Structural, spectroscopic, and theoretical analysis of a molecular system based on 2-((2-(4-chlorophenylhydrazone)methyl)quinolone
Análisis estructural, espectroscópico y teórico de un sistema molecular basado en la 2-((2-(4-clorofenilhidrazono)metil)quinolina
Análise estrutural, espectroscópico, e teórico de um sistema molecular com base na 2-((2-(4-Clorofenilhidrazona)metil)quinolina
DOI:
https://doi.org/10.15446/rev.colomb.quim.v47n2.67115Keywords:
Hydrazone derivatives, configurational dynamic, single XRD, DFT calculations, electrochemistry (en)derivados hidrazónicos, dinámica configuracional, cristalografía de rayos X, cálculos DFT, electroquímica (es)
derivados de hidrazonas, Dinâmica configuracional, Cristalografia de Raios-X, Cálculos DFT, Eletroquímica (pt)
A novel molecular system based on 2-((2-(4-chlorophenylhydrazone)methyl)quinoline (1-E) was synthesized. Interconversion of 1-E to its configurational isomer 1-Z was achieved using UV radiation (250 W Hg lamp). Such isomerization was monitored by 1H-NMR. The results suggest that the hydrazone derivative can act as a chemical brake in solution. This molecular system was structurally (Single Crystal X-Ray diffraction and DFT calculations) and spectroscopically (NMR, UV, and IR) characterized. Electrochemical measurements showed that configurational changes induce differential redox behavior. In this regard, the reported quinoline system exhibits different dynamic absorption and electrochemical properties that are modulated by the UV-light induced configurational change. Therefore, 1-E can be regarded as potential photo-electrochemical switch.
Se sintetizó un nuevo sistema molecular basado en 2-((2-(4-chlorofenilhidrazona)metil)quinolina. Del mismo modo, se evaluó la respuesta dinámica de este compuesto a radiación ultravioleta y formación de un enlace de hidrógeno intramolecular. Los resultados muestran que este derivado de hidrazona puede actuar como freno en solución. El sistema en mención es descrito estructural (Cristalografía de Rayos X y cálculos DFT) y espectroscópicamente (RMN, UV e IR). La interconversión de este sistema entre las configuraciones 1-E y 1-Z fue mediada por radiación UV y monitoreada a través de RMN-1H. El estudio electroquímico mostró un comportamiento diferencial en función de su configuración, aspecto fundamental en el desarrollo de sistemas foto- y electroquímicamente modulados.
Neste trabalho é apresentado um novo sistema molecular baseado na 2-((2-(4-clorofenilhidrazona)metil)quinolina, capaz de responder dinamicamente à radiação ultravioleta formando uma ligação de hidrogénio intramolecular que atua como um freio na solução. Este sistema é descrito estruturalmente (cristalografia de raios-X e DFT) e por diferentes técnicas espectroscópicas (RMN, de UV e de IV). Radiação UV foi usada para fazer a interconversão da hidrazona 1-E no seu isômero configuracional 1-Z. Este processo foi monitorado pelo RMN. As medidas eletroquímicas mostraram que as mudanças configuracionais entre os isômeros induzem a comportamentos redox diferentes, o que é uma caraterística chave no desenvolvimento de interruptores fotoelectroquímicos.

Fisicoquímica e Inorgánica
Structural, spectroscopic, and theoretical analysis of a molecular system based on 2-((2-(4-chlorophenylhydrazone)methyl)quinolone
Análisis estructural, espectroscópico y teórico de un sistema molecular basado en la 2-((2-(4-clorofenilhidrazono)metil)quinolina
Análise estrutural, espectroscópico, e teórico de um sistema molecular com base na 2-((2-(4-Clorofenilhidrazona)metil)quinolina
Structural, spectroscopic, and theoretical analysis of a molecular system based on 2-((2-(4-chlorophenylhydrazone)methyl)quinolone
Revista Colombiana de Química, vol. 47, no. 2, 2018
Universidad Nacional de Colombia
Abstract: A novel molecular system based on 2-((2-(4-chlorophenylhydrazone)methyl)quinoline (1-E ) was synthesized. Interconversion of 1-E to its configurational isomer 1-Z was achieved using UV radiation (250 W Hg lamp). Such isomerization was monitored by 1H-NMR. The results suggest that the hydrazone derivative can act as a chemical brake in solution. This molecular system was structurally (Single Crystal X-Ray diffraction and DFT calculations) and spectroscopically (NMR, UV, and IR) characterized. Electrochemical measurements showed that configurational changes induce differential redox behavior. In this regard, the reported quinoline system exhibits different dynamic absorption and electrochemical properties that are modulated by the UV-light induced configurational change. Therefore, 1-E can be regarded as potential photo-electrochemical switch.
Keywords: Hydrazone derivatives, configurational dynamic, single XRD, DFT calculations, electrochemistry.
Resumen: Se sintetizó un nuevo sistema molecular basado en 2-((2-(4-chlorofenilhidrazona)metil)quinolina. Del mismo modo, se evaluó la respuesta dinámica de este compuesto a radiación ultravioleta y formación de un enlace de hidrógeno intramolecular. Los resultados muestran que este derivado de hidrazona puede actuar como freno en solución. El sistema en mención es descrito estructural (Cristalografía de Rayos X y cálculos DFT) y espectroscópicamente (RMN, UV e IR). La interconversión de este sistema entre las configuraciones 1-E y 1-Z fue mediada por radiación UV y monitoreada a través de RMN-1H. El estudio electroquímico mostró un comportamiento diferencial en función de su configuración, aspecto fundamental en el desarrollo de sistemas foto- y electroquímicamente modulados.
Palabras clave: derivados hidrazónicos, dinámica configuracional, cristalografía de rayos X, cálculos DFT, electroquímica.
Resumo: Neste trabalho é apresentado um novo sistema molecular baseado na 2-((2-(4-clorofenilhidrazona)metil)quinolina, capaz de responder dinamicamente à radiação ultravioleta formando uma ligação de hidrogénio intramolecular que atua como um freio na solução. Este sistema é descrito estruturalmente (cristalografia de raios-X e DFT) e por diferentes técnicas espectroscópicas (RMN, de UV e de IV). Radiação UV foi usada para fazer a interconversão da hidrazona 1-E no seu isômero configuracional 1-Z . Este processo foi monitorado pelo RMN. As medidas eletroquímicas mostraram que as mudanças configuracionais entre os isômeros induzem a comportamentos redox diferentes, o que é uma caraterística chave no desenvolvimento de interruptores fotoelectroquímicos.
Palavras-chave: derivados de hidrazonas, Dinâmica configuracional, Cristalografia de Raios-X, Cálculos DFT, Eletroquímica.
Introduction
The synthesis, characterization, and identification of molecular systems potentially useful in the design of molecular machines is still a huge challenge for supramolecular chemistry researchers ( 1 , 2 ). In this regard, some organic compounds with double bonds (C=C, C=N, or N=N) are of special interest due to their reversible photochemical and thermal reactions. Such feature makes these compounds potentially useful for the synthesis of molecular machines ( 3 , 4 ). It is well known that hydrazone derivatives are one of the most used compounds as building blocks for the formation of supramolecular systems because of the physical and chemical properties that they exhibit. These properties can be reversibly modulated in response to an external stimulus, either light ( 5 – 9 ), addition of metal ions ( 10 – 14 ), and pH changes ( 15 ). Hydrazone derivatives based on 2-quinoline are particularly interesting molecules for the development of photo-activated switches as, upon UV-light irradiation, the quinoline-nitrogen may form an intramolecular hydrogen bond with the N-H proton from a hydrazine moiety, thus stabilizing the Z isomer making it even possible to isolate it and study its properties. Additionally, the E-isomer can act as a ligand similar to terpydine for metal-ion coordination ( 16 , 17 ).
Likewise, the study of electronic and electrochemical properties of configurational isomers has not been well documented, at least for hydrazone derivatives, where there are only a few recent reports ( 7 , 18 ). Electrochemistry of E/Z configurational isomers is important to demonstrate their potential use as molecular photoswitches and electronic devices. Therefore, to design molecular systems able to reversibly modulate the physical and chemical properties in response to light or metal centers, we have decided to study and compare through theoretical studies the electronic and electrochemical properties of the configurational isomers of 2-((2-(4-chlorophenylhydrazono)methyl)-quinoline.
Materials and methods
All starting reagents were acquired from Sigma-Aldrich (USA) and were used without additional purification. FT-IR, NMR (mono and bi-dimensional), and UV-vis spectra were taken in a Shimadzu FTIR-8400S instrument (Japan), in a 400 MHz Bruker Ultrashield spectrometer (USA), and in a PharmaSpec Shimadzu UV-Vis UV-1700 spectrophotometer (Japan), respectively. Electrochemical measurements were recorded in a bipotentiostat model 700B (USA) series electrochemical Analyzer/Workstation from CH Instruments coupled to a computer.
Synthesis of hydrazone derivatives
The hydrazone derivative (E)-2-((2-(4-Chlorophenylhydrazono)methyl)quinoline 1-E was synthesized following the same synthetic protocol as reported in the literature ( 7 ). A mixture of 2-methylquinoline (200 mg, 1.40 mmol) and selenium dioxide (263.5 mg, 2.37 mmol) was dissolved in dry dioxane (20 mL). Then, the reaction mixture was heated under reflux and monitored by thin-layer chromatography (TLC). After disappearance of the starting material, a solid was obtained which was filtered and washed with chloroform (3 x 5 mL). The 2-quinolinecarboxaldehyde was purified by column chromatography with a 95:5 CHCl3/MeOH solution getting an 80% yield. The aldehyde derivative was condensed with 4-chloropehnylhydrazine which was added in a 1:1 ratio in absolute ethanol and heated for 3 h until a red precipitate was formed. This precipitate was washed with cold ethanol and recrystallized from ethanol in 82% yield. Mp 206-208 °C. 205-207 (19) 1H NMR (400 MHz, DMSO-d6 ) δ 7.34-7.41 (m, 4H), 7.75 (t, 1 H, J 7.61), 7.91-8.02 (m, 1 H), 8.16 (d, 1 H, J 8.00), 8.25 (d, 1 H, J 8.39), 8.33-8.44 (m, 2 H), 8.72 (d, 1 H, J 8.98), 12.43 (s, 1 H); 13C NMR (100 MHz, DMSO-d6 ) δ 115.8. 118.4, 122.6, 125.9, 126.6, 127.4, 128.4, 128.8, 129.1, 129.7, 133.2, 142.03, 142.8, 152.5.
