Theoretical study of solvent effects on the hyperpolarizabilities of two chalcone derivatives
Estudio teórico de los efectos solventes sobre la hiperpolarizabilidad de dos derivados de la chalcona
Estudo teórico dos efeitos do solvente nas hiperpolarizabilidades de dois derivados da chalcona
DOI:
https://doi.org/10.15446/rev.colomb.quim.v1n49.82156Palabras clave:
first and second hyperpolarizabilities, solvent, DMSO, acetone (en)primera y segunda hiperpolarizabilidad, solvente, DMSO, acetona (es)
primeira e segunda hiperpolarizabilidades, solvente, DMSO, acetona (pt)
Descargas
The use of organic as nonlinear optical materials has been intensively explored in the recent years due to the ease of manipulation of the molecular structure and the synthetic flexibility regarding the change of substituent groups. In the present work, the linear and nonlinear properties of two chalcones derivatives (E)-1-(4-methylphenyl)-3-phenylprop-2-en-1-one (4MP3P) and (E)-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one (4NP3P), that differ by the substituent position at the phenyl ring, were studied in the presence of protic and aprotic solvents simulated by the Polarizable Continuum Model (PCM) at DFT/B3LYP/6-311+G(d) level. The static and dynamic (1064 nm) molecular parameters as the dipole moment, linear polarizability, first and second hyperpolarizabilities were studied as function of the solvent dielectric constant value. The geometrical behavior as the chemical bond angles, torsion angles, and partial charges distribution of the compounds were studied, including calculations of gap energies in various solvents. The obtained results revealed that the substituent change of CH3 (4MP3P) to NO2 (4NP3P) benefits the nonlinear optical properties of the compounds in the presence of the solvent media, the absolute values of the parallel first hyperpolarizability were the ones that present the greater variation.
El uso de materiales orgánicos como materiales ópticos no lineales se ha explorado intensamente en los últimos años, debido a la facilidad de manipulación de estas estructuras moleculares y la flexibilidad de síntesis en relación con el cambio de grupos sustituyentes. En el presente trabajo, las propiedades lineales y no lineales de dos derivados de chalcona (E)-1-(4-metilfenil)-3-fenilprop-2-en-1-ona (4MP3P) y (E)-1-(4-nitrofenil)-3-fenilprop-2-en-1-ona (4NP3P), los cuales difieren en la posición del sustituyente en el anillo de fenilo, se estudiaron en presencia de disolventes próticos y apróticos simulados por el Modelo Continuo Polarizable a nivel DFT/B3LYP/6-311+G(d). Además, se estudiaron parámetros moleculares estáticos y dinámicos (1064 nm) como el momento dipolar, la polarización lineal y la primera y la segunda hiperpolarización en función del valor constante dieléctrico del disolvente. El comportamiento geométrico se estudió como ángulos de enlace químico, ángulos de torsión y distribución de carga parcial de compuestos, incluidos los cálculos de energía de huecos en varios solventes. Los resultados mostraron que el cambio del sustituyente CH3 (4MP3P) a NO2 (4NP3P) beneficia las propiedades ópticas no lineales de los compuestos en presencia del medio solvente, los valores absolutos de la primera hiperpolarizabilidad paralela fueron los que presentaron la mayor variación.
O uso de materiais orgânicos como materiais ópticos não lineares tem sido intensamente explorado nos últimos anos, devido à facilidade de manipulação dessas estruturas moleculares e à flexibilidade de síntese em relação à mudança de grupos substituintes. No presente trabalho, as propriedades lineares e não lineares de dois derivados de chalconas (E)-1-(4-metilfenil)-3-fenilprop-2-en-1-ona (4MP3P) e (E)-1-(4-nitrofenil)-3-fenilprop-2-en-1-ona (4NP3P), que diferem pela posição do substituinte no anel fenil, foram estudados na presença de solventes próticos e apróticos simulados pelo Modelo Continuo Polarizável (PCM) no nível DFT/B3LYP/6-311+G(d). Os parâmetros moleculares estáticos e dinâmicos (1064 nm) como momento dipolar, polarizabilidade linear, primeira e segunda hiperpolarizabilidades foram estudados em função do valor da constante dielétrica do solvente. Estudou-se o comportamento geométrico como ângulos de ligação química, ângulos de torção e distribuição parcial de cargas dos compostos, incluindo cálculos de energias de gap em vários solventes. Os resultados obtidos revelaram que a mudança do substituinte de CH3 (4MP3P) para NO2 (4NP3P) beneficia as propriedades ópticas não lineares dos compostos na presença do meio solvente, os valores absolutos da primeira hiperpolarizabilidade paralela foram os que apresentaram a maior variação.

Fisicoquímica e inorgánica
Theoretical study of solvent effects on the hyperpolarizabilities of two chalcone derivatives
Estudio teórico de los efectos solventes sobre la hiperpolarizabilidad de dos derivados de la chalcona
Estudo teórico dos efeitos do solvente nas hiperpolarizabilidades de dois derivados da chalcona
Theoretical study of solvent effects on the hyperpolarizabilities of two chalcone derivatives
Revista Colombiana de Química, vol. 49, núm. 1, 2020
Universidad Nacional de Colombia
Recepción: 09 Septiembre 2019
Aprobación: 20 Noviembre 2019
Abstract: The use of organic as nonlinear optical materials has been intensively explored in the recent years due to the ease of manipulation of the molecular structure and the synthetic flexibility regarding the change of substituent groups. In the present work, the linear and nonlinear properties of two chalcones derivatives (E)-1-(4-methylphenyl)-3-phenylprop-2-en-1-one (4MP3P) and (E)-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one (4NP3P), that differ by the substituent position at the phenyl ring, were studied in the presence of protic and aprotic solvents simulated by the Polarizable Continuum Model (PCM) at DFT/B3LYP/6-311+G(d) level. The static and dynamic (1064 nm) molecular parameters as the dipole moment, linear polarizability, first and second hyperpolarizabilities were studied as function of the solvent dielectric constant value. The geometrical behavior as the chemical bond angles, torsion angles, and partial charges distribution of the compounds were studied, including calculations of gap energies in various solvents. The obtained results revealed that the substituent change of CH3 (4MP3P) to NO2 (4NP3P) benefits the nonlinear optical properties of the compounds in the presence of the solvent media, the absolute values of the parallel first hyperpolarizability were the ones that present the greater variation.
Keywords: first and second hyperpolarizabilities, solvent, DMSO, acetone.
Resumen: El uso de materiales orgánicos como materiales ópticos no lineales se ha explorado intensamente en los últimos años, debido a la facilidad de manipulación de estas estructuras moleculares y la flexibilidad de síntesis en relación con el cambio de grupos sustituyentes. En el presente trabajo, las propiedades lineales y no lineales de dos derivados de chalcona (E)-1-(4-metilfenil)-3-fenilprop-2-en-1-ona (4MP3P) y (E)-1-(4-nitrofenil)-3-fenilprop-2-en-1-ona (4NP3P), los cuales difieren en la posición del sustituyente en el anillo de fenilo, se estudiaron en presencia de disolventes próticos y apróticos simulados por el Modelo Continuo Polarizable a nivel DFT/B3LYP/6-311+G(d). Además, se estudiaron parámetros moleculares estáticos y dinámicos (1064 nm) como el momento dipolar, la polarización lineal y la primera y la segunda hiperpolarización en función del valor constante dieléctrico del disolvente. El comportamiento geométrico se estudió como ángulos de enlace químico, ángulos de torsión y distribución de carga parcial de compuestos, incluidos los cálculos de energía de huecos en varios solventes. Los resultados mostraron que el cambio del sustituyente CH3 (4MP3P) a NO2 (4NP3P) beneficia las propiedades ópticas no lineales de los compuestos en presencia del medio solvente, los valores absolutos de la primera hiperpolarizabilidad paralela fueron los que presentaron la mayor variación.
Palabras clave: primera y segunda hiperpolarizabilidad, solvente, DMSO, acetona.
Resumo: O uso de materiais orgânicos como materiais ópticos não lineares tem sido intensamente explorado nos últimos anos, devido à facilidade de manipulação dessas estruturas moleculares e à flexibilidade de síntese em relação à mudança de grupos substituintes. No presente trabalho, as propriedades lineares e não lineares de dois derivados de chalconas (E)-1-(4-metilfenil)-3-fenilprop-2-en-1-ona (4MP3P) e (E)-1-(4-nitrofenil)-3-fenilprop-2-en-1-ona (4NP3P), que diferem pela posição do substituinte no anel fenil, foram estudados na presença de solventes próticos e apróticos simulados pelo Modelo Continuo Polarizável (PCM) no nível DFT/B3LYP/6-311+G(d). Os parâmetros moleculares estáticos e dinâmicos (1064 nm) como momento dipolar, polarizabilidade linear, primeira e segunda hiperpolarizabilidades foram estudados em função do valor da constante dielétrica do solvente. Estudou-se o comportamento geométrico como ângulos de ligação química, ângulos de torção e distribuição parcial de cargas dos compostos, incluindo cálculos de energias de gap em vários solventes. Os resultados obtidos revelaram que a mudança do substituinte de CH3 (4MP3P) para NO2 (4NP3P) beneficia as propriedades ópticas não lineares dos compostos na presença do meio solvente, os valores absolutos da primeira hiperpolarizabilidade paralela foram os que apresentaram a maior variação.
Palavras-chave: primeira e segunda hiperpolarizabilidades, vent, solvente, DMSO, acetona.
Introduction
In the last few years the engineering of organic crystalline compound has become an important area of study and has attracted great interest from research centers [1], motivated by significant values of the nonlinear optical properties of these crystals, for instance, chalcone derivatives [2]. Nonlinear optics devices operate with high-speed information [3], as ultra-short pulse lasers, photonic devices, optical modulators, and others with applications in the areas of health and medicine [4, 5]. The advantages of organic crystals that have attracted much attention come from their ease of manipulation of the molecular structure and the synthetic flexibility regarding the change of substituent groups [6, 7]. The combination of the high nonlinearity characteristic of some organic compounds and the versatility of synthetic routes leads to maximization of the nonlinear optical properties [8]. Among the organic materials, the chalcones are known as promising compounds, since their derivatives have several biological properties that have been used as prototypes for new drugs [9]. Biosynthesis of flavonoids chalcones have been applied in several activities such as: antitumor [10], anti-inflammatory [11], antibacterial [12], antifungal [13], among others.
The chalcones consist of two aromatic rings joined by an unsaturated system so that their structure almost always acquires a linear or almost planar conformation [14]. Organic molecules with π-conjugated structures and donor and/or acceptor groups are compounds of great interest in the area of nonlinear optics [15]. The change of the substituent groups bond to the aromatic rings leads the chalcone molecules to acquire specific properties due the change of electronic distribution of the compound, hence new properties can be explored with each new substituent being added or substituted to the compound [16].
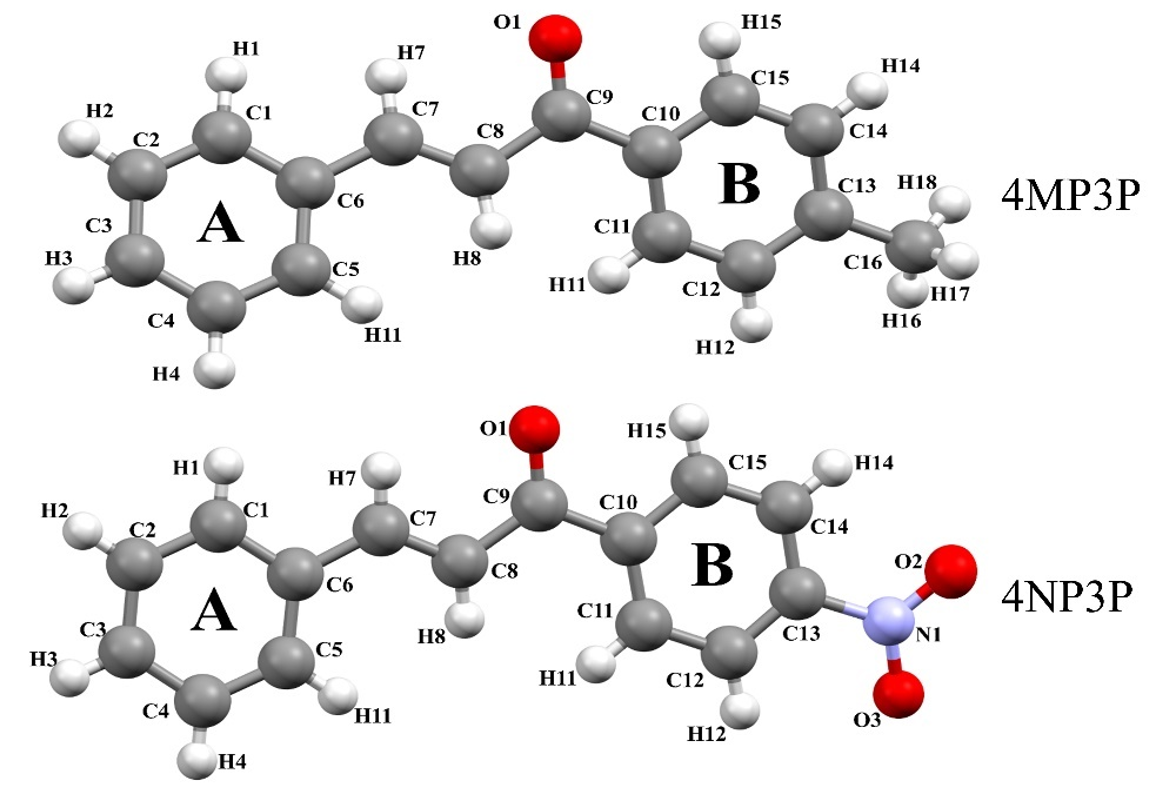
The purpose of the present paper is to study the nonlinear optical properties of two chalcone derivatives: (.)-1-(4-methylphenyl)-3-phenylprop-2-en-1-one (4MP3P) and (.)-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one (4NP3P), with formulas C16.14O and C15.11O., respectively. The structure 4MP3P was synthesized by Toda et al. [15] and the structure 4NP3P by Jing et al. [16]. These compounds differ by the substituent at position 4 of the phenyl ring (B) as shown in Figure 1.
