Tran, Dusan Losic. (2024). BioMagnetic-graphene-aminoclay nanocomposites for sustainable adsorption and precious metal recovery from industrial waste effluents. Advanced Nanocomposites, 1(1), p.304. https://doi.org/10.1016/j.adna.2024.09.001.
2. Iván Sorribes, David Ventura-Espinosa, Marcelo Assis, Santiago Martín, Patricia Concepción, Jefferson Bettini, Elson Longo, Jose A. Mata, Juan Andrés. (2021). Unraveling a Biomass-Derived Multiphase Catalyst for the Dehydrogenative Coupling of Silanes with Alcohols under Aerobic Conditions. ACS Sustainable Chemistry & Engineering, 9(7), p.2912. https://doi.org/10.1021/acssuschemeng.0c08953.
3. Weiwei Zheng, Wentao Tian, Xiaojun Liu, Qingquan Zhang, Chenghua Zong, Jia-Ping Lai, Wenfeng Zhao. (2021). In situ photochemical deposition of Ag nanoparticles on polyester fiber membranes as flexible SERS substrates for sensitive detection of sodium saccharin in soft drinks. Microchemical Journal, 164, p.106003. https://doi.org/10.1016/j.microc.2021.106003.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2018 Revista Colombiana de Química

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons (CC. Atribución 4.0) que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).




![Figure
1. Sequence of chemical reactions to synthesize
[Ag(sac)(pbi)].](https://revistas.unal.edu.co/index.php/rcolquim/article/download/68225/version/54127/67469/396838/309055565009_gf2.png)
![Figure 2. Numbering H and C atoms for [Ag(sac)(pbi)]
related to NMR spectra assignment.](https://revistas.unal.edu.co/index.php/rcolquim/article/download/68225/version/54127/67469/396839/309055565009_gf3.png)