(Z)-2-((2-(4-chlorophenylhydrazono)methyl)quinoline (1-Z): A solution of 1-E in methanol was irradiated with a 250 Watts mercury lamp for 30 min. This compound was purified by column chromatography using CHCl3 as eluent obtaining a yellow oil; yield 65%; 1H NMR (400 MHz, DMSO-d6 ) d 7.22-7.35 (m, 4H), 7.58-7.64 (m, 1H), 7.80 (t, 1H, J 7.7 Hz), 7.99-8.05 (m, 2H), 8.11-8.21 (m, 2H), 8.44 (d, 1H, J 8.98 Hz), 11.62 (s, 1H).
Single-Crystal structure determination
The data were collected on a Bruker APEX-II CCD diffractometer using MoKα radiation (0.71073 Å) monochromated by graphite at room temperature (296 K). The cell determination and the final cell parameters were acquired on all reflections using the Bruker SAINT software ( 20 ), included in APEX2 software suite. Data integration and scaled was carried out using the Bruker SAINT software ( 20 ).
The structure was solved using the SHELXS-2013 software and refined using SHELXL-2013 ( 21 ), contained in WinGX ( 22 ), and Olex2-1.2 ( 23 ). Non-hydrogen atoms of the molecules were clearly resolved and full-matrix least-squares refinements of these atoms with anisotropic thermal parameters were performed. All hydrogen atoms were stereochemically positioned and refined with the riding model ( 23 ), except for the hydrogen atom bonded to Nitrogen atom, which was found from the density map. ORTEP diagram was generated with Olex2 ( 23 ). Mercury software ( 24 ) was used to prepare the illustrations.
Electrochemical study of hydrazone derivatives
Cyclic voltammetry (CV) and Osteryoung Square Wave Voltammetry (OSWV) were performed using a 0.1 M solution of tetrabutylammonium hexafluorophosphate (TBAPF6) in DMF. A glassy carbon electrode, a platinum wire, and a silver wire were used as working, counter, and pseudo-reference electrode, respectively. The solutions were degassed with argon before each measurement (10 min). Ferrocene was added as an internal standard. The scan rate was 100 mVs-1.
Computational details
Theoretical calculations were performed using GaussView as graphic interface ( 24 ) and Gaussian 09 ( 26 ) for running calculations. Molecular geometry of 1-E in the ground state was optimized by DFT:B3LYP method with 6-311+G (d, p) basis set. NMR chemical shifts were computed at the same level of theory, using the gauge-including atomic orbital (GIAO) method without any solvent or solvation effect considerations. Vibrational frequencies were performed at the same level than vibrational analysis and make sure that global minima were achieved. Hartree-Fock/6-31+G (d) level of theory and Self-Consistent Isodensity Polarized Continuum Model (SCI-PCM) were employed to simulate solvation effect in optimizations, while UV-Vis spectrum calculations were carried out using same method and level of theory without solvation. For UV-Vis spectrum deconvolution, theoretical IR, and UV spectra graph generation GaussSum 3.0 software package was employed ( 27 ).
Results and discussion
Synthesis and characterization of hydrazone derivatives
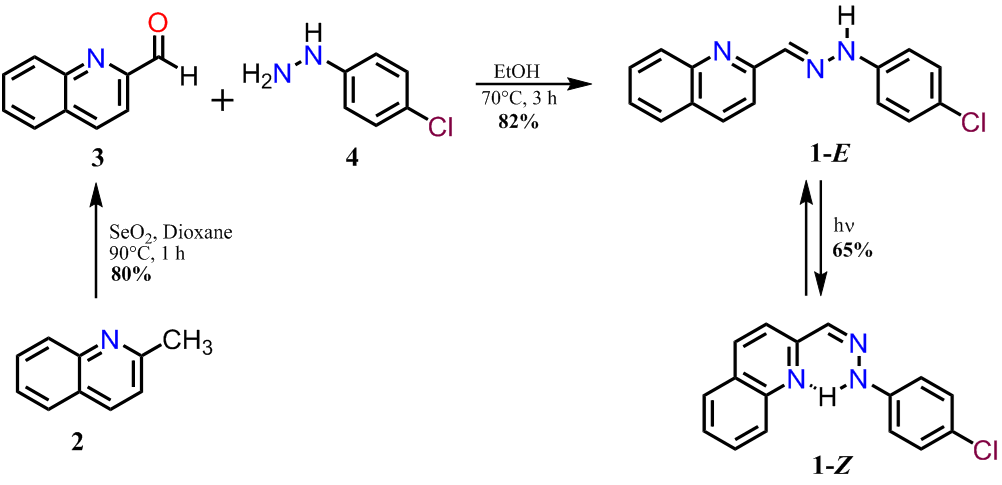
Details of the synthesis of 1-E and 1-Z are shown in Figure 1. Compound 1-E was synthesized by the partial oxidation of 2 with selenium dioxide in dioxane, followed by condensation of 3 with the hydrazine derivative 4 in ethanol ( 7 ). The condensation reaction led to the formation of the corresponding E-hydrazone, which was identified by the singlet at 12.43 ppm corresponding to the NH proton ( 3 ). The geometry optimization for 1 was also performed to compare the energy associated to each isomer, as it was expected the E isomer is 12.2 kJ/mol more stable than the Z isomer. The irradiation of 1-E with a 250 Watts mercury lamp produced the Z isomer, which was isolated by column chromatography in a 67% yield.
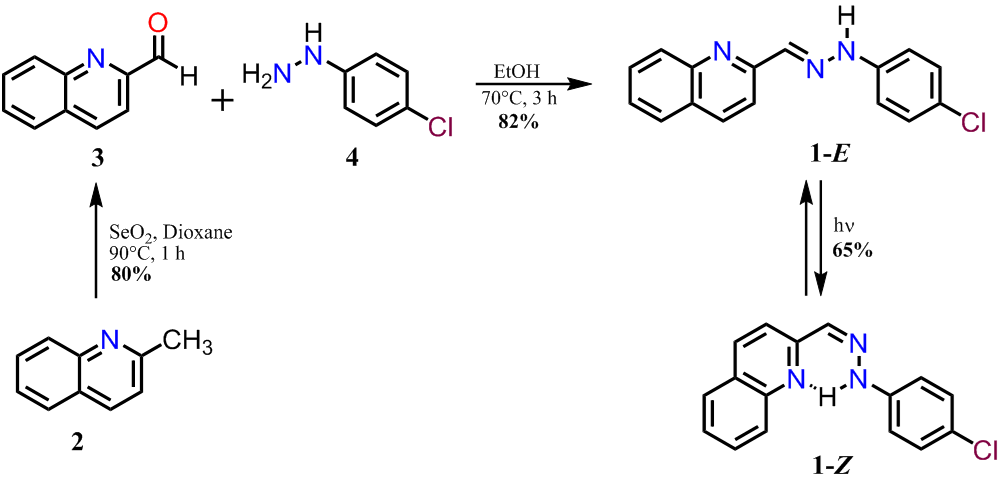
Figure 1. Synthetic route to obtain the hydrazone derivative 1.
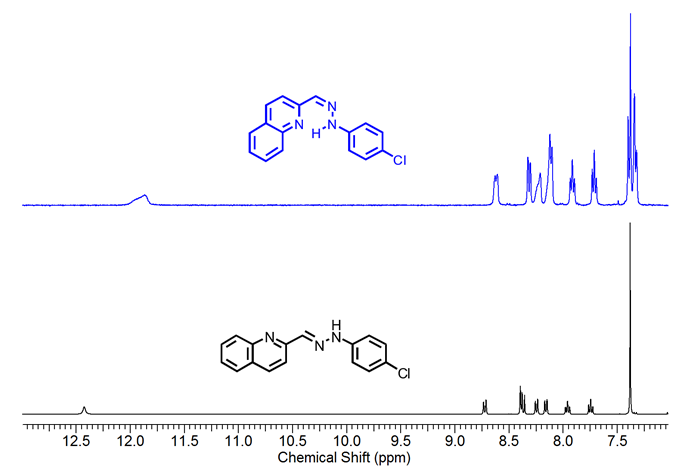
For 1-Z , the 1H-NMR spectra show the existence of an intramolecular H-bond after irradiation with UV light since all the signals changed their chemical shift. The lack of symmetry in the molecule is reflected by the different coupling between the signals and the shift of the N-H proton in 1-Z when compared to 1-E (See Figure 2). The most remarkable change occurs on the NH and phenyl protons, which are shifted to the up-field region and splitted as a multiplet, respectively.
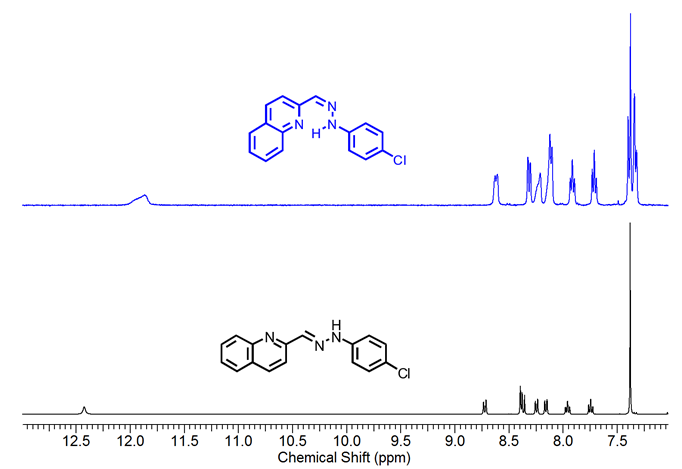
Figure 2.1H NMR spectra (400 MHz) of compound 1-E and 1-Z in DMSO-d6 .