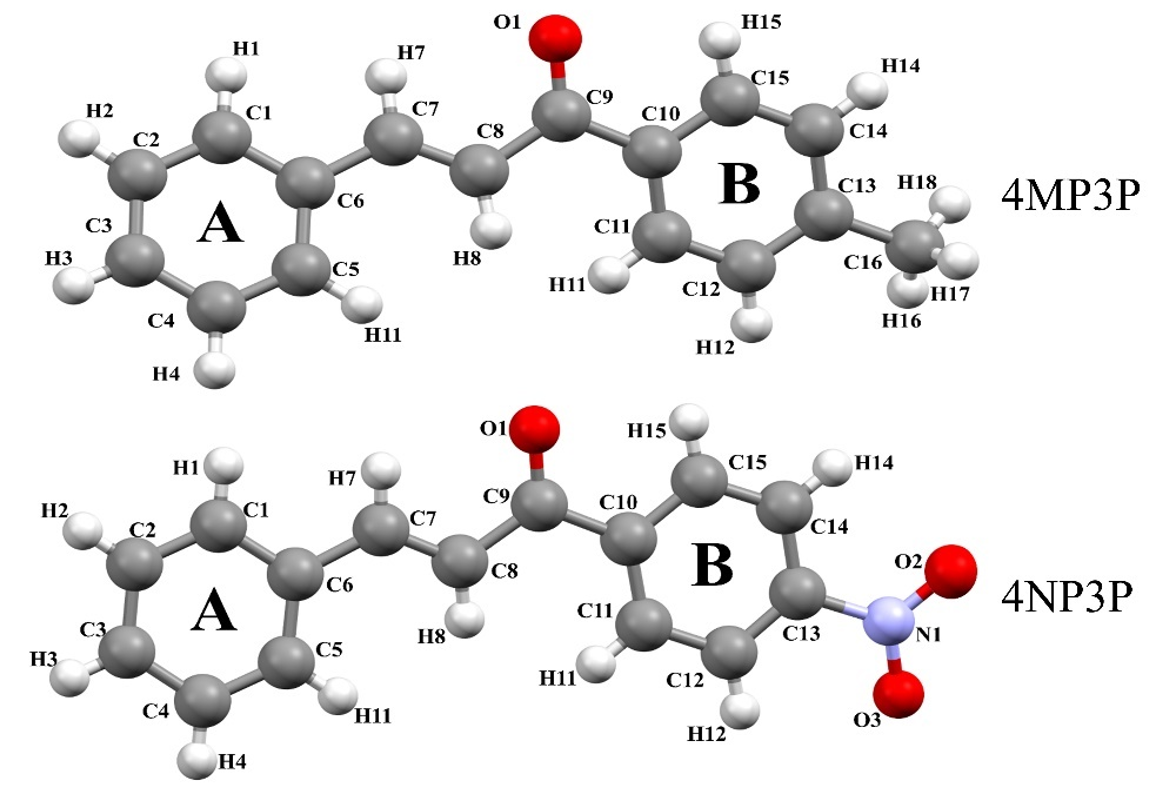
Figure 1
Molecular structure of the compounds 4MP3P and 4NP3P.
The electrical parameters of these compounds were calculated at DFT/CAM-B3LYP/6-311+G(d) level in the presence of several solvent media that are modeled by the Polarizable Continuum Model (PCM). The static and dynamic (1064 nm) molecular parameters as the dipole moment, linear polarizability, first and second hyperpolarizability were studied as function of the value of the solvent dielectric constant value. Also, the frontiers molecular orbital, HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital), and the respective gap energies in various solvents were calculated. The geometrical behavior of the compounds as the chemical bond angles, torsion angles, and partial charges distribution were also studied.
Materials and Methods
Computational procedures
Organic solvents belong to a class of liquid and volatile compounds and have the function of solubilizing, extracting, treating, and facilitating a chemical reaction among other functions. This group of compounds is divided into: polar and nonpolar solvents. Polar protic solvents are characterized by the presence of hydrogen bonded to electronegative elements, usually, atoms of O, N, and F, which can lead to the formation of hydrogen bonds [17] and polar aprotic solvents are characterized by the absence of electronegative atoms so that only bonds between carbon and hydrogen atoms are present. The values of the dielectric constant and of the dipole moments for polar solvents are higher than those for the nonpolar solvents [18]. The dielectric constant of a solvent medium is a good indicator of the ability to accommodate a charge separation that increases with the values of the dipole moment and with the polarizability of the molecule [19].
In this work the quantitative concept of the solvent polarity was considered through the normalized transition energy ( scale of Dimroth and Reichardt [19, 20]. The -value is based on the transition energy to the solvatochromic absorption band for the greatest wavelenght of the nitrile betaine pyridynium dye. Table 1 shows a list of the solvent media, here considered with the respective values of and ε. Reactions involving charge separation in the -scale advance more slowly in polar aprotic solvents due to the small dipole moments and the absence of hydrogen bonds which make them few effective in the development of the separation and the stabilization of charges when compared to the polar protic solvents[19, 21].
| Solvents | Type | ||
| Water | 1.000 | 78.355 | Protic |
| Methanol | 0.762 | 32.613 | Protic |
| Ethanol | 0.654 | 24.852 | Protic |
| DMSO | 0.444 | 46.826 | Aprotic |
| Acetone | 0.355 | 20.493 | Aprotic |
| Dichloroethane | 0.327 | 10.125 | Aprotic |
| Chloroform | 0.259 | 4.711 | nonpolar |
| Tetrahydrofuran | 0.207 | 7.430 | Aprotic |
| Heptane | 0.012 | 1.911 | nonpolar |
Here the solvent chloroform must be considered nonpolar because the value of its dielectric constant ε is less than 5.
The total dipole moment and the average linear polarizability were calculated from the components x, y, and z, through the expressions:
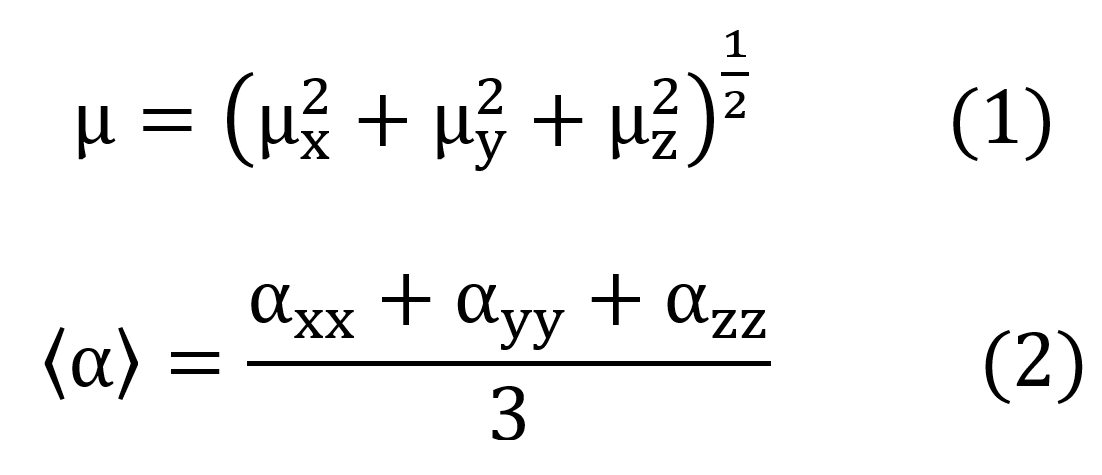
The first hyperpolarizabilities considered concerned the parallel components to the direction of dipole moment (taken as z-direction) given by,
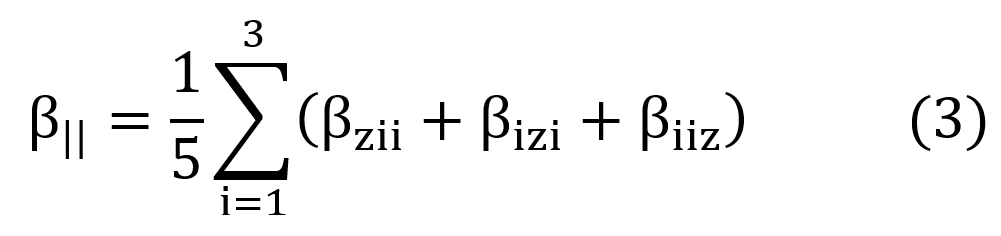
and the Hyper-Rayleigh Scattering first hyperpolarizability (BHRS) defined by,

where the laboratory system of reference is adopted as X, Y, and Z coordinates. The X-direction is assumed as the fundamental light beam propagation, polarized in the Z-direction. The (BZ2ZZ) and (BX2ZZ) are macroscopic averages calculated from the first hyperpolarizability components (Bijk) through the expressions,

in which the coefficients are defined in Table 2.
Using the Kleymann symmetry, the average value of the second hyperpolarizability ( ) can be calculated through the following expression,

The linear and nonlinear optical (NLO) parameters of the molecules of chalcone derivatives in several solvent media were calculated using the PCM/DFT/CAM-B3LYP/6-311+G(d) level with the Gaussian 09 software package.
Results and discussions
Solvent effects on the molecular properties
In this section, the similarities between the molecular geometry of 4MP3P and 4NP3P, obtained via X-ray diffraction [15, 16], and the theoretical results in several solvent media are analyzed through the root mean square deviation (RMSD) of the overlap of the two structures. As can be seen, in Table 3, the value of RMSD is 0.156 a.u. (0.217 a.u.) for gas phase of 4MP3P (4NP3P) and this value presents small changes when the solvent dielectric constant increases, until reaching the value 0.167 a.u. (0.295 a.u.), therefore the effects of solvent media on the molecular geometry is more significant for 4NP3P. Figure 2 shows a schematic representation of the overlap of 4MP3P and 4NP3P molecular structure determined by X-ray (in blue) and in water (in red), where the phenyl ring (A) was used as anchorage for the molecular structures. The H-atoms were disregarded in view of their uncertainties in X-ray position refinement.
Table 3. RMSD (a.u.) and Gap energies (eV) of 4MP3P and 4NP3P.
| Solvent | RMSD4MP3P | RMSD4NP3P | Gap (eV)4MP3P | Gap (eV)4NP3P | |
| Gas-phase | 1.00 | 0.156 | 0.217 | 6.636 | 6.094 |
| Heptane | 1.91 | 0.158 | 0.285 | 6.587 | 5.980 |
| Chloroform | 4.71 | 0.162 | 0.292 | 6.537 | 5.886 |
| Tetrahydrofuran | 7.43 | 0.163 | 0.293 | 6.520 | 5.859 |
| Dichloroethane | 10.13 | 0.164 | 0.294 | 6.511 | 5.846 |
| Acetone | 20.49 | 0.165 | 0.295 | 6.499 | 5.829 |
| Ethanol | 24.85 | 0.166 | 0.295 | 6.496 | 5.825 |
| Methanol | 32.61 | 0.166 | 0.295 | 6.494 | 5.822 |
| DMSO | 46.71 | 0.166 | 0.295 | 6.491 | 5.819 |
| Water | 78.36 | 0.167 | 0.295 | 6.489 | 5.815 |
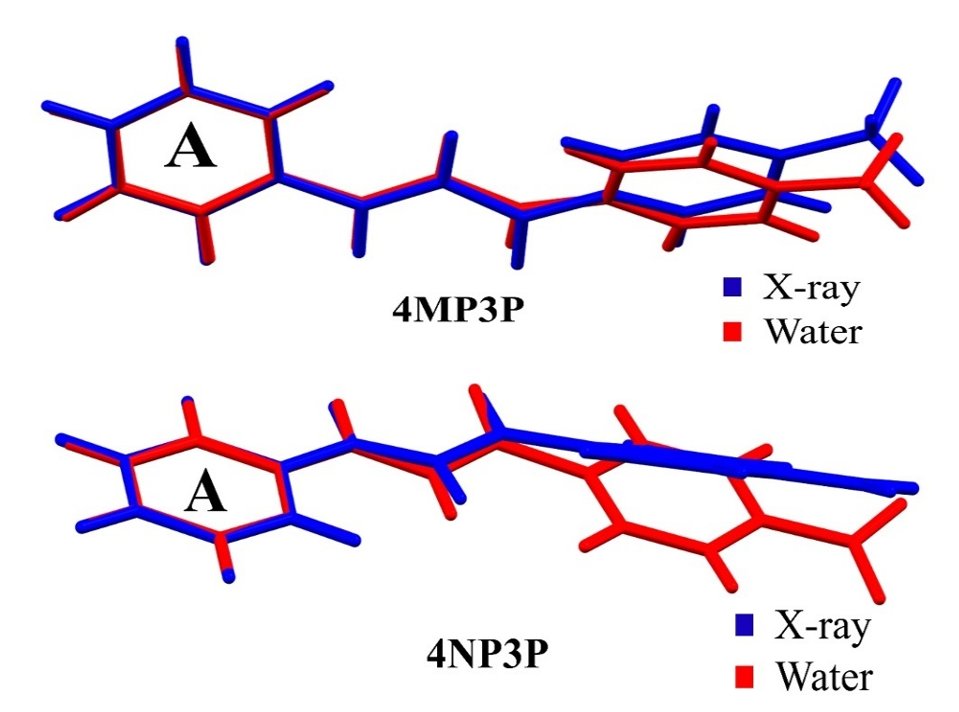
Figure 2.
Overlap of the theoretical molecular structures of the compounds (4MP3P and 4NP3P) in water (red) and that determined by X-ray diffraction (blue).
Table 4 shows the (O1-C9-C8-C7), (C8-C9-C10-C11), (C8-C9-C10-C15), (C8-C7-C6-C5), (C8-C7-C6-C5), and (01-C9-C10-C15) torsion angles for various solvents and for the gas-phase. As can be seen, the torsion angles present a small variations in all solvent media, independent of the solvent polarity or the protic or aprotic character for both compunds 4MP3P and 4NP3P.
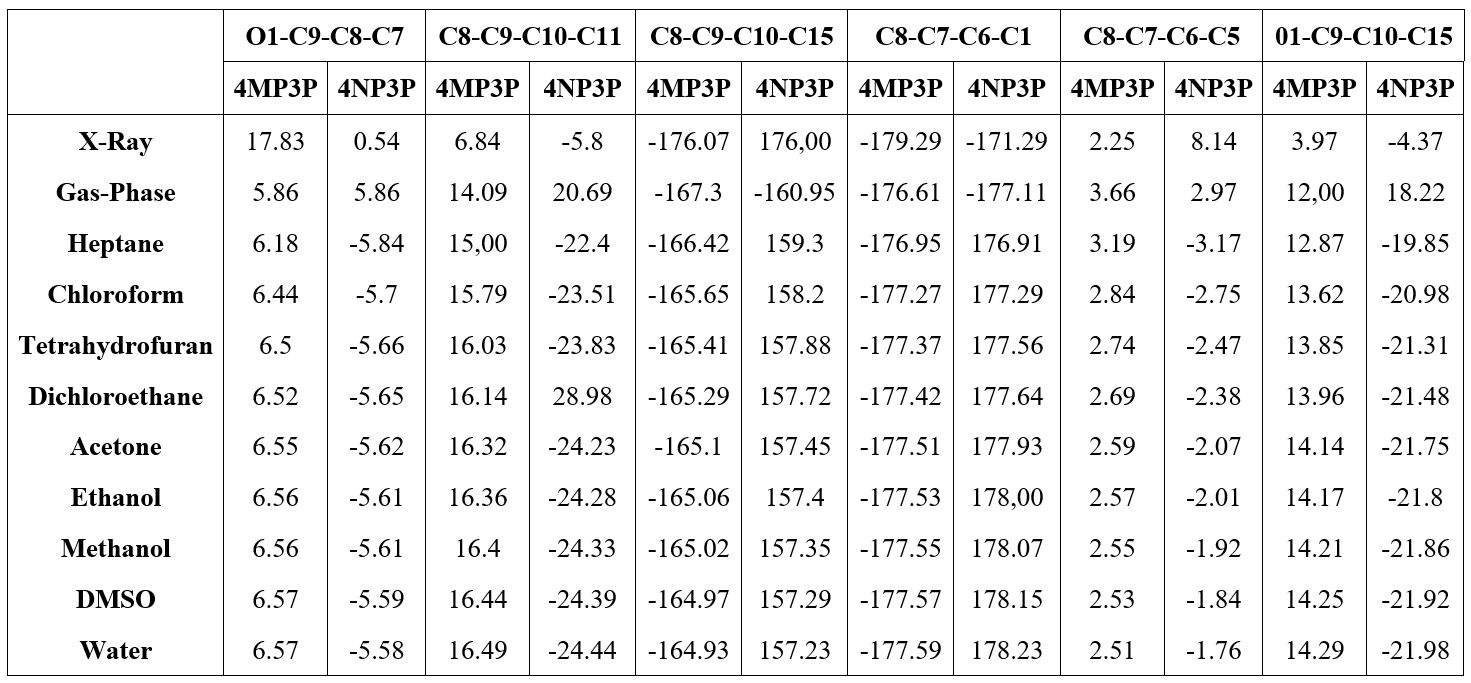
The results of the solvent torsion angles as compared with the gas–phase present a small variation in their absolute values and for 4NP3P a signal inversion occurs. In addition, in Table 4 the X-ray results for the torsion angles for the compounds in crystalline phase are presented. Comparing these angles with the results obtained in gas-phase one can see that greater differences occur for 4NP3P, whose variations can reach 27..
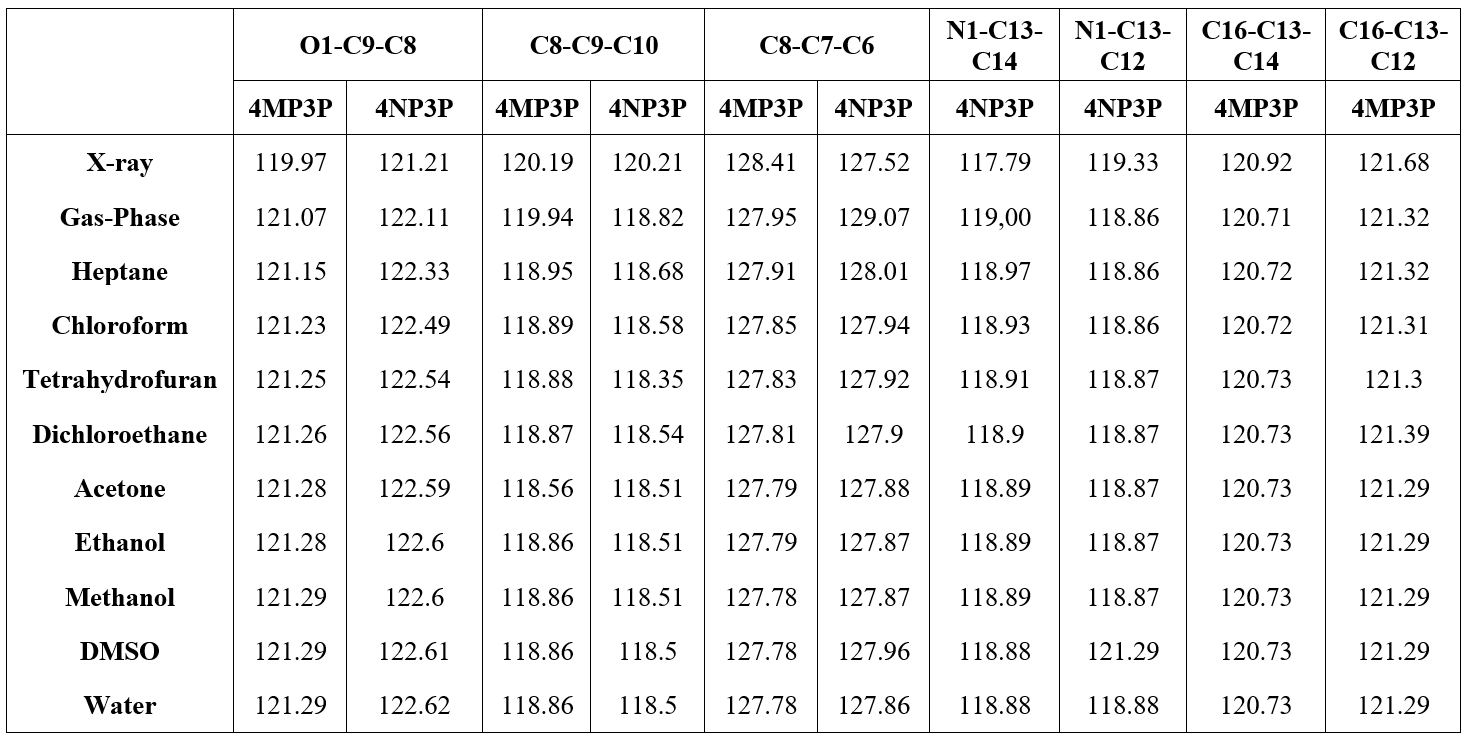
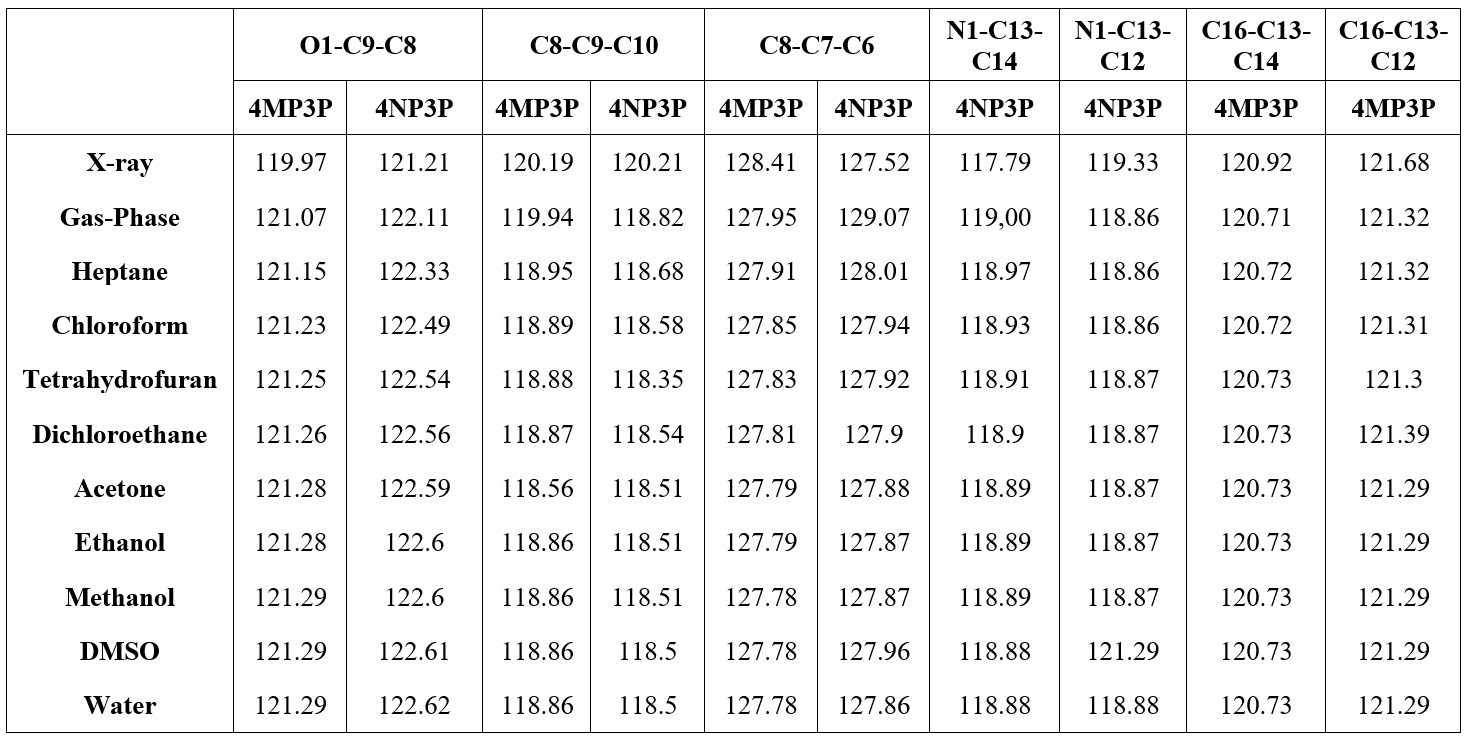
Results for the angles (O1-C9-C8), (C8-C9-C10), (C8-C7-C6) for both compounds and the angles (N1-C13-C14) and (N1-C13-C12) for 4NP3P, and the angles (C16-C13-C14) and (C16-C13-C12) for 4MP3P are presented in Table 5. As can be seen, the results for these angles presents no significative variation caused by the presence of the solvent media.
The asymmetric distribution of electrons in the chemical bonds of the molecular compounds can be visualized by the partial charges calculations in gas-phase. For 4MP3P the total charges of the groups (C4-C5-C6-C7-C8-C9-C16-H3-H4-H5-H6-H12-H13-H14), (C10-C11-C12-C13-C14-C15-H7-H8-H9-H10-H11), and (C1-C2-C3-H1-H2-O1) are 0.012 e, 0.148 e and -0.159 e, respectively. For 4NP3P the total charges of the (C10-C15-H15-C14-H14-C13-C12-H12-C11-H11), (C1-H1-C2-H2-C3-H3-C4-H4-C5-H5-C6), (C7-H7-C8-H8-C9-H9-O1), and ( N1-O2-O3) are 0.170 e, 0.152 e, -0.101 e and -0.221 e, respectively. The transfer of charge is greater for the polar solvent media than that for the nonpolar solvent (e<5)
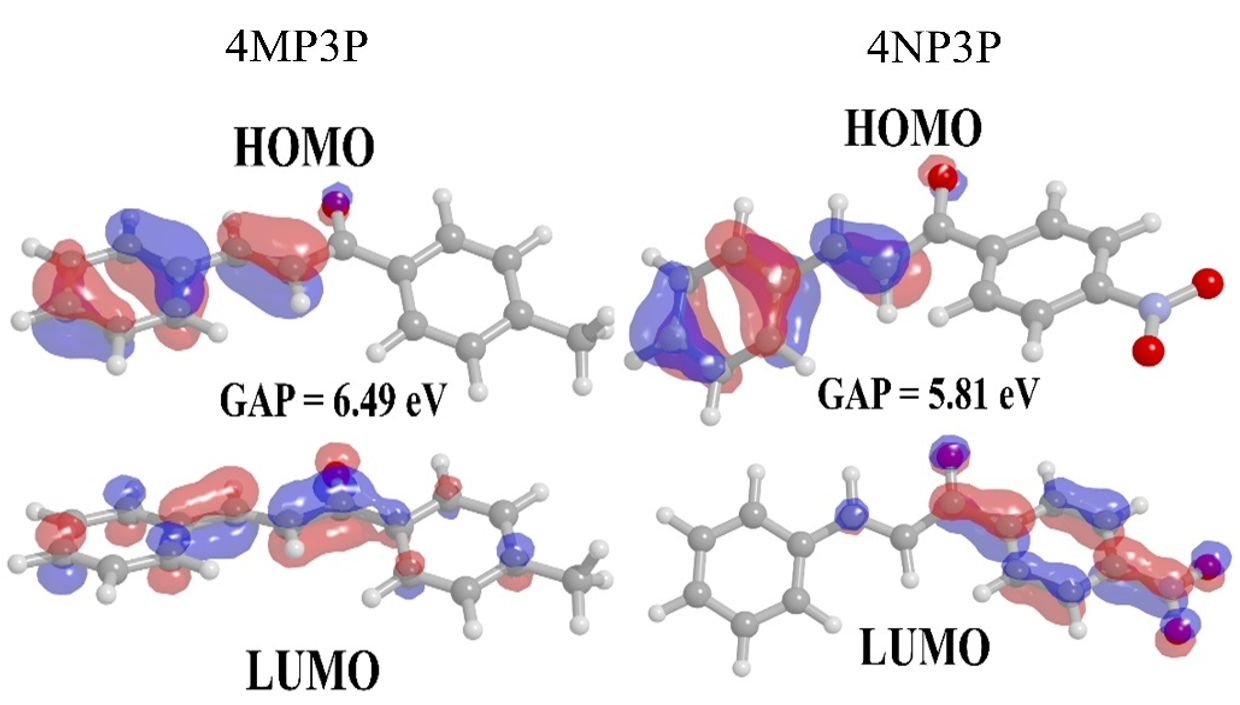
The gap energy (EG) calculated from the difference between the HOMO and LUMO energies is also shown in Table 3 for several solvent media. As can be noted, the substituent change, from CH3 (4MP3P) by the NO2 (4NP3P), causes a decreasing (~11%) in the gap energy in all solvent media.