Single-crystal structure determination
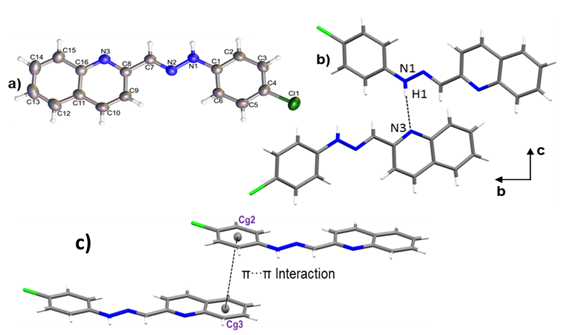
Crystals of 1-E were obtained by slow evaporation of ethanol, resulting in fine, orange needlelike crystals. 1-E crystalized in the monoclinic space group P21/c. The molecule presents an almost planar configuration with a torsion angle between C6–C1–N1– N2 of 5.89(1)° and a dihedral angle between the quinoline and benzene planes of 1.91°. The Mogul geometry check shows that all bond lengths and angles are within the normal range ( 28 ). ORTEP representation of the 1-E structure is presented in the Figure 3a; Crystal data collection and structure refinement details are summarized in Table 1.
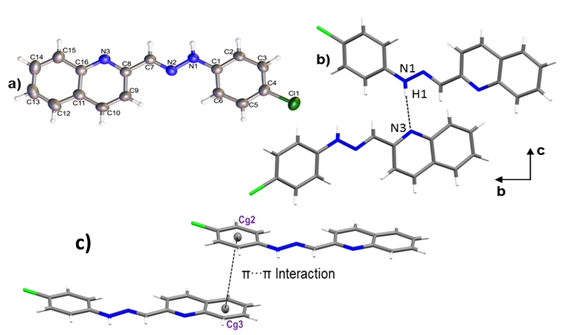
Figure 3. a) ORTEP representation of title compound, b) Hydrogen bond N1–H1···N3 and c) a) π···π interaction between Cg2 and Cg3.
| Identification Code | 1-E |
| Empirical formula | C16H11ClN3 |
| Formula weight | 280.73 |
| Temperature/K | 296(2) K |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a/Å | 8.2023(3) |
| b/Å | 7.1828(2) |
| c/Å | 23.2593(8) |
| β(°) | 90.5180(10) |
| Volume/ Å3 | 1370.28(8) |
| Z | 4 |
| ρcalc mg/ mm3 | 1.361 |
| μ/mm−1 | 0.271 |
| F(000) | 580 |
| Crystal size/ mm3 | 0.522 x 0.178 x 0.171 |
| 2θ range for data collection/° | 1.751 to 26.412 |
| Index ranges | -10≤h≤10, -8≤k≤8, -29≤l≤29 |
| Reflections collected | 38252 |
| Independent reflections | 2810[R(int) = 0.0241] |
| Data / restraints / parameters | 2810 / 0 / 185 |
| Goodness-of-fit on F2 | 1.050 |
| Final R indices [I>2sigma(I)] | R1 = 0.0371, wR2 = 0.0999 |
| R indices (all data) | R1 = 0.0442, wR2 = 0.1067 |
| Largest diff. Peak/hole/ e.Å-3 | 0.204 and -0.209 |
The crystal packing is driven by N1–H1···N3 hydrogen bond, along [010] direction with 3.0855(1) Å of distance. As shown in Figure 3b, the interactions give rise to zig-zag chains growing along the [010] direction and the connected molecules are placed on the two planes (931) and (12-41). A 3D supramolecular network is formed by the joining of the chains along [100] direction by π-π interactions between the aromatic rings with an intercentroids distance of Cg2···Cg3= 3.8903(1) Å (Figure 3 c). It was also observed a C9–H9 ···Cl1 interaction ( 29 ) along [001] direction with a distance of 3.7168(15) Å.
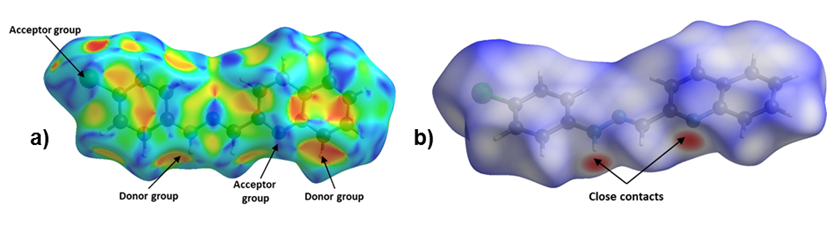
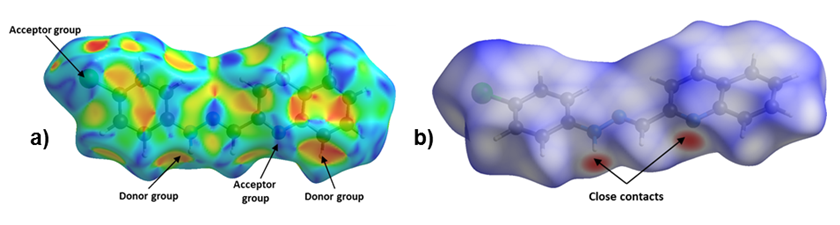
Figure 4. Hirshfeld Surface of title compound. Left: a) Shape Index and Right: b) dnorm.
The Hirshfeld surface for 1-E showed that the chloride and the deprotonated nitrogen atoms in the quinoline and hydrazone present mainly, a hydrogen acceptor behavior, while the N–H and C–H act as donor groups (see Figure 4). Furthermore, an observation over the aromatic rings in the shape index surface blue and red regions that indicates the presence of π-π interactions, is confirmed by the fingerprint plot. The greatest contribution comes from Van der Waals interactions (H···H and C···H) followed by hydrogen bonds as C l ···H and N···H (see Figure 4b), that contribute with 15.6% and 7.9%, respectively. The π-π interactions contribute with 10.9% . Other contacts were also observed, however, their contribution is less than 5% each.
Molecular geometry optimization
Ab initio calculations yielded a planar structure with a dihedral angle equals to 0.001º between the quinoline and benzene planes and º torsion angles between the quinoline or benzene rings and the hydrazine group. As shown in Figure 5, these dihedral and torsional angles are not significantly deviated concerning to the results discussed in the crystallographic section.
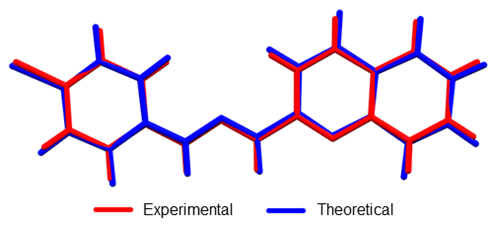
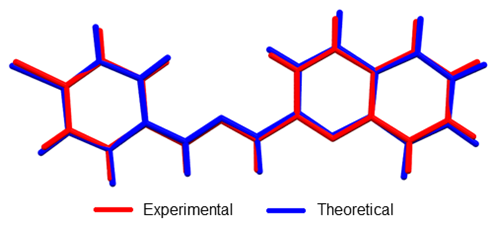
Figure 5. Overlap of crystallographic and optimized (DFT: B3LYP/6-311+G (d, p) structures of 1-E . RMSD was 0.0985.
Linear regression analysis was also carried out to establish a systematic comparison between the optimized and crystallographic structures. The slope represents a ratio between the set of variations of calculated-experimental data. The intercept corresponds to the value of the dependent variable (calculated) when the independent variable (experimental) is equal to zero: meaning an over or underestimation value. Thus, perfect correlation is given when the slope and intercept values are 1 and 0, respectively (selected structural parameters and linear regression are shown in Table 2). The analysis reveals that theoretical calculations tend to a subtle overestimation respect to the crystallographic data set as shown in the intercept values (see Table 2). The highest deviation from experimental bond length measurements was observed in N1–H bond (17.17% error) since this group is highly influenced by hydrogen bond interaction (See Figure 3). However, other bond angles and length show less than 1.4% and 0.9% bond length and angle errors respectively.