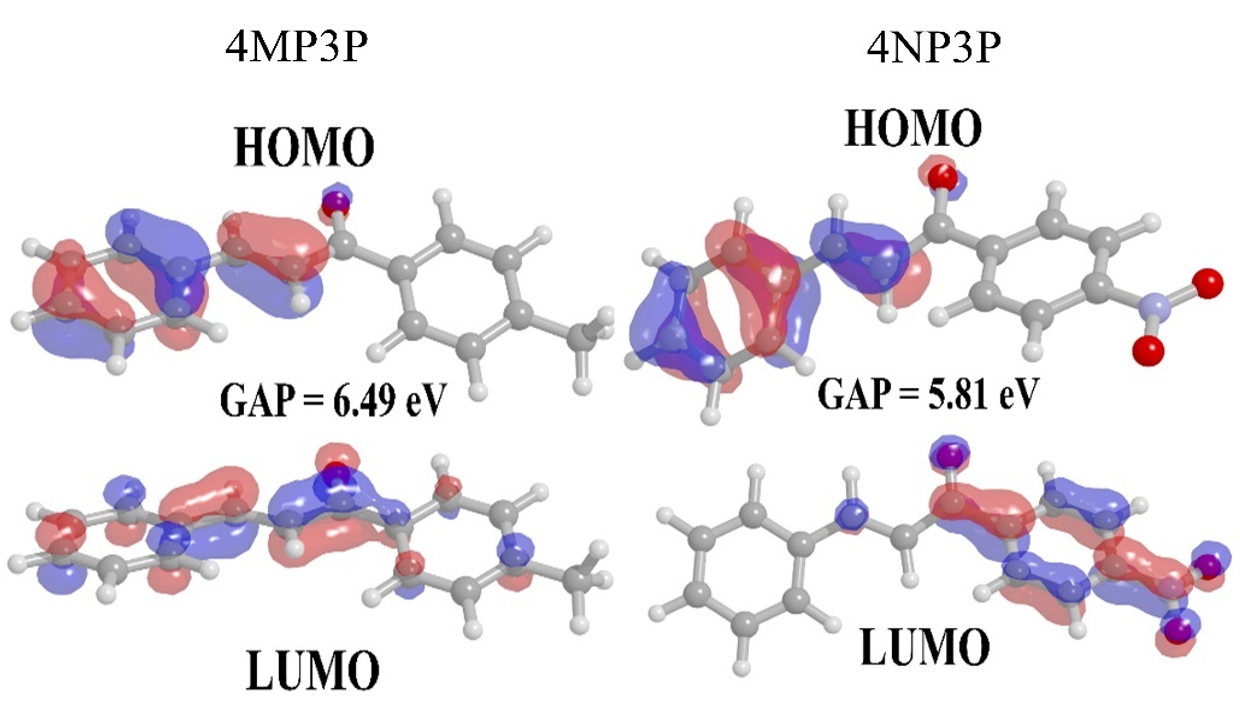
Figure 3.
Frontiers orbital HOMO and LUMO for 4MP3P and 4NP3P in water.
Figure 3 shows the HOMO and LUMO orbitals for 4MP3P and 4NP3P in water, presenting the lowest values of gap energy. Also it shows that the smaller the value of gap energy the better the nonlinear optical properties of the compound [7]. Both HOMO and LUMO present π-bonds.
Nonlinear optical properties
Table 6 shows the DFT/CAM-B3LYP/6-311+G(d) results for the dipole moment (μ) in debye units (D) for both compounds in the gas-phase and in various solvents. As can be seen the μ-value increases with the increasing of the ε-value. For 4MP3P (4NP3P) the dipole moment increases 48.5% (23.6%) as compared with the gas-phase. As example, in water the substituent changes from 4-methylphenyl to 4-nitrophenyl and increases the μ-value of 62.5%, evidencing a greater negative charge transfer for the NO2 group.
| Solvent | ε | 4MP3P μ | 4NP3P μ |
| Gas-phase | 1 | 3.34 | 6.52 |
| Heptane | 1.91 | 3.84 | 7.12 |
| Chloroform | 4.71 | 4.39 | 7.64 |
| Tetrahydrofuran | 7.43 | 4.58 | 7.79 |
| Dichloroethane | 10.13 | 4.68 | 7.87 |
| Acetone | 20.49 | 4.84 | 7.98 |
| Ethanol | 24.85 | 4.86 | 8.00 |
| Methanol | 32.61 | 4.89 | 8.02 |
| DMSO | 46.7 | 4.92 | 8.05 |
| Water | 78.36 | 4.96 | 8.06 |
The behavior of the static values of the average linear polarizability (<a(0,0,0)>), first hyperpolarizability (<b(0,0,0)>), and average second hyperpolarizability (ô<(0,0,0)) as function of ε-values for both compounds are shown in the Table 7. As can be seen, all absolute values of these parameters increase when the e-values for both compounds also increase, the result being greater for 4NP3P. Comparing the absolute values of these parameters for 4MP3P with those for 4NP3P, we can see that the static linear polarizability exhibits the smallest variation (~3%) and the absolute values of the parallel first hyperpolarizability present the greatest variation (more than 150%). So, the substituent change benefits the nonlinear optical properties of the compounds; this fact can be seen for the <ô(0,0,0)>-value that is greater (~20%) for 4NP3P than for 4MP3P, showing that accumulation of negative charges in the NO2 group favors the electron cloud distortion.
The increasing of the a(0;0) values is greater for the nonpolar solvents (~14%) than for the aprotic (~4%) and protic (~1.5%) solvents for both compounds. However, the increasing of the static absolute β-values and γ-values are basically the same for both 4MP3P and 4NP3P, in protic solvents around of 5%, in aprotic solvents around of ~14% and for nonpolar solvents around 60%. These results show that for the nonpolar solvents the dielectric properties of the medium are the strongest characteristic to be considered, but for the aprotic and protic solvents considered here, despite the great increasing of values of the solvent dielectric constant between then, the effect on the electric parameters is small.
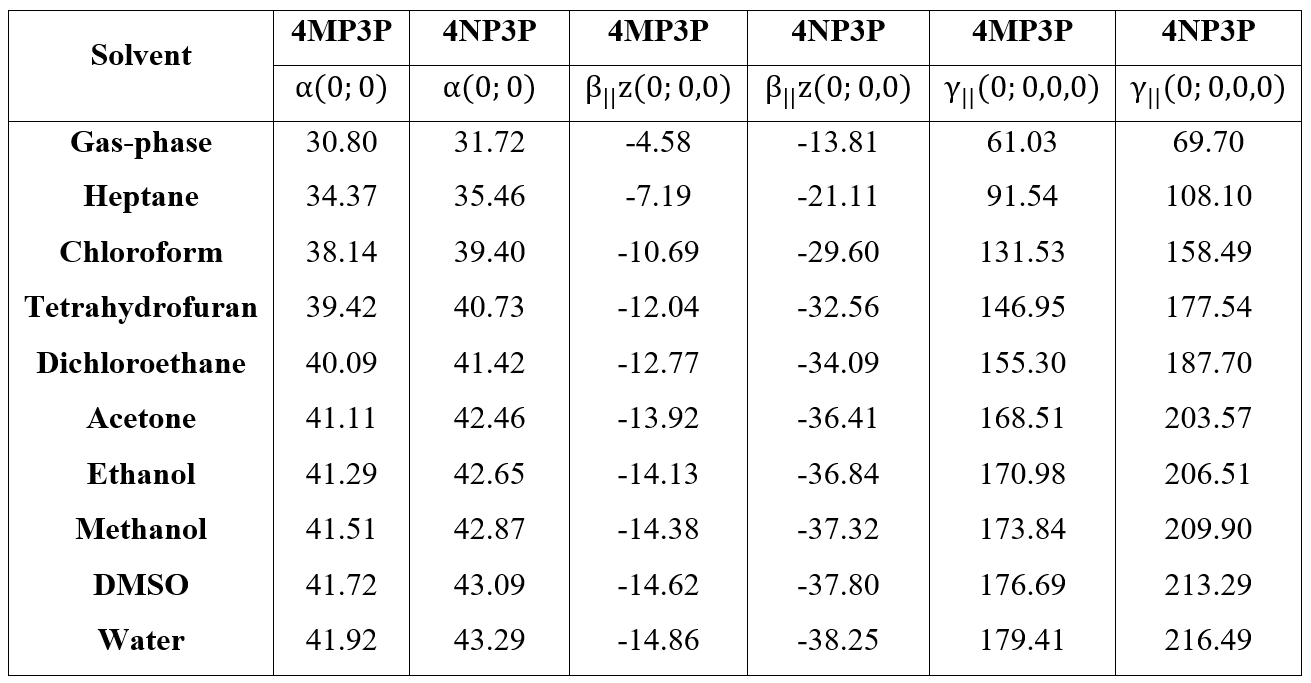
Table 8 shows the static and dynamic (w=0.0428 a.u.) results for HRS hyperpolarizabilities (BHRS) as function of the dielectric constant value; as can be seen, the BHRS(0,0,0,)-value increases when the ε-value also increases. The highest values of the static-BHRS occur in water 26.53*10-30 esu and 15.846*10-30 esu for 4NP3P and 4MP3P, respectively, indicating a significant difference of 67% due the substituent change at position 4 of the phenyl ring. The highest values of the dynamic BHRS(-2w,w,w) occur in DMSO, namely 15.21*10-30 esu (4MP3P) and 38.145*10-30 esu (4NP3P).
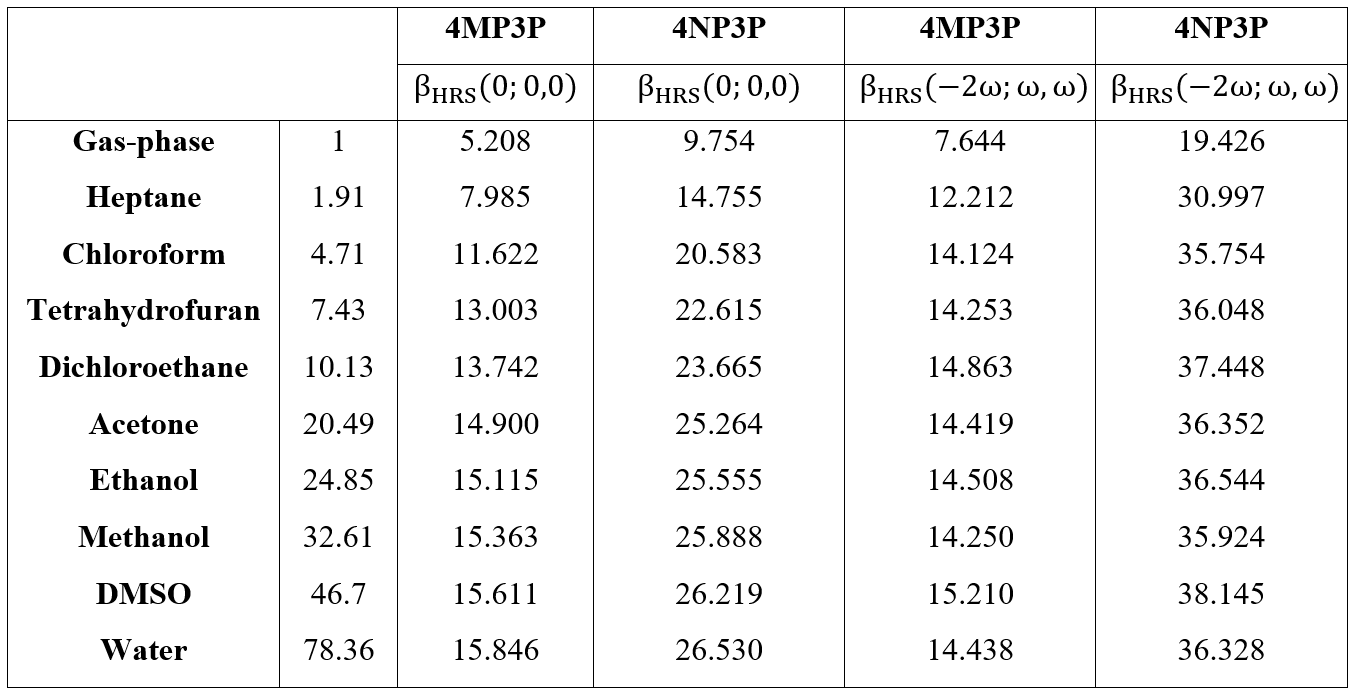
In Figure 4, for the two chalcone derivatives, the static and dynamic results for BHRS are plotted as function of the ε-values; as we can observe, the values for 4NP3P are greater than those of 4MP3P.
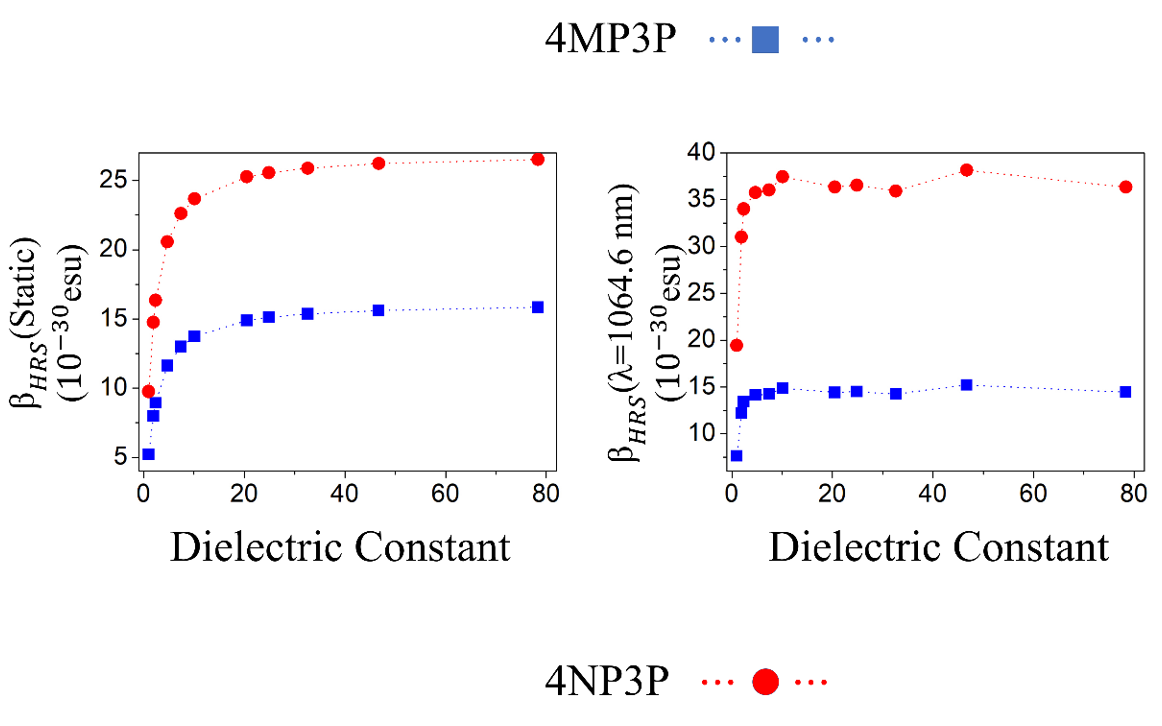
Figure 4.
Static and dynamic HRS first-hyperpolarizabilities as function of the dielectric constant values.
The BHRS-ratio between the compounds 4NP3P ( BHRS4NP3P) and 4MP3P (BHRS4MP3P) as function of ε for the dynamic (ω = 0.0428 a.u) and static case are shown in Figure 5. As can be seen, in the dynamic case, the BHRS-ratio is around 2.5 and for the static case the value lies between 1.7 and 1.9.