| Bond | Experimental length (Å) | Calculated length (Å) | Error (%) | |
| C1-C2 | 1,388 | 1.4020 | 1.01 | |
| C2-C3 | 1.377 | 1.3897 | 0.92 | |
| C3-C4 | 1.378 | 1.3912 | 0.96 | |
| C4-C1 | 1.742 | 1.7605 | 1.06 | |
| C4-C5 | 1.391 | 1.3914 | 1.12 | |
| C5-C6 | 1.380 | 1.3904 | 0.75 | |
| C6-C1 | 1.391 | 1.4009 | 0.71 | |
| C1-N1 | 1.388 | 1.3938 | 0.42 | |
| N1-H* | 0.867 | 1.0159 | 17.17 | |
| N1-N2 | 1.348 | 1.3388 | 0.68 | |
| N2-C7 | 1.279 | 1.2859 | 0.54 | |
| C7-C8 | 1.456 | 1.4622 | 0.43 | |
| C8-N3 | 1.323 | 1.3240 | 0.08 | |
| C8-C9 | 1.42 | 1.4266 | 0.46 | |
| C9-C10 | 1.352 | 1.3664 | 1.07 | |
| C10-C11 | 1.414 | 1.4202 | 0.44 | |
| C11-C16 | 1.417 | 1.4293 | 0.87 | |
| C11-C12 | 1.410 | 1.4155 | 0.39 | |
| C12-C13 | 1.359 | 1.3763 | 1.27 | |
| C13-C14 | 1.395 | 1.4144 | 1.39 | |
| C14-C15 | 1.366 | 1.3753 | 0.68 | |
| C15-C16 | 1.408 | 1.4186 | 0.75 | |
| C16-N3 | 1.372 | 1.3610 | 0.80 | |
| Linear regression | ||||
| Slope | 1.0300 | |||
| Intercept | 0.0337 | |||
| R2 | 0.99301 | |||
| Bond | Crystallographic angle (º) | Calculated angle (º) | Error (%) | |
| C3-C4-C5 | 120.51 | 120.49 | 0.02 | |
| C5-C4-C1 | 119.62 | 119.79 | 0.14 | |
| C6-C1-N1 | 121.84 | 121.95 | 0.09 | |
| N1-N2-C7 | 117.53 | 118.28 | 0.64 | |
| C7-C8-N3 | 116.41 | 115.38 | 0.89 | |
| C9-C10-C11 | 120.33 | 119.85 | 0.40 | |
| C11-C12-C13 | 120.72 | 120.47 | 0.20 | |
| C14-C15-C16 | 120.46 | 120.44 | 0.02 | |
| C16-N3-C8 | 118.19 | 118.68 | 0.42 | |
| Linear regression | ||||
| Slope | 1.0242 | |||
| Intercept | 2.9178 | |||
| R2 | 0.9223 | |||
UV-Vis Spectroscopy analysis
The electronic transitions of 1-E were studied by UV-Vis. As shown in Figure 6, the hydrazone derivative exhibits a high absorption band around 425 nm, which present a bathochromic shift with the increasing polarity of the solvent. Additionally, a new band around 380 nm corresponding to π-π* transitions related to the imine bond was observed using methanol as solvent ( 6 ). An optimized structure by DFT: B3LYP/6-311+G (d, p) calculation was used as input geometry for a second optimization at HF/6-31+G (d) level of theory including solvent effects through the SCI-PCM approximation to simulate chloroform solvation yielding an equivalent conformation with no differences in the electronic structure. From this optimized structure, TD-SCF method was employed to calculate excited states energy at HF/6-31+G (d) level of theory neglecting solvation. Besides, the theoretical UV-Visible spectrum presents a transition in the visible region and two transitions in the ultraviolet region (absorptions at 427, 319, and 278 nm respectively) with an absolute error percentage respect to experimental observations equal to 6.57, 10.34, and 11.15%, respectively. These results tend to an underestimation in a range between 28 to 33 nm.
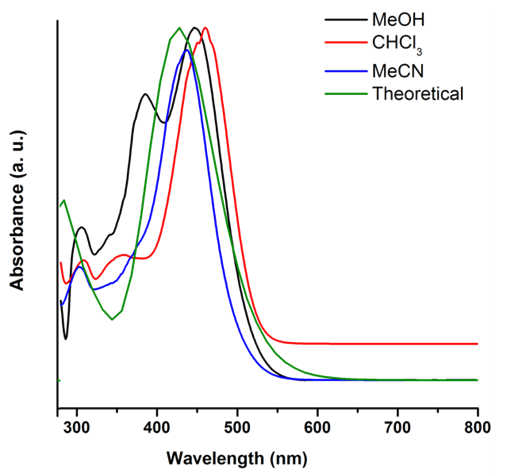
Figure 6. Theoretical and Experimental UV-vis spectra of 1-E (1.0 x 10-5 M) in different solvents.
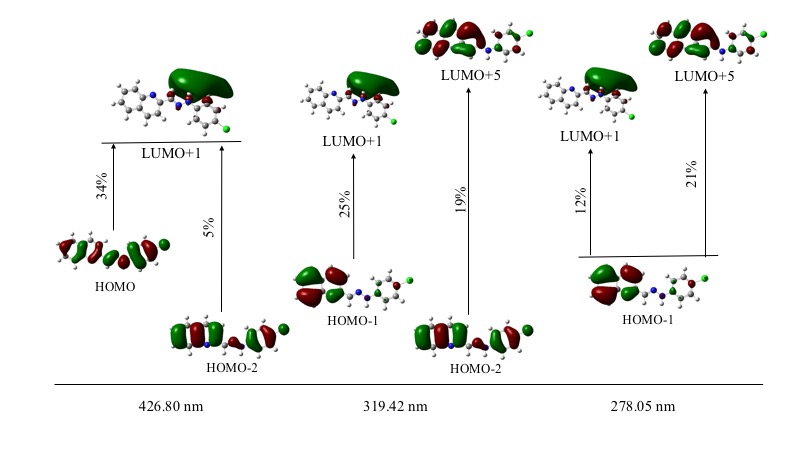
GaussSum 3.0 software package was employed to deconvolute the computed electronic transitions using Gaussian 09 and to determine the contribution of the main electronic transitions (see Figure 7). Higher oscillator strength was observed at 427 nm signal (0.3465). This absorption maximum was characterized by a main electronic transition from HOMO to LUMO+1 energy levels (34%), where a homogeneous electronic distribution is displaced to a MO over the hydrazone and phenyl groups. A second main electronic transition from HOMO-2 to LUMO+1 (5%) was also observed. Second higher oscillator strength was observed at 278 nm (0.1705), where the main electronic transition (HOMO-1®LUMO+5, 21%) is characterized by a partial electronic displacement from the quinoline to hydrazone and chlorophenyl moieties.
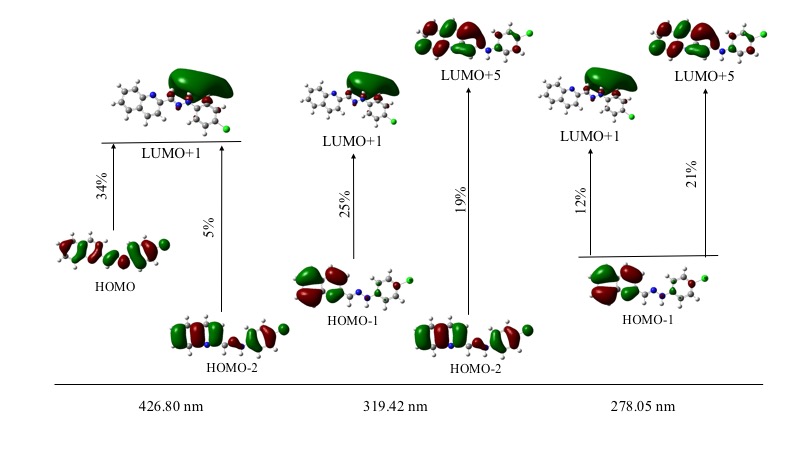
Figure 7. Main electronic transitions in ultraviolet-visible and contribution percentage.
NMR and FT-IR spectroscopy analysis
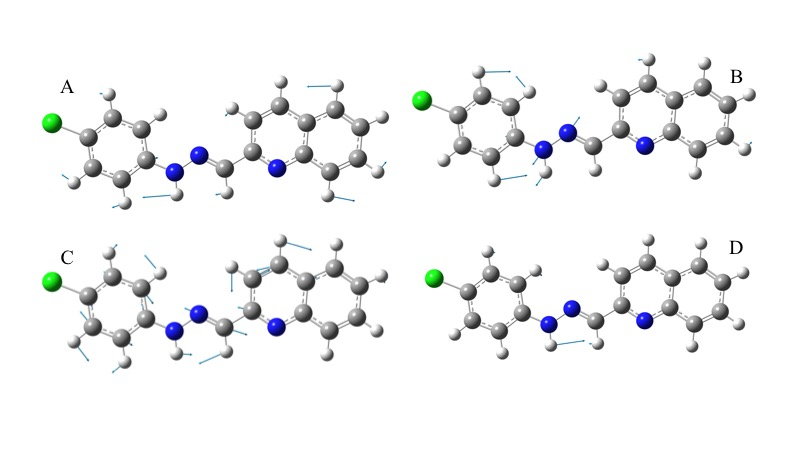
Theoretical-experimental comparison of FT-IR and NMR spectra of 1-E was also carried out. The four most prominent bands are C=N stretching (1579 cm-1), N-H rocking (1508 cm-1), N-N stretching (1142 cm-1), and C-H bending (1241cm-1). Displacement vectors for these vibrations are shown in Figure 8. These vibrational modes are degenerated except N-H bending since displacement vectors clearly showed other vibrational modes coexisting simultaneously in each frequency.
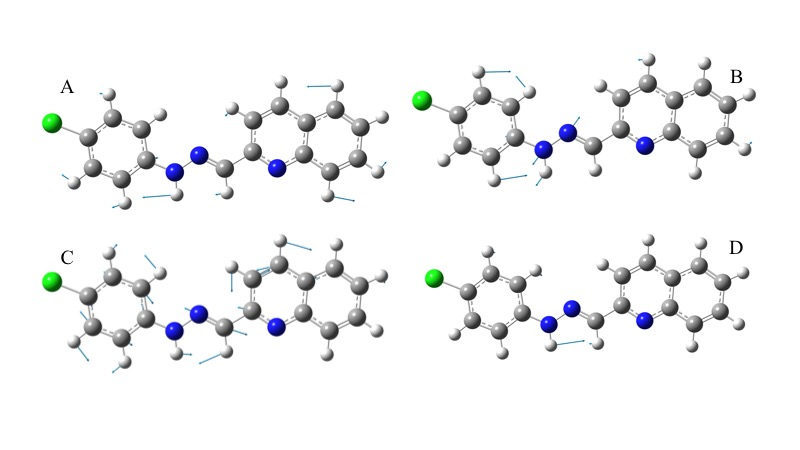
Figure 8. Displacement vectors for selected vibrational modes. A) C-H bending. B) N-N stretching. C) C=N stretching. D) N-H bending.