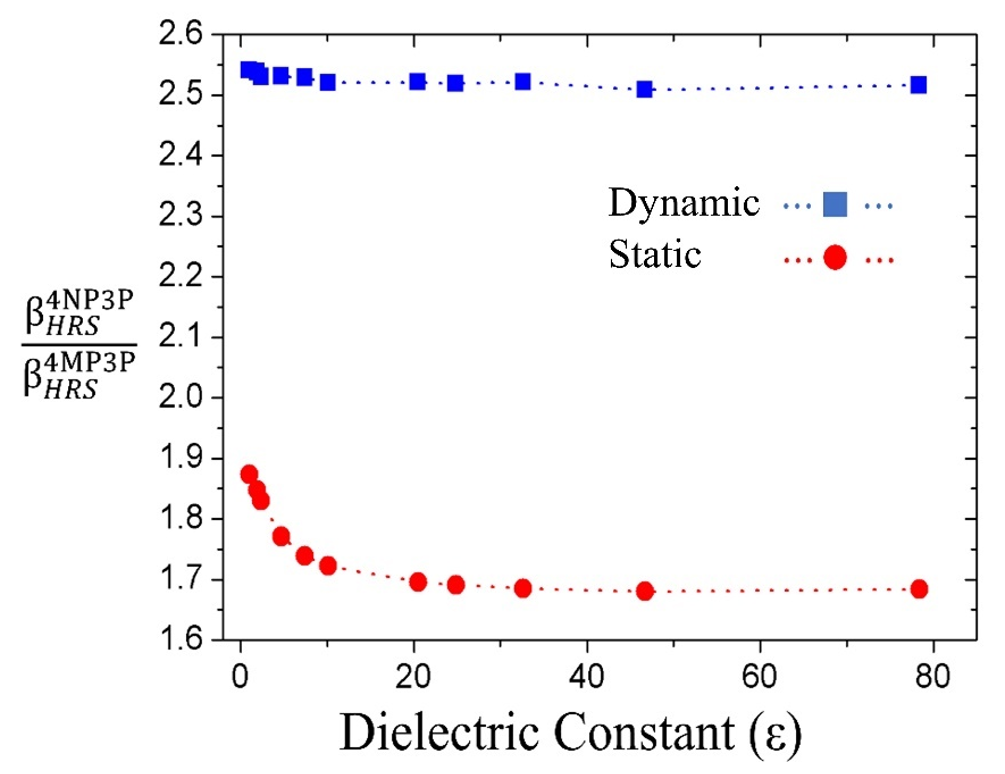
Figure 5.
βHRS-ratio for the compounds 4MP3P and 4NP3P as function of ε for the static and dynamic (ω = 0.428 a.u) cases.
Conclusions
We have reported the geometrical and nonlinear optical properties of two chalcone derivatives: (E)-1-(4-methylphenyl)-3-phenylprop-2-en-1-one (4MP3P) and (E)-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one (4NP3P), within the DFT/CAM-B3LYP/6-311+G(d) level. The effects of the various solvents on the electrical parameters of the two chalcone derivatives were considered via the Polarizable Continuum Model (PCM). The values of the dipole moment (u) increase with the increasing of the solvent dielectric constant values and this increasing is greater for 4MP3P than for 4NP3P. However, the substituent change of CH3 to NO2 increases the m-value for all solvents; as an example, in water this increase is of 62.5%, evidencing the greater negative charge transfer for the NO2 group. The static linear polarizability presents the smaller variation (~3%) due to the solvent medium presence; however, the absolute values of the parallel first hyperpolarizability present the greater variation (more than 150%). Therefore, the substituent change of CH3 (4MP3P) to NO2 (4NP3P) benefits the nonlinear optical properties of the compounds. The HRS hyperpolarizability (BHRS) as function of the solvent dielectric constant value were calculated as in static and dynamic cases. The static BHRS-values also increase when the ε-value increases. The highest values of the static-BHRS occur in water 26.53*10-30 esu and 15.846*10-30 esu for 4NP3P and 4MP3P, respectively, indicating a significant difference of 67% due the substituent change.
Acknowledgements
The authors would like to thank the partial supports of the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES); Pró-Reitoria de Pesquisa e Pós-Graduação da PUC-GO (Prope) e Fundação de Apoio Pesquisa do Estado de Goiás (FAPEG). Research developed with support from the High Performance Computing Center at Universidade Estadual de Goiás (UEG).
References
[1] M. Anis, G. G. Muley, V. G. Pahurkar, M. I. Baig, and S. R. Dagdale, “Influence of Nd 3+ on zinc tris-thiourea sulphate single crystal: a comparative crystal growth, structural, linear–nonlinear optical and dielectric study to explore NLO device applications”, Mater. Res. Innov., vol. 22, no. 2, pp. 99–106, 2018. DOI: https://doi.org/10.1080/14328917.2016.1264848.
[2] X. Fan et al., “Broken Symmetry Induced Strong Nonlinear Optical Effects in Spiral WS 2 Nanosheets,” ACS Nano, vol. 11, no. 5, pp. 4892–4898, 2017. DOI: https://doi.org/10.1021/acsnano.7b01457.
[3] P. K. Johansson, L. Schmüser, and D. G. Castner, “Nonlinear Optical Methods for Characterization of Molecular Structure and Surface Chemistry,” Top. Catal., vol. 61, no. 9–11, pp. 1101–1124, 2018. DOI: https://doi.org/10.1007/s11244-018-0924-3.
[4] L. Lu et al., “Few-layer Bismuthene: Sonochemical Exfoliation, Nonlinear Optics and Applications for Ultrafast Photonics with Enhanced Stability”, Laser Photon. Rev., vol. 12, no. 1, p. 1700221, 2018. DOI: https://doi.org/10.1002/lpor.201700221.
[5] M. Luo et al., “M 2 B 10 O 14 F 6 (M = Ca, Sr): Two Noncentrosymmetric Alkaline Earth Fluorooxoborates as Promising Next-Generation Deep-Ultraviolet Nonlinear Optical Materials”, J. Am. Chem. Soc., vol. 140, no. 11, pp. 3884–3887, 2018. DOI: https://doi.org/10.1021/jacs.8b01263.
[6] A. N. Castro et al., “Theoretical study on the third-order nonlinear optical properties and structural characterization of 3-Acetyl-6-Bromocoumarin,” Chem. Phys. Lett., vol. 653, pp. 122–130, 2016. DOI: https://doi.org/10.1016/j.cplett.2016.04.070.
[7] A. N. Castro, F. A. P. Osório, R. R. Ternavisk, H. B. Napolitano, C. Valverde, and B. Baseia, “Theoretical investigations of nonlinear optical properties of two crystalline acetamides structures including polarization effects of their environment”, Chem. Phys. Lett., vol. 681, pp. 110–123, 2017. DOI: https://doi.org/10.1016/j.cplett.2017.05.066.
[8] T. Chandra Shekhara Shetty, S. Raghavendra, C. S. Chidan Kumar, and S. M. Dharmaprakash, “Nonlinear absorption, optical limiting behavior and structural study of a new chalcone derivative-1-(3, 4-dimethylphenyl)-3-[4(methylsulfanyl) phenyl] prop-2-en-1-one”, Opt. Laser Technol., vol. 77, pp. 23–30, 2016. DOI: https://doi.org/10.1016/j.optlastec.2015.08.015.
[9] S. Bag et al., “Design, synthesis and biological activity of multifunctional α,β-unsaturated carbonyl scaffolds for Alzheimer’s disease”, Bioorg. Med. Chem. Lett., vol. 23, no. 9, pp. 2614–2618, 2013. DOI: https://doi.org/10.1016/j.bmcl.2013.02.103.
[10] Y. Wang, S. Xue, R. Li, Z. Zheng, H. Yi, and Z. Li, “Synthesis and biological evaluation of novel synthetic chalcone derivatives as anti-tumor agents targeting Cat L and Cat K”, Bioorg. Med. Chem., vol. 26, no. 1, pp. 8–16, 2018. DOI: https://doi.org/10.1016/j.bmc.2017.09.019.
[11] J. Li et al., “Design, synthesis, biological evaluation, and molecular docking of chalcone derivatives as anti-inflammatory agents”, Bioorg. Med. Chem. Lett., vol. 27, no. 3, pp. 602–606, 2017. DOI: https://doi.org/10.1016/j.bmcl.2016.12.008.
[12] D. Choi et al., “In Vitro Osteogenic Differentiation and Antibacterial Potentials of Chalcone Derivatives”, Mol. Pharm., vol. 15, no. 8, pp. 3197–3204, 2018. DOI: https://doi.org/10.1021/acs.molpharmaceut.8b00288.
[13] L. Illicachi et al., “Synthesis and DFT Calculations of Novel Vanillin-Chalcones and Their 3-Aryl-5-(4-(2-(dimethylamino)-ethoxy)-3-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde Derivatives as Antifungal Agents”, Molecules, vol. 22, no. 9, p. 1476, 2017. DOI: https://doi.org/10.3390/molecules22091476.
[14] L. M. G. Abegão et al., “Second- and third-order nonlinear optical properties of unsubstituted and mono-substituted chalcones”, Chem. Phys. Lett., vol. 648, pp. 91–96, 2016. DOI: https://doi.org/10.1016/j.cplett.2016.02.009.
[15] F. Toda, K. Tanaka, and M. Kato, “Stereoselective photodimerisation of chalcones in the molten state”, J. Chem. Soc. Perkin Trans. 1, no. 7, pp. 1315–1318, 1998. DOI: https://doi.org/10.1039/a707380a.
[16] L.-H. Jing, “( E )-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one”, Acta Crystallogr. Sect. E Struct. Reports Online, vol. 65, no. 10, pp. o2510–o2510, 2009. DOI: https://doi.org/10.1107/S1600536809037556.
[17] C. Laane, S. Boeren, K. Vos, and C. Veeger, “Rules for optimization of biocatalysis in organic solvents”, Biotechnol. Bioeng., vol. 30, no. 1, pp. 81–87, 1987. DOI: https://doi.org/10.1002/bit.260300112.
[18] W. J. Geary, “The use of conductivity measurements in organic solvents for the characterisation of coordination compounds”, Coord. Chem. Rev., vol. 7, no. 1, pp. 81–122, 1971. DOI: https://doi.org/10.1016/S0010-8545(00)80009-0.
[19] C. Valverde, Í. N. Ribeiro, J. V. B. Soares, B. Baseia, and F. A. P. Osório, “Prediction of the Linear and Nonlinear Optical Properties of a Schiff Base Derivatives via DFT”, Adv. Condens. Matter Phys., vol. 2019, pp. 1–12, 2019. DOI: https://doi.org/10.1155/2019/8148392.
[20] S. Backus et al., “16-fs, 1-μJ ultraviolet pulses generated by third-harmonic conversion in air”, Opt. Lett., vol. 21, no. 9, p. 665, 1996. DOI: https://doi.org/10.1364/OL.21.000665.
[21] J. M. F. Custodio et al., “Chalcone as Potential Nonlinear Optical Material: A Combined Theoretical, Structural, and Spectroscopic Study”, J. Phys. Chem. C, vol. 123, no. 10, pp. 5931–5941, 2019. DOI: https://doi.org/10.1021/acs.jpcc.9b01063.
[22] C. Reichardt, Solvents and Related Titles from WILEY-VCH Organic Synthesis Workbook II Chemical Synthesis Using Supercritical Fluids. 2003.
Notas de autor
valverde@ueg.br
Recibido: 9 de septiembre de 2019; Aceptado: 20 de noviembre de 2019
Abstract
The use of organic as nonlinear optical materials has been intensively explored in the recent years due to the ease of manipulation of the molecular structure and the synthetic flexibility regarding the change of substituent groups. In the present work, the linear and nonlinear properties of two chalcones derivatives (E)-1-(4-methylphenyl)-3-phenylprop-2-en-1-one (4MP3P) and (E)-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one (4NP3P), that differ by the substituent position at the phenyl ring, were studied in the presence of protic and aprotic solvents simulated by the Polarizable Continuum Model (PCM) at DFT/B3LYP/6-311+G(d) level. The static and dynamic (1064 nm) molecular parameters as the dipole moment, linear polarizability, first and second hyperpolarizabilities were studied as function of the solvent dielectric constant value. The geometrical behavior as the chemical bond angles, torsion angles, and partial charges distribution of the compounds were studied, including calculations of gap energies in various solvents. The obtained results revealed that the substituent change of CH3 (4MP3P) to NO2 (4NP3P) benefits the nonlinear optical properties of the compounds in the presence of the solvent media, the absolute values of the parallel first hyperpolarizability were the ones that present the greater variation.
Keywords
first and second hyperpolarizabilities, solvent, DMSO, acetone.Resumen
El uso de materiales orgánicos como materiales ópticos no lineales se ha explorado intensamente en los últimos años, debido a la facilidad de manipulación de estas estructuras moleculares y la flexibilidad de síntesis en relación con el cambio de grupos sustituyentes. En el presente trabajo, las propiedades lineales y no lineales de dos derivados de chalcona (E)-1-(4-metilfenil)-3-fenilprop-2-en-1-ona (4MP3P) y (E)-1-(4-nitrofenil)-3-fenilprop-2-en-1-ona (4NP3P), los cuales difieren en la posición del sustituyente en el anillo de fenilo, se estudiaron en presencia de disolventes próticos y apróticos simulados por el Modelo Continuo Polarizable a nivel DFT/B3LYP/6-311+G(d). Además, se estudiaron parámetros moleculares estáticos y dinámicos (1064 nm) como el momento dipolar, la polarización lineal y la primera y la segunda hiperpolarización en función del valor constante dieléctrico del disolvente. El comportamiento geométrico se estudió como ángulos de enlace químico, ángulos de torsión y distribución de carga parcial de compuestos, incluidos los cálculos de energía de huecos en varios solventes. Los resultados mostraron que el cambio del sustituyente CH3 (4MP3P) a NO2 (4NP3P) beneficia las propiedades ópticas no lineales de los compuestos en presencia del medio solvente, los valores absolutos de la primera hiperpolarizabilidad paralela fueron los que presentaron la mayor variación.