The absence of imaginary frequencies confirms that the optimized structure is a global minimum. A comparison of selected computed vibrational frequencies and assigned values experimentally is shown in Table 3 from supporting information. Calculated values tend to overestimation even though the scale factor used is less than 1.00 ( 30 ). The maximum error rate was observed in C-Cl frequency (5.45%), C=N and N-H frequencies are the second and third biggest deviation respect to experimental observations. C-Cl and N-H errors may be explained because these moieties are typically involved in hydrogen bonding interactions in the solid state and those were neglected in theoretical calculations.
| Vibrational mode | Theoretical (cm-1) | Scaled value (cm-1) | Experimental frequency (cm-1) | Error rate (%) |
| N-H stretching | 3499 | 3387 | 3209 | 5,26 |
| N=C-H stretching | 3062 | 2964 | 2985 | -0,71 |
| C=N stretching | 1631 | 1579 | 1495 | 5,32 |
| N-N stretching | 1180 | 1142 | 1091 | 4,47 |
| C-Cl stretching | 1100 | 1065 | 1007 | 5,45 |
| C=C stretch. Aryl | 1640 | 1587 | 1587 | 0.02 |
| N-H rocking | 1558 | 1508 | 1555 | -3,12 |
| C-H bending | 1282 | 1241 | 1236 | 0,40 |
The correlation between the computed and experimental 1H-NMR chemical shifts was unacceptable as the signal of N-H, showed the biggest deviation (see Table 4), mainly due to its high influence by solvation ( 31 ). Therefore, the neglected solvation effect should help to improve the quality of 1H-NMR chemical shift calculations. The 13C-NMR chemical shifts showed acceptable correlation respect to experimental values yielding an average rate of error equal to 2.61%.
| 1H-NMR Chemical shift (ppm) | 13C-NMR Chemical shift (ppm) | ||||||
| Experimental | Calculated | Error | Experimental | Calculated | Error | ||
| 7.38 | 7.00 | -5.43 | 115.8 | 113.81 | 1.72 | ||
| 7.38 | 7.73 | 4.53 | 118.4 | 114.54 | 3.26 | ||
| 7.38 | 7.94 | 7.05 | 122.6 | 129.09 | -5.29 | ||
| 7.38 | 8.26 | 10.65 | 125.9 | 129.41 | -2.79 | ||
| 7.75 | 8.10 | 4.32 | 126.6 | 130.46 | -3.05 | ||
| 7.97 | 8.35 | 4.55 | 127.4 | 131.88 | -3.52 | ||
| 8.16 | 8.25 | 1.09 | 128.4 | 132.08 | -2.87 | ||
| 8.25 | 8.55 | 3.51 | 128.8 | 134.87 | -4.71 | ||
| 8.35 | 8.82 | 5.33 | 129.1 | 137.89 | -6.81 | ||
| 8.72 | 8.71 | -0.11 | 129.7 | 136.57 | -5.30 | ||
| 12.43 | 7.47 | -66.40 | 133.2 | 138.54 | -4.01 | ||
| - | - | - | 142.03 | 139.14 | 2.03 | ||
Electrochemical studies
The electrochemical properties of compounds 1-E and 1-Z were studied by cyclic voltammetry (CV) and Osteryoung Square Wave Voltammetry (OSWV) (See Experimental Section).
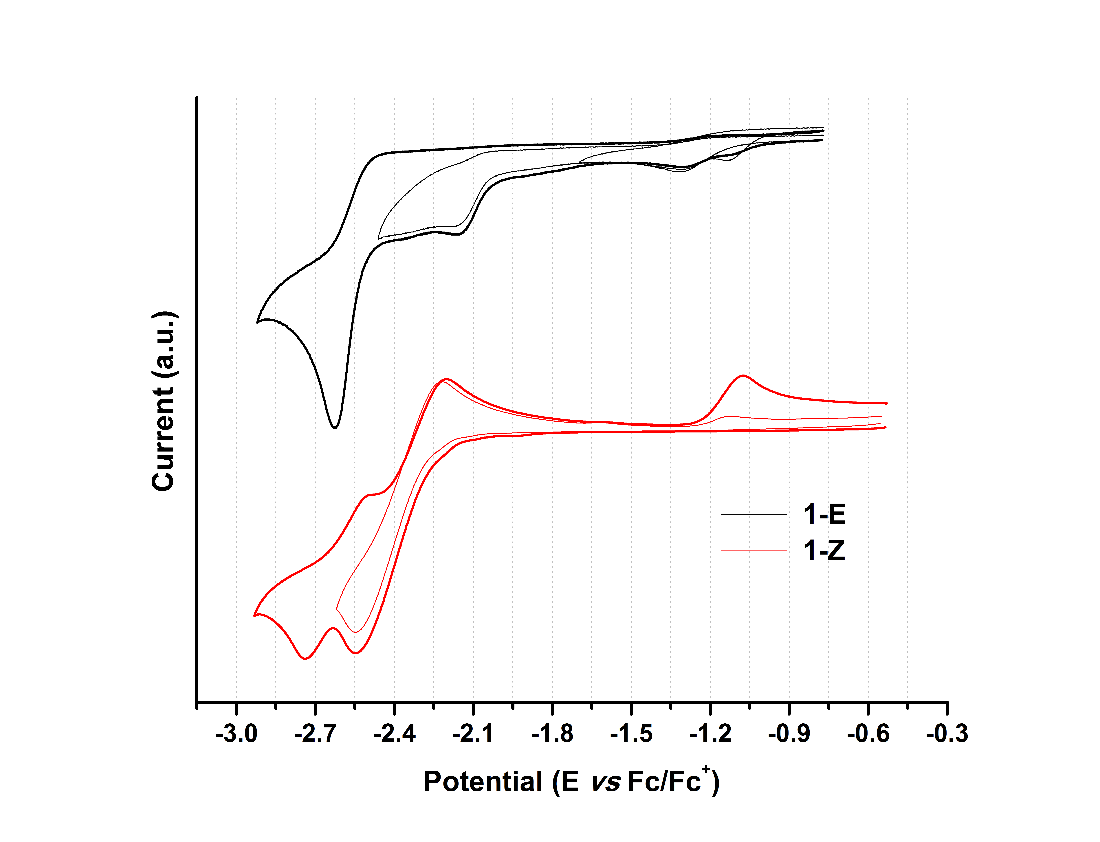
Figure 9. Cyclic voltammogram of hydrazone derivatives at 100 mVs-1. Glassy carbon as working electrode and using ferrocene as an internal reference.
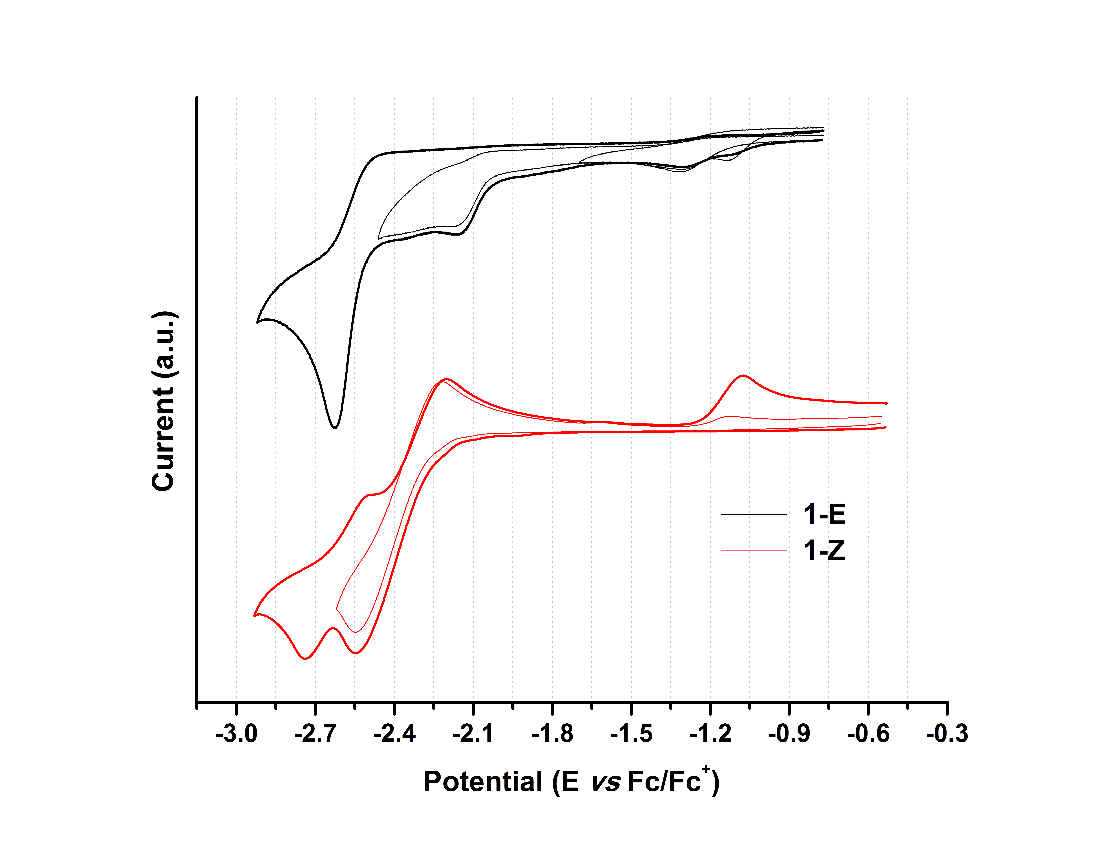
When analyzing the cathodic potentials (see Figure 9), three irreversible reduction potentials for the 1-E isomer are observed. These potentials remained irreversible upon increasing scan rates. OSWV revealed two additional reductions (see Figure 10) which are located very close to those observed in CV. Presumably, the closed peak potentials correspond to the processes occurring on the surface of the electrode and the corresponding cathodically shifted potential to the process occurring in the solution. The first reduction in compound 1-E is located at -1.09 V and corresponds to the imine reduction whereas for 1-Z is cathodically shifted towards -2.54 V (see Table 5). These results indicate that upon reduction the [1-E ]· - radical anion is stabilized by π delocalization in the quinoline system. However, in the Z configuration the nitrogen from the quinoline is acting as a Lewis base (forming the intramolecular hydrogen bond) and, therefore, its participation in the radical anion stabilization is restricted thus shifting cathodically the reduction potential. Likewise, the differences in the current intensity for the cathodic/anodic potentials suggest different reduction mechanism for both configurational isomers.
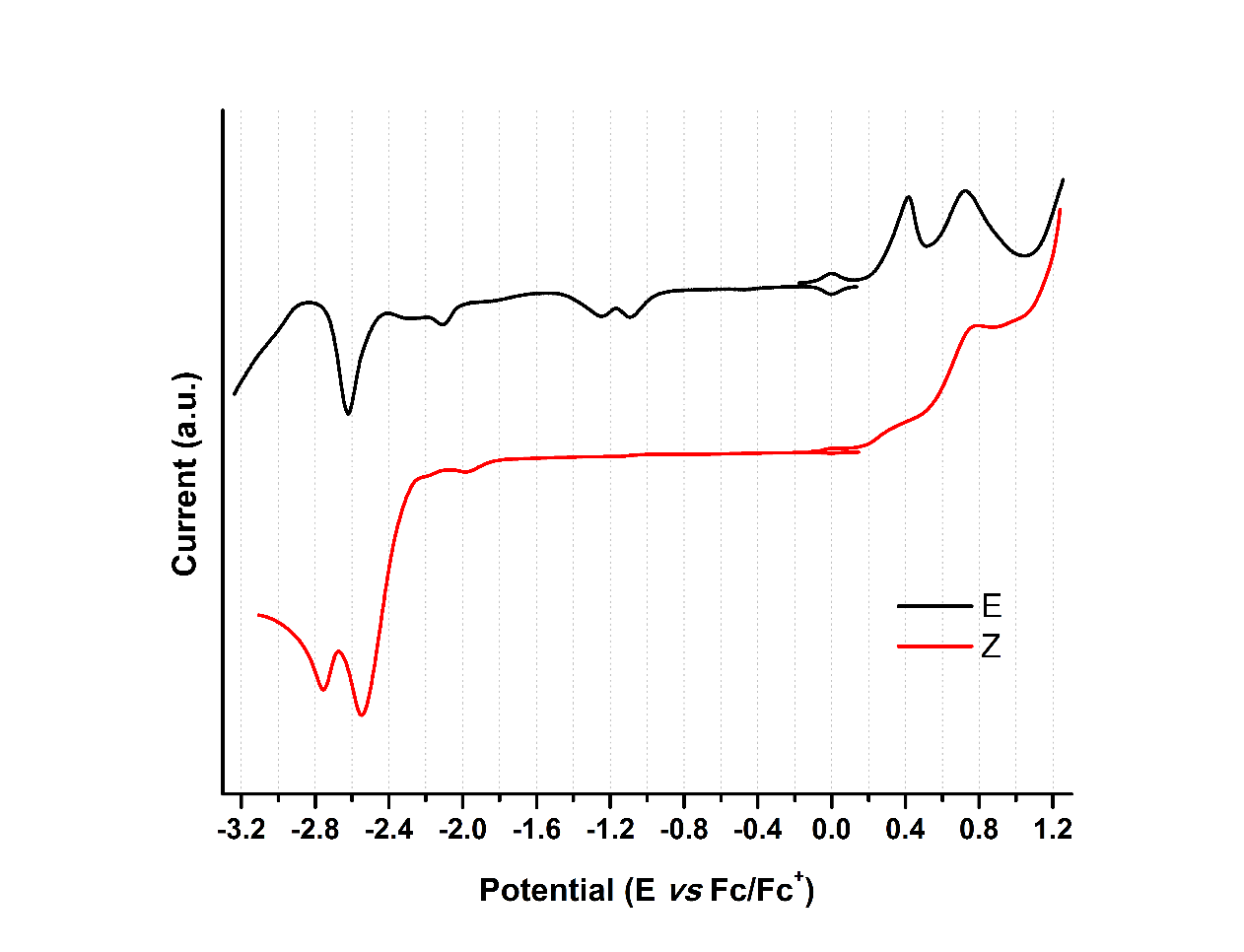
Figure 10. OSW of hydrazone derivatives 1-E and 1-Z at 100 mVs-1. Glassy carbon as working electrode and using ferrocene as an internal reference.
| Compound | Ep,oxd(1) | Ep,oxd(2) | Ep,red(1) | Ep,red(2) | Ep,red(3) | Ep,red(4) | Ep ,red(5) |
| 1-E | 0.42 | 0.73 | -1.09 | -1.24 | -2.10 | -2.28 | -2.62 |
| 1-Z | 0.31 | 0.76 | -2.54 | -2.75 |
Compounds 1-E and 1-Z exhibited two irreversible oxidation potentials which are attributed to the –NH groups of the hydrazone framework. The first reduction potential of 1-E is anodically shifted, when compared to its 1-Z counterpart. This behavior indicates a larger acidity of 1-Z gained by the intramolecular hydrogen bond formation.
Conclusions
The solid-state structural investigation trough single-crystal X-ray diffraction reveals a crystal packaging governed by N1–H1···N3 hydrogen bond, forming inverted dimers along [010] direction. The theoretical calculations carried out at DFT: B3LYP/6-311+G (d, p) level of theory showed good correlation with experimental data, yielding error rates less than 1.4% and 0.9% error for bond length and angle. Also, spectroscopic properties were successfully calculated employing ab initio calculations. The UV-Vis spectrum was deconvoluted giving the main electronic transitions from HOMO to LUMO+1 and HOMO-1 to LUMO+5.
In solution, this novel compound exhibited UV radiation-mediated configurational isomerism conversion from 1-E to 1-Z confirmed by 1H-NMR evidence: chemical shift change and new signals appearance owing to system asymmetry. Finally, modified electrochemical behavior induced by configurational changes was observed, supporting the applicability of these type of molecular systems as an electrochemical template.
Acknowledgements
The authors are grateful to the Vicerrectoria de Investigaciones and Centro de Excelencia en Nuevos Materiales (CENM) from the Universidad del Valle (Colombia), as well as the Departamento Administrativo de Ciencia, Tecnología e Innovación (COLCIENCIAS) for the financial support of this work. M.S.-M. and R. D. acknowledges Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Conselho Nacional de Desenvolvimento Cientfico y Tecnológico for the CNPq and CAPES/PNPD scholarships from Brazilian Ministry of Education, and R. de Almeida Santos to facilitate the measurements and FAPESP (2009/54011-8) for providing Apex-II equipment. R.F.D. acknowledges to the Dirección General de Investigaciones (DGI) of the Universidad Santiago de Cali. G.G. thanks to the Laboratorio de Computación Científica from Facultad de Ciencias Naturales (Icesi University) for the computational resources support as well as Prof. Carlos A. Arango for his invaluable guidance.
References
1. Lehn, J.-M. Perspectives in Chemistry—Aspects of Adaptive Chemistry and Materials. Angew. Chemie Int. Ed. 2015, 54 (11), 3276–3289. DOI https://doi.org/10.1002/anie.201409399.
2. Li, J.; Nowak, P.; Otto, S. Dynamic Combinatorial Libraries: From Exploring Molecular Recognition to Systems Chemistry. J. Am. Chem. Soc. 2013, 135 (25), 9222–9239. DOI: https://doi.org/10.1021/ja402586c.
3. Tatum, L. A.; Su, X.; Aprahamian, I. Simple Hydrazone Building Blocks for Complicated Functional Materials. Acc. Chem. Res. 2014, 47 (7), 2141–2149. DOI: https://doi.org/10.1021/ar500111f.
4. Su, X.; Aprahamian, I. Hydrazone-Based Switches, Metallo-Assemblies and Sensors. Chem. Soc. Rev. 2014, 43 (6), 1963–1981. DOI: https://doi.org/10.1039/C3CS60385G.
5. Lehn, J.-M. Conjecture: Imines as Unidirectional Photodriven Molecular Motors -Motional and Constitutional Dynamic Devices. Chemistry. 2006, 12 (23), 5910–5915. DOI: https://doi.org/10.1002/chem.200600489.
6. Chaur, M. N.; Collado, D.; Lehn, J.-M. Configurational and Constitutional Information Storage: Multiple Dynamics in Systems Based on Pyridyl and Acyl Hydrazones. Chem. A Eur. J. 2011, 17 (1), 248–258. DOI: https://doi.org/10.1002/chem.201002308.
7. Romero, E. L.; D´Vries, R. F.; Zuluaga, F.; Chaur, M. N. Multiple Dynamics of Hydrazone Based Compounds. J. Brazilian Chem. Soc. 2015, 26 (6), 1265–1273. DOI: https://doi.org/10.5935/0103-5053.20150092.
8. Gordillo, M. A.; Soto-Monsalve, M.; Gutiérrez, G.; D’Vries, R. F.; Chaur, M. N. Theoretical and Experimental Comparative Study of a Derivative from 2-Pyridinecarboxaldehyde Which Exhibits Configurational Dynamics. J. Mol. Struct. 2016, 1119. DOI: https://doi.org/10.1016/j.molstruc.2016.04.055.
9. Chaur, M. N. Aroylhydrazones as potential systems for information storage: photoisomerization and metal complexation. Rev. Colomb. Quim. 2012, 41 (3), 349-358. DOI: https://doi.org/10.15446/rev.colomb.quim.v45n3.57351
10. Ulrich, S.; Buhler, E.; Lehn, J.-M. Reversible Constitutional Switching between Macrocycles and Polymers Induced by Shape Change in a Dynamic Covalent System. New J. Chem. 2009, 33 (2), 271–292. https://doi.org/10.1039/B817261G.
11. Carmona-Vargas, C. C.; Váquiro, I. Y.; Jaramillo-Gómez, L. M.; Lehn, J.-M.; Chaur, M. N. Grid-Type Complexes of M2+ (M=Co, Ni, and Zn) with Highly Soluble Bis(hydrazone)thiopyrimidine-Based Ligands: Spectroscopy and Electrochemical Properties. Inorganica Chim. Acta. 2017, In Press. DOI: https://doi.org/10.1016/j.ica.2017.05.002.