Palabras clave
primera y segunda hiperpolarizabilidad, solvente, DMSO, acetona.Resumo
O uso de materiais orgânicos como materiais ópticos não lineares tem sido intensamente explorado nos últimos anos, devido à facilidade de manipulação dessas estruturas moleculares e à flexibilidade de síntese em relação à mudança de grupos substituintes. No presente trabalho, as propriedades lineares e não lineares de dois derivados de chalconas (E)-1-(4-metilfenil)-3-fenilprop-2-en-1-ona (4MP3P) e (E)-1-(4-nitrofenil)-3-fenilprop-2-en-1-ona (4NP3P), que diferem pela posição do substituinte no anel fenil, foram estudados na presença de solventes próticos e apróticos simulados pelo Modelo Continuo Polarizável (PCM) no nível DFT/B3LYP/6-311+G(d). Os parâmetros moleculares estáticos e dinâmicos (1064 nm) como momento dipolar, polarizabilidade linear, primeira e segunda hiperpolarizabilidades foram estudados em função do valor da constante dielétrica do solvente. Estudou-se o comportamento geométrico como ângulos de ligação química, ângulos de torção e distribuição parcial de cargas dos compostos, incluindo cálculos de energias de gap em vários solventes. Os resultados obtidos revelaram que a mudança do substituinte de CH3 (4MP3P) para NO2 (4NP3P) beneficia as propriedades ópticas não lineares dos compostos na presença do meio solvente, os valores absolutos da primeira hiperpolarizabilidade paralela foram os que apresentaram a maior variação.
Palavras-chave
primeira e segunda hiperpolarizabilidades, vent, solvente, DMSO, acetona.Introduction
In the last few years the engineering of organic crystalline compound has become an important area of study and has attracted great interest from research centers [1], motivated by significant values of the nonlinear optical properties of these crystals, for instance, chalcone derivatives [2]. Nonlinear optics devices operate with high-speed information [3], as ultra-short pulse lasers, photonic devices, optical modulators, and others with applications in the areas of health and medicine [4, 5]. The advantages of organic crystals that have attracted much attention come from their ease of manipulation of the molecular structure and the synthetic flexibility regarding the change of substituent groups [6, 7]. The combination of the high nonlinearity characteristic of some organic compounds and the versatility of synthetic routes leads to maximization of the nonlinear optical properties [8]. Among the organic materials, the chalcones are known as promising compounds, since their derivatives have several biological properties that have been used as prototypes for new drugs [9]. Biosynthesis of flavonoids chalcones have been applied in several activities such as: antitumor [10], anti-inflammatory [11], antibacterial [12], antifungal [13], among others.
The chalcones consist of two aromatic rings joined by an unsaturated system so that their structure almost always acquires a linear or almost planar conformation [14]. Organic molecules with π-conjugated structures and donor and/or acceptor groups are compounds of great interest in the area of nonlinear optics [15]. The change of the substituent groups bond to the aromatic rings leads the chalcone molecules to acquire specific properties due the change of electronic distribution of the compound, hence new properties can be explored with each new substituent being added or substituted to the compound [16].
The purpose of the present paper is to study the nonlinear optical properties of two chalcone derivatives: (.)-1-(4-methylphenyl)-3-phenylprop-2-en-1-one (4MP3P) and (.)-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one (4NP3P), with formulas C16.14O and C15.11O., respectively. The structure 4MP3P was synthesized by Toda et al. [15] and the structure 4NP3P by Jing et al. [16]. These compounds differ by the substituent at position 4 of the phenyl ring (B) as shown in Figure 1.
Figure 1: Molecular structure of the compounds 4MP3P and 4NP3P.
The electrical parameters of these compounds were calculated at DFT/CAM-B3LYP/6-311+G(d) level in the presence of several solvent media that are modeled by the Polarizable Continuum Model (PCM). The static and dynamic (1064 nm) molecular parameters as the dipole moment, linear polarizability, first and second hyperpolarizability were studied as function of the value of the solvent dielectric constant value. Also, the frontiers molecular orbital, HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital), and the respective gap energies in various solvents were calculated. The geometrical behavior of the compounds as the chemical bond angles, torsion angles, and partial charges distribution were also studied.
Materials and Methods
Computational procedures
Organic solvents belong to a class of liquid and volatile compounds and have the function of solubilizing, extracting, treating, and facilitating a chemical reaction among other functions. This group of compounds is divided into: polar and nonpolar solvents. Polar protic solvents are characterized by the presence of hydrogen bonded to electronegative elements, usually, atoms of O, N, and F, which can lead to the formation of hydrogen bonds [17] and polar aprotic solvents are characterized by the absence of electronegative atoms so that only bonds between carbon and hydrogen atoms are present. The values of the dielectric constant and of the dipole moments for polar solvents are higher than those for the nonpolar solvents [18]. The dielectric constant of a solvent medium is a good indicator of the ability to accommodate a charge separation that increases with the values of the dipole moment and with the polarizability of the molecule [19].
In this work the quantitative concept of the solvent polarity was considered through the normalized transition energy ( scale of Dimroth and Reichardt [19, 20]. The -value is based on the transition energy to the solvatochromic absorption band for the greatest wavelenght of the nitrile betaine pyridynium dye. Table 1 shows a list of the solvent media, here considered with the respective values of and ε. Reactions involving charge separation in the -scale advance more slowly in polar aprotic solvents due to the small dipole moments and the absence of hydrogen bonds which make them few effective in the development of the separation and the stabilization of charges when compared to the polar protic solvents[19, 21].
The first two parameters are dimensionless.Table 1: Polarity of several solvent media following
Solvents
Type
Water
1.000
78.355
Protic
Methanol
0.762
32.613
Protic
Ethanol
0.654
24.852
Protic
DMSO
0.444
46.826
Aprotic
Acetone
0.355
20.493
Aprotic
Dichloroethane
0.327
10.125
Aprotic
Chloroform
0.259
4.711
nonpolar
Tetrahydrofuran
0.207
7.430
Aprotic
Heptane
0.012
1.911
nonpolar
Here the solvent chloroform must be considered nonpolar because the value of its dielectric constant ε is less than 5.
The total dipole moment and the average linear polarizability were calculated from the components x, y, and z, through the expressions:
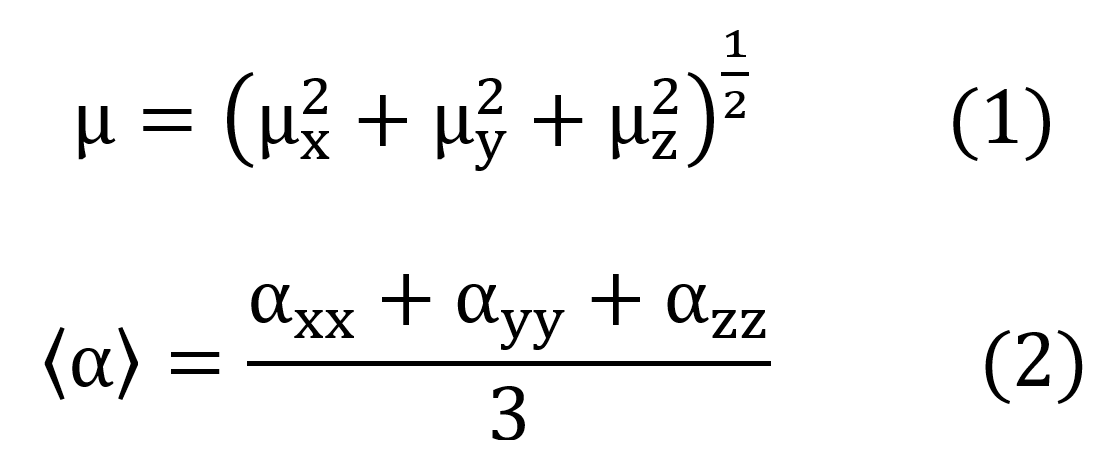
The first hyperpolarizabilities considered concerned the parallel components to the direction of dipole moment (taken as z-direction) given by,
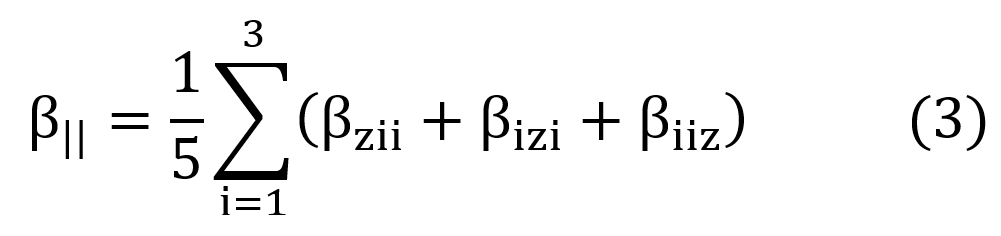
and the Hyper-Rayleigh Scattering first hyperpolarizability (BHRS) defined by,
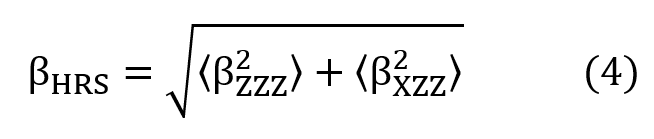
where the laboratory system of reference is adopted as X, Y, and Z coordinates. The X-direction is assumed as the fundamental light beam propagation, polarized in the Z-direction. The (BZ 2 ZZ) and (BX 2 ZZ) are macroscopic averages calculated from the first hyperpolarizability components (Bijk) through the expressions,
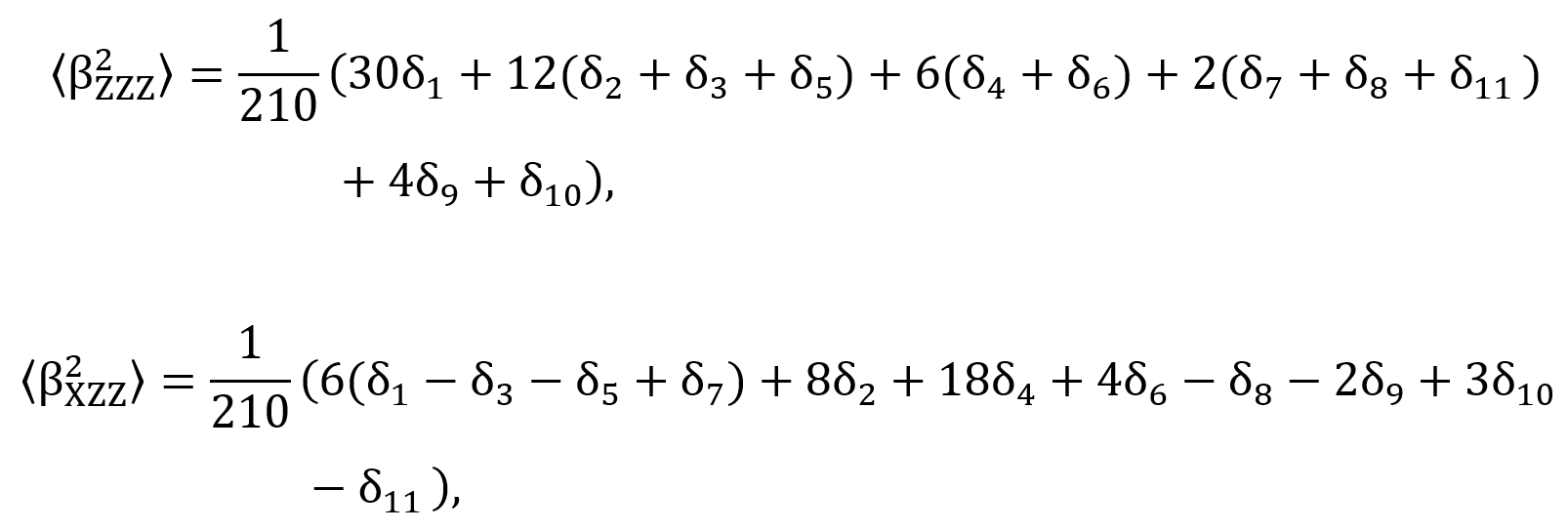
in which the coefficients are defined in Table 2.
Table 2.: The HRS first hyperpolarizability coefficients (dn).
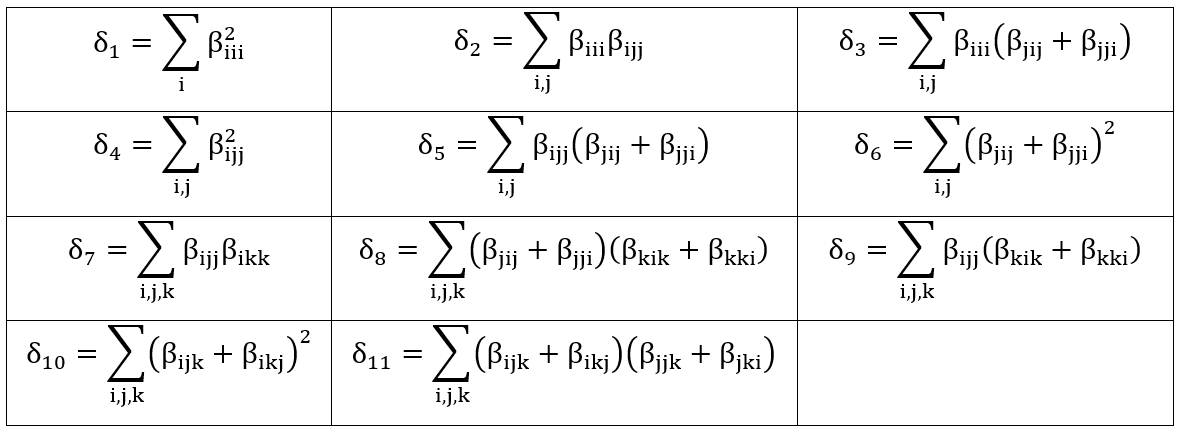
Using the Kleymann symmetry, the average value of the second hyperpolarizability ( ) can be calculated through the following expression,
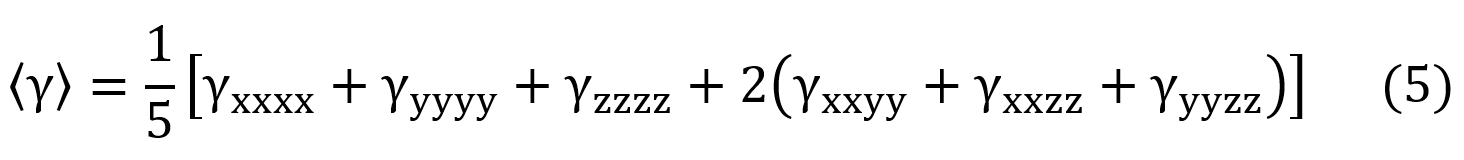
The linear and nonlinear optical (NLO) parameters of the molecules of chalcone derivatives in several solvent media were calculated using the PCM/DFT/CAM-B3LYP/6-311+G(d) level with the Gaussian 09 software package.