12. Su, X.; Robbins, T. F.; Aprahamian, I. Switching through Coordination-Coupled Proton Transfer. Angew. Chemie Int. Ed. 2011, 50 (8), 1841–1844. https://doi.org/10.1002/anie.201006982.
13. Romero, E. L.; Cabrera-Espinoza, A.; Ortiz-Peña, N.; Soto-Monsalve, M.; Zuluaga, F.; D’Vries, R. F.; Chaur, M. N. New Pyrazolino and pyrrolidino[60]fullerenes: The Introduction of the Hydrazone Moiety for the Formation of Metal Complexes. J. Phys. Org. Chem. 2017, 30 (2), e3601–n/a. DOI: https://doi.org/10.1002/poc.3601.
14. Gutiérrez, G; Gordillo, M.A.;Chaur, M. N. A DFT study on Dichloro {(E)-4-dimethylamino-N'-[(pyridin-2-yl) methylideneKN] benzohydrazide-k0}M2+ (M = Zn, Cu, Ni, Fe, Mn, Ca and Co) complexes: Effect of the metal over association energy and complex geometry. Rev. Colomb. Quim. 2016, 45, 3, 28-32. DOI: https://doi.org/10.15446/rev.colomb.quim.v45n3.57351
15. Landge, S. M.; Tkatchouk, E.; Benítez, D.; Lanfranchi, D. A.; Elhabiri, M.; Goddard, W. A.; Aprahamian, I. Isomerization Mechanism in Hydrazone-Based Rotary Switches: Lateral Shift, Rotation, or Tautomerization? J. Am. Chem. Soc. 2011, 133 (25), 9812–9823. DOI: https://doi.org/10.1021/ja200699v.
16. Castro Agudelo, B.; Ochoa-Puentes, C.; Rodríguez-Córdoba, W.; Reiber, A.; Sierra, C. A. Synthesis, characterization, X-ray crystal structure and DFT calculations of 4-([2,2':6',2"-terpyridin]-4'-yl)phenol. Rev. Colomb. Quím. 2018, 47 (1). In press. DOI: https://doi.org/10.15446/rev.colomb.quim.v47n1.66281.
17. Parada, G.; Fernández, D.; Reyes, A.; Suárez, M.F.; Fadini, L. Sinteis y Estudio Teorico de Compuesto de Ru(II) con Ligantes Ferrocennílicos para Aplicaciones Electroquímicas. Rev. Colomb. Quim. 2007, 36 (2), 199-211. DOI: https://doi.org/10.15446/rev.colomb.quim
18. Fernández, M. A.; Barona, J. C.; Polo-Cerón, D.; Chaur, M. N. Photochemical and Electrochemical Studies on Lanthanide Complexes of 6-(Hydroxymethyl)pyridine- 2-carboxaldehyde[2- Methyl-Pyrimidine-4,6-Diyl] Bis-Hydrazone. Rev. Colomb. Quím. 2014, 43 (1), 5–11. DOI: https://doi.org/10.15446/rev.colomb.quim.v43n1.50540.
19. Puskullu, M. O.; Shirinzadeh, H.: Nenni, M.; Gurer-Orhan, H.; Suzen, S. Synthesis and evaluation of antioxidant activity of new quinoline-2-carbaldehyde hydrazone derivatives: bioisosteric melatonin analogues. J. Enzyme Inhib. Med. Chem. 2016, 31(1): 121–125. DOI: https://doi.org/10.3109/14756366.2015.1005012.
20. Bruker-AXS. SAINT. Bruker-Siemens: Madison, Wisconsin,USA 2006.
21. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71 (1), 3–8. DOI: https://doi.org/10.1107/S2053229614024218.
22. Farrugia, L. J. WinGX and ORTEP for Windows : An Update. J. Appl. Crystallogr. 2012, 45 (4), 849–854. DOI: https://doi.org/10.1107/S0021889812029111.
23. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2 : A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42 (2), 339–341. DOI: https://doi.org/10.1107/S0021889808042726.
24. Macrae, C. F.; Bruno, I. J.; Chisholm, J. A.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. A. Mercury CSD 2.0 – New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41 (2), 466–470. DOI: https://doi.org/10.1107/S0021889807067908.
25. Dennington, R.; Keith, T.; Millam, J. GaussView. Semichem Inc.: Shawnee Mission 2009.
26. Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; et al. Gaussian 09. Gaussian, Inc.: Wallingford CT 2009.
27. O’Boyle, N. M.; Tenderholt, A. L.; Langner, K. M. Cclib: A Library for Package-Independent Computational Chemistry Algorithms. J. Comput. Chem. 2008, 29 (5), 839–845. DOI: https://doi.org/10.1002/jcc.20823.
28. Bruno, I. J.; Cole, J. C.; Kessler, M.; Luo, J.; Motherwell, W. D. S.; Purkis, L. H.; Smith, B. R.; Taylor, R.; Cooper, R. I.; Harris, S. E.; et al. Retrieval of Crystallographically-Derived Molecular Geometry Information. J. Chem. Inf. Comput. Sci. 2004, 44 (6), 2133–2144. DOI: https://doi.org/10.1021/ci049780b.
29. B. Aakeroy, C.; A. Evans, T.; R. Seddon, K.; Palinko, I. The C-H···Cl Hydrogen Bond: Does It Exist? New J. Chem. 1999, 23 (2), 145–152. DOI: https://doi.org/10.1039/A809309A.
30. Andersson, M. P.; Uvdal, P. New Scale Factors for Harmonic Vibrational Frequencies Using the B3LYP Density Functional Method with the Triple-ζ Basis Set 6-311+G(d,p). J. Phys. Chem. A. 2005, 109 (12), 2937–2941. DOI: https://doi.org/10.1021/jp045733a.
31. Friebolin, H. Basic One-and Two-Dimensional NMR Spectroscopy; John Wiley & Sons, Inc: Weinheim, 1993.
Author notes
manuel.chaur@correounivalle.edu.co
Abstract
A novel molecular system based on 2-((2-(4-chlorophenylhydrazone)methyl)quinoline (1-E ) was synthesized. Interconversion of 1-E to its configurational isomer 1-Z was achieved using UV radiation (250 W Hg lamp). Such isomerization was monitored by 1H-NMR. The results suggest that the hydrazone derivative can act as a chemical brake in solution. This molecular system was structurally (Single Crystal X-Ray diffraction and DFT calculations) and spectroscopically (NMR, UV, and IR) characterized. Electrochemical measurements showed that configurational changes induce differential redox behavior. In this regard, the reported quinoline system exhibits different dynamic absorption and electrochemical properties that are modulated by the UV-light induced configurational change. Therefore, 1-E can be regarded as potential photo-electrochemical switch.
Keywords
Hydrazone derivatives, configurational dynamic, single XRD, DFT calculations, electrochemistry.Resumen
Se sintetizó un nuevo sistema molecular basado en 2-((2-(4-chlorofenilhidrazona)metil)quinolina. Del mismo modo, se evaluó la respuesta dinámica de este compuesto a radiación ultravioleta y formación de un enlace de hidrógeno intramolecular. Los resultados muestran que este derivado de hidrazona puede actuar como freno en solución. El sistema en mención es descrito estructural (Cristalografía de Rayos X y cálculos DFT) y espectroscópicamente (RMN, UV e IR). La interconversión de este sistema entre las configuraciones 1-E y 1-Z fue mediada por radiación UV y monitoreada a través de RMN-1H. El estudio electroquímico mostró un comportamiento diferencial en función de su configuración, aspecto fundamental en el desarrollo de sistemas foto- y electroquímicamente modulados.
Palabras clave
derivados hidrazónicos, dinámica configuracional, cristalografía de rayos X, cálculos DFT, electroquímica.Resumo
Neste trabalho é apresentado um novo sistema molecular baseado na 2-((2-(4-clorofenilhidrazona)metil)quinolina, capaz de responder dinamicamente à radiação ultravioleta formando uma ligação de hidrogénio intramolecular que atua como um freio na solução. Este sistema é descrito estruturalmente (cristalografia de raios-X e DFT) e por diferentes técnicas espectroscópicas (RMN, de UV e de IV). Radiação UV foi usada para fazer a interconversão da hidrazona 1-E no seu isômero configuracional 1-Z . Este processo foi monitorado pelo RMN. As medidas eletroquímicas mostraram que as mudanças configuracionais entre os isômeros induzem a comportamentos redox diferentes, o que é uma caraterística chave no desenvolvimento de interruptores fotoelectroquímicos.
Palavras-chave
derivados de hidrazonas, Dinâmica configuracional, Cristalografia de Raios-X, Cálculos DFT, Eletroquímica.Introduction
The synthesis, characterization, and identification of molecular systems potentially useful in the design of molecular machines is still a huge challenge for supramolecular chemistry researchers ( 1 , 2 ). In this regard, some organic compounds with double bonds (C=C, C=N, or N=N) are of special interest due to their reversible photochemical and thermal reactions. Such feature makes these compounds potentially useful for the synthesis of molecular machines ( 3 , 4 ). It is well known that hydrazone derivatives are one of the most used compounds as building blocks for the formation of supramolecular systems because of the physical and chemical properties that they exhibit. These properties can be reversibly modulated in response to an external stimulus, either light ( 5 – 9 ), addition of metal ions ( 10 – 14 ), and pH changes ( 15 ). Hydrazone derivatives based on 2-quinoline are particularly interesting molecules for the development of photo-activated switches as, upon UV-light irradiation, the quinoline-nitrogen may form an intramolecular hydrogen bond with the N-H proton from a hydrazine moiety, thus stabilizing the Z isomer making it even possible to isolate it and study its properties. Additionally, the E-isomer can act as a ligand similar to terpydine for metal-ion coordination ( 16 , 17 ).