Results and discussions
Solvent effects on the molecular properties
In this section, the similarities between the molecular geometry of 4MP3P and 4NP3P, obtained via X-ray diffraction [15, 16], and the theoretical results in several solvent media are analyzed through the root mean square deviation (RMSD) of the overlap of the two structures. As can be seen, in Table 3, the value of RMSD is 0.156 a.u. (0.217 a.u.) for gas phase of 4MP3P (4NP3P) and this value presents small changes when the solvent dielectric constant increases, until reaching the value 0.167 a.u. (0.295 a.u.), therefore the effects of solvent media on the molecular geometry is more significant for 4NP3P. Figure 2 shows a schematic representation of the overlap of 4MP3P and 4NP3P molecular structure determined by X-ray (in blue) and in water (in red), where the phenyl ring (A) was used as anchorage for the molecular structures. The H-atoms were disregarded in view of their uncertainties in X-ray position refinement.
Table 3. RMSD (a.u.) and Gap energies (eV) of 4MP3P and 4NP3P.
Table 3: RMSD au and Gap energies eV of 4MP3P and 4NP3P
Solvent
RMSD
4MP3P
RMSD
4NP3P
Gap (eV)
4MP3P
Gap (eV)
4NP3P
Gas-phase
1.00
0.156
0.217
6.636
6.094
Heptane
1.91
0.158
0.285
6.587
5.980
Chloroform
4.71
0.162
0.292
6.537
5.886
Tetrahydrofuran
7.43
0.163
0.293
6.520
5.859
Dichloroethane
10.13
0.164
0.294
6.511
5.846
Acetone
20.49
0.165
0.295
6.499
5.829
Ethanol
24.85
0.166
0.295
6.496
5.825
Methanol
32.61
0.166
0.295
6.494
5.822
DMSO
46.71
0.166
0.295
6.491
5.819
Water
78.36
0.167
0.295
6.489
5.815
Figure 2.: Overlap of the theoretical molecular structures of the compounds (4MP3P and 4NP3P) in water (red) and that determined by X-ray diffraction (blue).
Table 4 shows the (O1-C9-C8-C7), (C8-C9-C10-C11), (C8-C9-C10-C15), (C8-C7-C6-C5), (C8-C7-C6-C5), and (01-C9-C10-C15) torsion angles for various solvents and for the gas-phase. As can be seen, the torsion angles present a small variations in all solvent media, independent of the solvent polarity or the protic or aprotic character for both compunds 4MP3P and 4NP3P.
Table 4.: Geometrical parameters related to torsion angle (º).
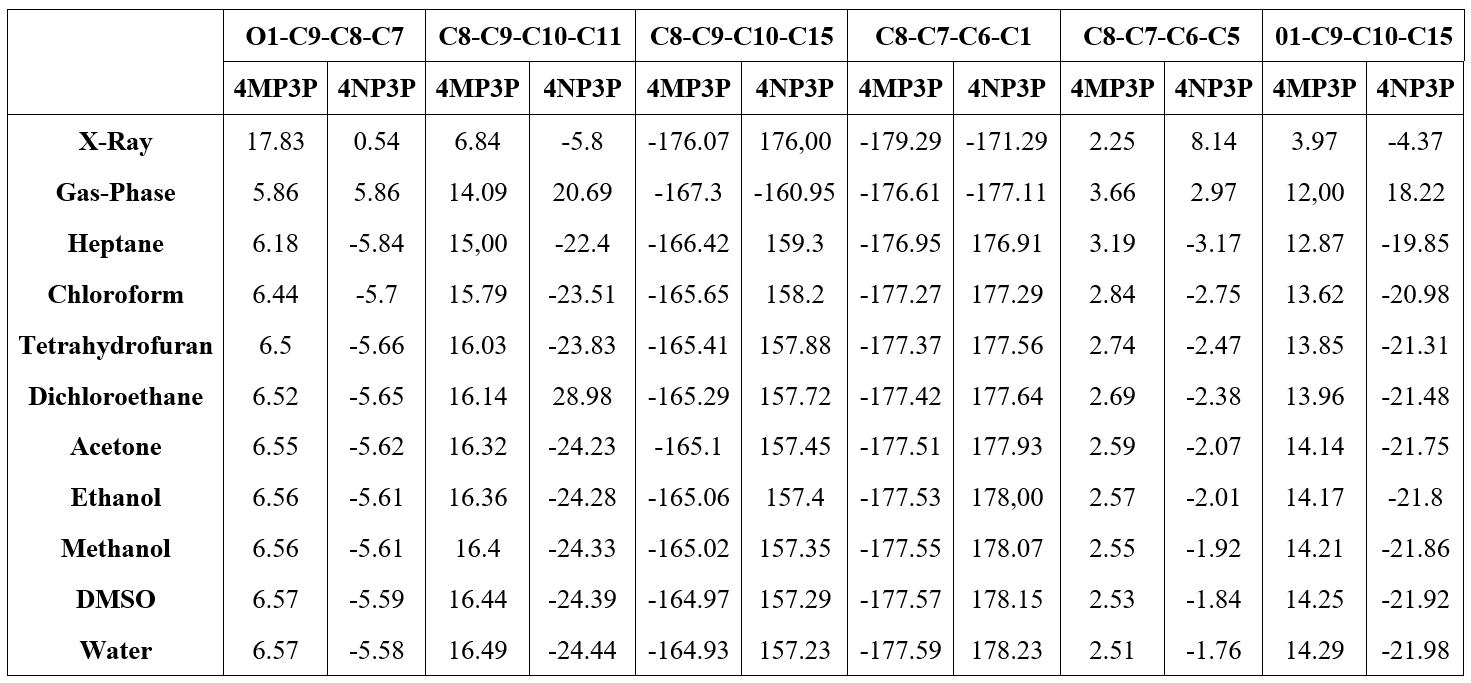
The results of the solvent torsion angles as compared with the gas–phase present a small variation in their absolute values and for 4NP3P a signal inversion occurs. In addition, in Table 4 the X-ray results for the torsion angles for the compounds in crystalline phase are presented. Comparing these angles with the results obtained in gas-phase one can see that greater differences occur for 4NP3P, whose variations can reach 27..
Results for the angles (O1-C9-C8), (C8-C9-C10), (C8-C7-C6) for both compounds and the angles (N1-C13-C14) and (N1-C13-C12) for 4NP3P, and the angles (C16-C13-C14) and (C16-C13-C12) for 4MP3P are presented in Table 5. As can be seen, the results for these angles presents no significative variation caused by the presence of the solvent media.
Table 5: Geometrical parameters related to angle (º).
The asymmetric distribution of electrons in the chemical bonds of the molecular compounds can be visualized by the partial charges calculations in gas-phase. For 4MP3P the total charges of the groups (C4-C5-C6-C7-C8-C9-C16-H3-H4-H5-H6-H12-H13-H14), (C10-C11-C12-C13-C14-C15-H7-H8-H9-H10-H11), and (C1-C2-C3-H1-H2-O1) are 0.012 e, 0.148 e and -0.159 e, respectively. For 4NP3P the total charges of the (C10-C15-H15-C14-H14-C13-C12-H12-C11-H11), (C1-H1-C2-H2-C3-H3-C4-H4-C5-H5-C6), (C7-H7-C8-H8-C9-H9-O1), and ( N1-O2-O3) are 0.170 e, 0.152 e, -0.101 e and -0.221 e, respectively. The transfer of charge is greater for the polar solvent media than that for the nonpolar solvent (e<5)
The gap energy (EG) calculated from the difference between the HOMO and LUMO energies is also shown in Table 3 for several solvent media. As can be noted, the substituent change, from CH3 (4MP3P) by the NO2 (4NP3P), causes a decreasing (~11%) in the gap energy in all solvent media.
Figure 3.: Frontiers orbital HOMO and LUMO for 4MP3P and 4NP3P in water.
Figure 3 shows the HOMO and LUMO orbitals for 4MP3P and 4NP3P in water, presenting the lowest values of gap energy. Also it shows that the smaller the value of gap energy the better the nonlinear optical properties of the compound [7]. Both HOMO and LUMO present π-bonds.
Nonlinear optical properties
Table 6 shows the DFT/CAM-B3LYP/6-311+G(d) results for the dipole moment (μ) in debye units (D) for both compounds in the gas-phase and in various solvents. As can be seen the μ-value increases with the increasing of the ε-value. For 4MP3P (4NP3P) the dipole moment increases 48.5% (23.6%) as compared with the gas-phase. As example, in water the substituent changes from 4-methylphenyl to 4-nitrophenyl and increases the μ-value of 62.5%, evidencing a greater negative charge transfer for the NO2 group.
Table 6: Dipole moment values D for 4MP3P and 4NP3P in several solvent media
Solvent
ε
4MP3P μ
4NP3P
μ
Gas-phase
1
3.34
6.52
Heptane
1.91
3.84
7.12
Chloroform
4.71
4.39
7.64
Tetrahydrofuran
7.43
4.58
7.79
Dichloroethane
10.13
4.68
7.87
Acetone
20.49
4.84
7.98
Ethanol
24.85
4.86
8.00
Methanol
32.61
4.89
8.02
DMSO
46.7
4.92
8.05
Water
78.36
4.96
8.06
The behavior of the static values of the average linear polarizability (<a(0,0,0)>), first hyperpolarizability (<b(0,0,0)>), and average second hyperpolarizability (ô<(0,0,0)) as function of ε-values for both compounds are shown in the Table 7. As can be seen, all absolute values of these parameters increase when the e-values for both compounds also increase, the result being greater for 4NP3P. Comparing the absolute values of these parameters for 4MP3P with those for 4NP3P, we can see that the static linear polarizability exhibits the smallest variation (~3%) and the absolute values of the parallel first hyperpolarizability present the greatest variation (more than 150%). So, the substituent change benefits the nonlinear optical properties of the compounds; this fact can be seen for the <ô(0,0,0)>-value that is greater (~20%) for 4NP3P than for 4MP3P, showing that accumulation of negative charges in the NO2 group favors the electron cloud distortion.
The increasing of the a(0;0) values is greater for the nonpolar solvents (~14%) than for the aprotic (~4%) and protic (~1.5%) solvents for both compounds. However, the increasing of the static absolute β-values and γ-values are basically the same for both 4MP3P and 4NP3P, in protic solvents around of 5%, in aprotic solvents around of ~14% and for nonpolar solvents around 60%. These results show that for the nonpolar solvents the dielectric properties of the medium are the strongest characteristic to be considered, but for the aprotic and protic solvents considered here, despite the great increasing of values of the solvent dielectric constant between then, the effect on the electric parameters is small.
Table 7.: Static values of the average linear polarizability (in 10-24 esu), first hyperpolarizability (in 10-30 esu) and average second hyperpolarizability (in 10-36 esu) for 4MP3P and 4NP3P.
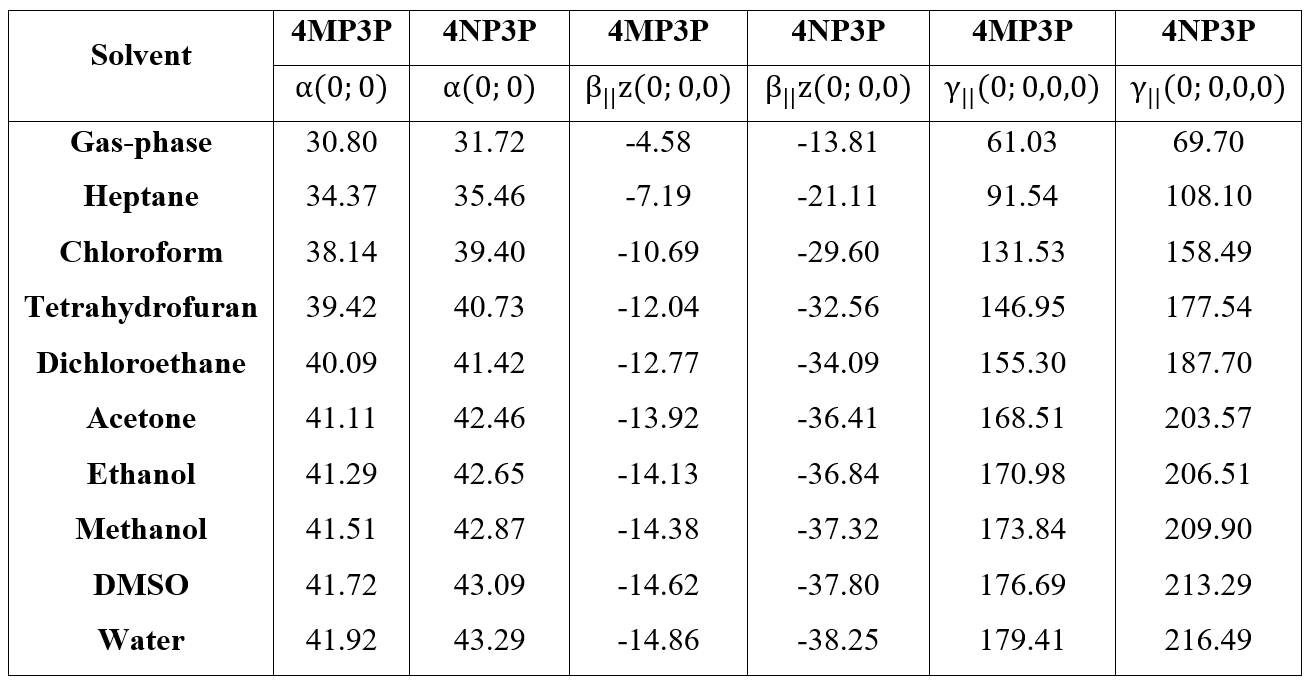
Table 8 shows the static and dynamic (w=0.0428 a.u.) results for HRS hyperpolarizabilities (BHRS) as function of the dielectric constant value; as can be seen, the BHRS(0,0,0,)-value increases when the ε-value also increases. The highest values of the static-BHRS occur in water 26.53*10-30 esu and 15.846*10-30 esu for 4NP3P and 4MP3P, respectively, indicating a significant difference of 67% due the substituent change at position 4 of the phenyl ring. The highest values of the dynamic BHRS(-2w,w,w) occur in DMSO, namely 15.21*10-30 esu (4MP3P) and 38.145*10-30 esu (4NP3P).
Table 8: Static and dynamic values of the HRS first hyperpolarizabilities (in 10-30 esu) for 4MP3P and 4NP3P.
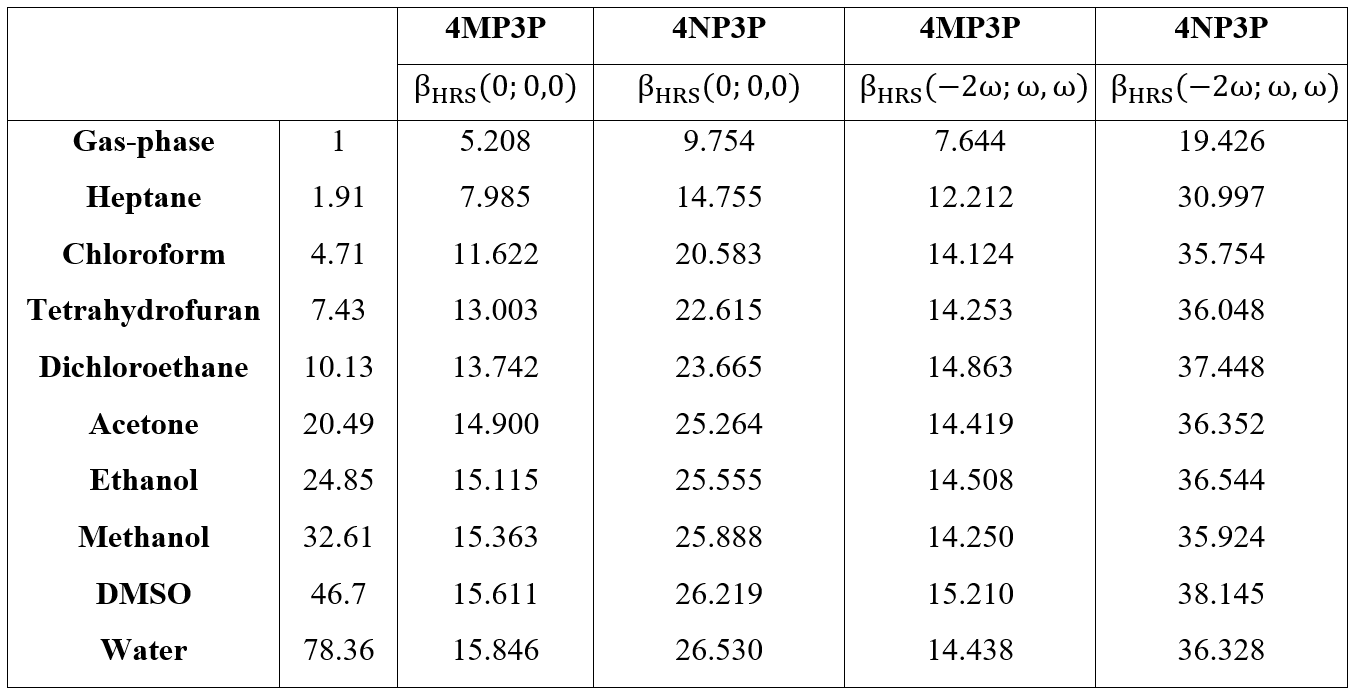
In Figure 4, for the two chalcone derivatives, the static and dynamic results for BHRS are plotted as function of the ε-values; as we can observe, the values for 4NP3P are greater than those of 4MP3P.
Figure 4.: Static and dynamic HRS first-hyperpolarizabilities as function of the dielectric constant values.
The BHRS-ratio between the compounds 4NP3P ( BHRS 4NP3P) and 4MP3P (BHRS 4MP3P) as function of ε for the dynamic (ω = 0.0428 a.u) and static case are shown in Figure 5. As can be seen, in the dynamic case, the BHRS-ratio is around 2.5 and for the static case the value lies between 1.7 and 1.9.
Figure 5.: βHRS-ratio for the compounds 4MP3P and 4NP3P as function of ε for the static and dynamic (ω = 0.428 a.u) cases.
Conclusions
We have reported the geometrical and nonlinear optical properties of two chalcone derivatives: (E)-1-(4-methylphenyl)-3-phenylprop-2-en-1-one (4MP3P) and (E)-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one (4NP3P), within the DFT/CAM-B3LYP/6-311+G(d) level. The effects of the various solvents on the electrical parameters of the two chalcone derivatives were considered via the Polarizable Continuum Model (PCM). The values of the dipole moment (u) increase with the increasing of the solvent dielectric constant values and this increasing is greater for 4MP3P than for 4NP3P. However, the substituent change of CH3 to NO2 increases the m-value for all solvents; as an example, in water this increase is of 62.5%, evidencing the greater negative charge transfer for the NO2 group. The static linear polarizability presents the smaller variation (~3%) due to the solvent medium presence; however, the absolute values of the parallel first hyperpolarizability present the greater variation (more than 150%). Therefore, the substituent change of CH3 (4MP3P) to NO2 (4NP3P) benefits the nonlinear optical properties of the compounds. The HRS hyperpolarizability (BHRS) as function of the solvent dielectric constant value were calculated as in static and dynamic cases. The static BHRS-values also increase when the ε-value increases. The highest values of the static-BHRS occur in water 26.53*10-30 esu and 15.846*10-30 esu for 4NP3P and 4MP3P, respectively, indicating a significant difference of 67% due the substituent change.
Acknowledgements
The authors would like to thank the partial supports of the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES); Pró-Reitoria de Pesquisa e Pós-Graduação da PUC-GO (Prope) e Fundação de Apoio Pesquisa do Estado de Goiás (FAPEG). Research developed with support from the High Performance Computing Center at Universidade Estadual de Goiás (UEG).
References
Referencias
M. Anis, G. G. Muley, V. G. Pahurkar, M. I. Baig, and S. R. Dagdale, “Influence of Nd 3+ on zinc tris-thiourea sulphate single crystal: a comparative crystal growth, structural, linear–nonlinear optical and dielectric study to explore NLO device applications”, Mater. Res. Innov., vol. 22, no. 2, pp. 99–106, 2018. DOI: https://doi.org/10.1080/14328917.2016.1264848.
X. Fan et al., “Broken Symmetry Induced Strong Nonlinear Optical Effects in Spiral WS 2 Nanosheets,” ACS Nano, vol. 11, no. 5, pp. 4892–4898, 2017. DOI: https://doi.org/10.1021/acsnano.7b01457.
P. K. Johansson, L. Schmüser, and D. G. Castner, “Nonlinear Optical Methods for Characterization of Molecular Structure and Surface Chemistry,” Top. Catal., vol. 61, no. 9–11, pp. 1101–1124, 2018. DOI: https://doi.org/10.1007/s11244-018-0924-3.
L. Lu et al., “Few-layer Bismuthene: Sonochemical Exfoliation, Nonlinear Optics and Applications for Ultrafast Photonics with Enhanced Stability”, Laser Photon. Rev., vol. 12, no. 1, p. 1700221, 2018. DOI: https://doi.org/10.1002/lpor.201700221.
M. Luo et al., “M 2 B 10 O 14 F 6 (M = Ca, Sr): Two Noncentrosymmetric Alkaline Earth Fluorooxoborates as Promising Next-Generation Deep-Ultraviolet Nonlinear Optical Materials”, J. Am. Chem. Soc., vol. 140, no. 11, pp. 3884–3887, 2018. DOI: https://doi.org/10.1021/jacs.8b01263.
A. N. Castro et al., “Theoretical study on the third-order nonlinear optical properties and structural characterization of 3-Acetyl-6-Bromocoumarin,” Chem. Phys. Lett., vol. 653, pp. 122–130, 2016. DOI: https://doi.org/10.1016/j.cplett.2016.04.070.
A. N. Castro, F. A. P. Osório, R. R. Ternavisk, H. B. Napolitano, C. Valverde, and B. Baseia, “Theoretical investigations of nonlinear optical properties of two crystalline acetamides structures including polarization effects of their environment”, Chem. Phys. Lett., vol. 681, pp. 110–123, 2017. DOI: https://doi.org/10.1016/j.cplett.2017.05.066.
T. Chandra Shekhara Shetty, S. Raghavendra, C. S. Chidan Kumar, and S. M. Dharmaprakash, “Nonlinear absorption, optical limiting behavior and structural study of a new chalcone derivative-1-(3, 4-dimethylphenyl)-3-[4(methylsulfanyl) phenyl] prop-2-en-1-one”, Opt. Laser Technol., vol. 77, pp. 23–30, 2016. DOI: https://doi.org/10.1016/j.optlastec.2015.08.015.
S. Bag et al., “Design, synthesis and biological activity of multifunctional α,β-unsaturated carbonyl scaffolds for Alzheimer’s disease”, Bioorg. Med. Chem. Lett., vol. 23, no. 9, pp. 2614–2618, 2013. DOI: https://doi.org/10.1016/j.bmcl.2013.02.103.
Y. Wang, S. Xue, R. Li, Z. Zheng, H. Yi, and Z. Li, “Synthesis and biological evaluation of novel synthetic chalcone derivatives as anti-tumor agents targeting Cat L and Cat K”, Bioorg. Med. Chem., vol. 26, no. 1, pp. 8–16, 2018. DOI: https://doi.org/10.1016/j.bmc.2017.09.019.
J. Li et al., “Design, synthesis, biological evaluation, and molecular docking of chalcone derivatives as anti-inflammatory agents”, Bioorg. Med. Chem. Lett., vol. 27, no. 3, pp. 602–606, 2017. DOI: https://doi.org/10.1016/j.bmcl.2016.12.008.
D. Choi et al., “In Vitro Osteogenic Differentiation and Antibacterial Potentials of Chalcone Derivatives”, Mol. Pharm., vol. 15, no. 8, pp. 3197–3204, 2018. DOI: https://doi.org/10.1021/acs.molpharmaceut.8b00288.
L. Illicachi et al., “Synthesis and DFT Calculations of Novel Vanillin-Chalcones and Their 3-Aryl-5-(4-(2-(dimethylamino)-ethoxy)-3-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde Derivatives as Antifungal Agents”, Molecules, vol. 22, no. 9, p. 1476, 2017. DOI: https://doi.org/10.3390/molecules22091476.
L. M. G. Abegão et al., “Second- and third-order nonlinear optical properties of unsubstituted and mono-substituted chalcones”, Chem. Phys. Lett., vol. 648, pp. 91–96, 2016. DOI: https://doi.org/10.1016/j.cplett.2016.02.009.
F. Toda, K. Tanaka, and M. Kato, “Stereoselective photodimerisation of chalcones in the molten state”, J. Chem. Soc. Perkin Trans. 1, no. 7, pp. 1315–1318, 1998. DOI: https://doi.org/10.1039/a707380a.
L.-H. Jing, “( E )-1-(4-Nitrophenyl)-3-phenylprop-2-en-1-one”, Acta Crystallogr. Sect. E Struct. Reports Online, vol. 65, no. 10, pp. o2510–o2510, 2009. DOI: https://doi.org/10.1107/S1600536809037556.
C. Laane, S. Boeren, K. Vos, and C. Veeger, “Rules for optimization of biocatalysis in organic solvents”, Biotechnol. Bioeng., vol. 30, no. 1, pp. 81–87, 1987. DOI: https://doi.org/10.1002/bit.260300112.
W. J. Geary, “The use of conductivity measurements in organic solvents for the characterisation of coordination compounds”, Coord. Chem. Rev., vol. 7, no. 1, pp. 81–122, 1971. DOI: https://doi.org/10.1016/S0010-8545(00)80009-0.
C. Valverde, Í. N. Ribeiro, J. V. B. Soares, B. Baseia, and F. A. P. Osório, “Prediction of the Linear and Nonlinear Optical Properties of a Schiff Base Derivatives via DFT”, Adv. Condens. Matter Phys., vol. 2019, pp. 1–12, 2019. DOI: https://doi.org/10.1155/2019/8148392.
S. Backus et al., “16-fs, 1-μJ ultraviolet pulses generated by third-harmonic conversion in air”, Opt. Lett., vol. 21, no. 9, p. 665, 1996. DOI: https://doi.org/10.1364/OL.21.000665.
J. M. F. Custodio et al., “Chalcone as Potential Nonlinear Optical Material: A Combined Theoretical, Structural, and Spectroscopic Study”, J. Phys. Chem. C, vol. 123, no. 10, pp. 5931–5941, 2019. DOI: https://doi.org/10.1021/acs.jpcc.9b01063.
C. Reichardt, Solvents and Related Titles from WILEY-VCH Organic Synthesis Workbook II Chemical Synthesis Using Supercritical Fluids. 2003.
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Hayam A. Abd El Salam, Gehad G. Mohamed, Ehab M. Zayed. (2023). Synthesis, spectroscopic characterization, biological application and molecular docking studies of some transition metal complexes of isophthalamide ligand. Journal of Molecular Structure, 1273, p.134231. https://doi.org/10.1016/j.molstruc.2022.134231.
2. Faiza Lehraki, Nadjib Melkemi. (2021). Solvent effect on the Molecular structure and Global, Local and Dual Descriptors: A Density Functional Theory Study. Asian Journal of Research in Chemistry, , p.305. https://doi.org/10.52711/0974-4150.2021.00052.
3. Qin Ai Wong, Ching Kheng Quah, Xin Ai Wong, Yip-Foo Win, Huey Chong Kwong, Parutagouda Shankaragouda Patil, Neelamma B. Gummagol, Venugopal Rao S.. (2022). A comprehensive study on the photophysical and non-linear optical properties of thienyl-chalcone derivatives. Physical Chemistry Chemical Physics, 24(36), p.21927. https://doi.org/10.1039/D2CP02127G.
4. Ehab M. Zayed, Gehad G. Mohamed, Hayam A. Abd El Salam. (2023). Ni(II), Co(II), Fe(III), and Zn(II) mixed ligand complexes of indoline-dione and naphthalene-dione: Synthesis, characterization, thermal, antimicrobial, and molecular modeling studies. Inorganic Chemistry Communications, 147, p.110276. https://doi.org/10.1016/j.inoche.2022.110276.
5. Dhanya P.K., Renjith Raveendran Pillai, Jamelah S. Al-Otaibi, Y. Sheena Mary. (2024). DFT insights into the nonlinear optical properties of azulene-stilbene dyads for photonics and optoelectronics. Journal of Molecular Liquids, 414, p.126053. https://doi.org/10.1016/j.molliq.2024.126053.
6. Ehab M. Zayed, Hayam A. Abd ElSallam, Maha I. Fathala, Farid M. Sroor. (2023). Naphthalene‐1,2‐dione and indoline‐2,3‐dione as Convenient Ligands to Prepare Mixed Ligand Complexes of Mn, Cu And Cd: Synthesis, Spectroscopic Characterization, DFT Study and Antibacterial Activity. ChemistrySelect, 8(15) https://doi.org/10.1002/slct.202300145.
7. Dhanya P. K., Prakash Chandran R., Sobha Vijayan Nair, Renjith Raveendran Pillai. (2024). Non-linear optical properties of 2,7-naphthyridine derivatives for optical switching applications: a DFT study. New Journal of Chemistry, 48(6), p.2689. https://doi.org/10.1039/D3NJ04885C.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2020 Revista Colombiana de Química

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons (CC. Atribución 4.0) que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).