Likewise, the study of electronic and electrochemical properties of configurational isomers has not been well documented, at least for hydrazone derivatives, where there are only a few recent reports ( 7 , 18 ). Electrochemistry of E/Z configurational isomers is important to demonstrate their potential use as molecular photoswitches and electronic devices. Therefore, to design molecular systems able to reversibly modulate the physical and chemical properties in response to light or metal centers, we have decided to study and compare through theoretical studies the electronic and electrochemical properties of the configurational isomers of 2-((2-(4-chlorophenylhydrazono)methyl)-quinoline.
Materials and methods
All starting reagents were acquired from Sigma-Aldrich (USA) and were used without additional purification. FT-IR, NMR (mono and bi-dimensional), and UV-vis spectra were taken in a Shimadzu FTIR-8400S instrument (Japan), in a 400 MHz Bruker Ultrashield spectrometer (USA), and in a PharmaSpec Shimadzu UV-Vis UV-1700 spectrophotometer (Japan), respectively. Electrochemical measurements were recorded in a bipotentiostat model 700B (USA) series electrochemical Analyzer/Workstation from CH Instruments coupled to a computer.
Synthesis of hydrazone derivatives
The hydrazone derivative (E)-2-((2-(4-Chlorophenylhydrazono)methyl)quinoline 1-E was synthesized following the same synthetic protocol as reported in the literature ( 7 ). A mixture of 2-methylquinoline (200 mg, 1.40 mmol) and selenium dioxide (263.5 mg, 2.37 mmol) was dissolved in dry dioxane (20 mL). Then, the reaction mixture was heated under reflux and monitored by thin-layer chromatography (TLC). After disappearance of the starting material, a solid was obtained which was filtered and washed with chloroform (3 x 5 mL). The 2-quinolinecarboxaldehyde was purified by column chromatography with a 95:5 CHCl3/MeOH solution getting an 80% yield. The aldehyde derivative was condensed with 4-chloropehnylhydrazine which was added in a 1:1 ratio in absolute ethanol and heated for 3 h until a red precipitate was formed. This precipitate was washed with cold ethanol and recrystallized from ethanol in 82% yield. Mp 206-208 °C. 205-207 (19) 1H NMR (400 MHz, DMSO-d6 ) δ 7.34-7.41 (m, 4H), 7.75 (t, 1 H, J 7.61), 7.91-8.02 (m, 1 H), 8.16 (d, 1 H, J 8.00), 8.25 (d, 1 H, J 8.39), 8.33-8.44 (m, 2 H), 8.72 (d, 1 H, J 8.98), 12.43 (s, 1 H); 13C NMR (100 MHz, DMSO-d6 ) δ 115.8. 118.4, 122.6, 125.9, 126.6, 127.4, 128.4, 128.8, 129.1, 129.7, 133.2, 142.03, 142.8, 152.5.
(Z)-2-((2-(4-chlorophenylhydrazono)methyl)quinoline (1-Z): A solution of 1-E in methanol was irradiated with a 250 Watts mercury lamp for 30 min. This compound was purified by column chromatography using CHCl3 as eluent obtaining a yellow oil; yield 65%; 1H NMR (400 MHz, DMSO-d6 ) d 7.22-7.35 (m, 4H), 7.58-7.64 (m, 1H), 7.80 (t, 1H, J 7.7 Hz), 7.99-8.05 (m, 2H), 8.11-8.21 (m, 2H), 8.44 (d, 1H, J 8.98 Hz), 11.62 (s, 1H).
Single-Crystal structure determination
The data were collected on a Bruker APEX-II CCD diffractometer using MoKα radiation (0.71073 Å) monochromated by graphite at room temperature (296 K). The cell determination and the final cell parameters were acquired on all reflections using the Bruker SAINT software ( 20 ), included in APEX2 software suite. Data integration and scaled was carried out using the Bruker SAINT software ( 20 ).
The structure was solved using the SHELXS-2013 software and refined using SHELXL-2013 ( 21 ), contained in WinGX ( 22 ), and Olex2-1.2 ( 23 ). Non-hydrogen atoms of the molecules were clearly resolved and full-matrix least-squares refinements of these atoms with anisotropic thermal parameters were performed. All hydrogen atoms were stereochemically positioned and refined with the riding model ( 23 ), except for the hydrogen atom bonded to Nitrogen atom, which was found from the density map. ORTEP diagram was generated with Olex2 ( 23 ). Mercury software ( 24 ) was used to prepare the illustrations.
Electrochemical study of hydrazone derivatives
Cyclic voltammetry (CV) and Osteryoung Square Wave Voltammetry (OSWV) were performed using a 0.1 M solution of tetrabutylammonium hexafluorophosphate (TBAPF6) in DMF. A glassy carbon electrode, a platinum wire, and a silver wire were used as working, counter, and pseudo-reference electrode, respectively. The solutions were degassed with argon before each measurement (10 min). Ferrocene was added as an internal standard. The scan rate was 100 mVs-1.
Computational details
Theoretical calculations were performed using GaussView as graphic interface ( 24 ) and Gaussian 09 ( 26 ) for running calculations. Molecular geometry of 1-E in the ground state was optimized by DFT:B3LYP method with 6-311+G (d, p) basis set. NMR chemical shifts were computed at the same level of theory, using the gauge-including atomic orbital (GIAO) method without any solvent or solvation effect considerations. Vibrational frequencies were performed at the same level than vibrational analysis and make sure that global minima were achieved. Hartree-Fock/6-31+G (d) level of theory and Self-Consistent Isodensity Polarized Continuum Model (SCI-PCM) were employed to simulate solvation effect in optimizations, while UV-Vis spectrum calculations were carried out using same method and level of theory without solvation. For UV-Vis spectrum deconvolution, theoretical IR, and UV spectra graph generation GaussSum 3.0 software package was employed ( 27 ).
Results and discussion
Synthesis and characterization of hydrazone derivatives
Details of the synthesis of 1-E and 1-Z are shown in Figure 1. Compound 1-E was synthesized by the partial oxidation of 2 with selenium dioxide in dioxane, followed by condensation of 3 with the hydrazine derivative 4 in ethanol ( 7 ). The condensation reaction led to the formation of the corresponding E-hydrazone, which was identified by the singlet at 12.43 ppm corresponding to the NH proton ( 3 ). The geometry optimization for 1 was also performed to compare the energy associated to each isomer, as it was expected the E isomer is 12.2 kJ/mol more stable than the Z isomer. The irradiation of 1-E with a 250 Watts mercury lamp produced the Z isomer, which was isolated by column chromatography in a 67% yield.
Figure
1. Synthetic route to obtain the hydrazone derivative 1.
For 1-Z , the 1H-NMR spectra show the existence of an intramolecular H-bond after irradiation with UV light since all the signals changed their chemical shift. The lack of symmetry in the molecule is reflected by the different coupling between the signals and the shift of the N-H proton in 1-Z when compared to 1-E (See Figure 2). The most remarkable change occurs on the NH and phenyl protons, which are shifted to the up-field region and splitted as a multiplet, respectively.
Figure 2.1H NMR spectra (400 MHz) of compound 1-E
and 1-Z
in DMSO-d6
.
Single-crystal structure determination
Crystals of 1-E were obtained by slow evaporation of ethanol, resulting in fine, orange needlelike crystals. 1-E crystalized in the monoclinic space group P21/c. The molecule presents an almost planar configuration with a torsion angle between C6–C1–N1– N2 of 5.89(1)° and a dihedral angle between the quinoline and benzene planes of 1.91°. The Mogul geometry check shows that all bond lengths and angles are within the normal range ( 28 ). ORTEP representation of the 1-E structure is presented in the Figure 3a; Crystal data collection and structure refinement details are summarized in Table 1.
Figure 3. a) ORTEP representation of title compound, b)
Hydrogen bond N1–H1···N3 and c) a) π···π interaction between Cg2 and Cg3.
Table 1. Crystal data and structure refinement for 1-E
.
Identification
Code
1-E
Empirical formula
C16H11ClN3
Formula weight
280.73
Temperature/K
296(2)
K
Crystal system
Monoclinic
Space group
P21/c
a/Å
8.2023(3)
b/Å
7.1828(2)
c/Å
23.2593(8)
β(°)
90.5180(10)
Volume/ Å3
1370.28(8)
Z
4
ρcalc
mg/ mm3
1.361
μ/mm−1
0.271
F(000)
580
Crystal size/ mm3
0.522
x 0.178 x 0.171
2θ range for data
collection/°
1.751 to
26.412
Index ranges
-10≤h≤10,
-8≤k≤8, -29≤l≤29
Reflections
collected
38252
Independent
reflections
2810[R(int)
= 0.0241]
Data /
restraints / parameters
2810 / 0
/ 185
Goodness-of-fit on F2
1.050
Final R indices [I>2sigma(I)]
R1 =
0.0371, wR2 = 0.0999
R indices (all
data)
R1
= 0.0442, wR2 = 0.1067
Largest diff. Peak/hole/ e.Å-3
0.204 and -0.209
The crystal packing is driven by N1–H1···N3 hydrogen bond, along [010] direction with 3.0855(1) Å of distance. As shown in Figure 3b, the interactions give rise to zig-zag chains growing along the [010] direction and the connected molecules are placed on the two planes (931) and (12-41). A 3D supramolecular network is formed by the joining of the chains along [100] direction by π-π interactions between the aromatic rings with an intercentroids distance of Cg2···Cg3= 3.8903(1) Å (Figure 3 c). It was also observed a C9–H9 ···Cl1 interaction ( 29 ) along [001] direction with a distance of 3.7168(15) Å.
Figure 4. Hirshfeld Surface of title compound. Left: a) Shape Index and Right:
b) dnorm.
The Hirshfeld surface for 1-E showed that the chloride and the deprotonated nitrogen atoms in the quinoline and hydrazone present mainly, a hydrogen acceptor behavior, while the N–H and C–H act as donor groups (see Figure 4). Furthermore, an observation over the aromatic rings in the shape index surface blue and red regions that indicates the presence of π-π interactions, is confirmed by the fingerprint plot. The greatest contribution comes from Van der Waals interactions (H···H and C···H) followed by hydrogen bonds as C l ···H and N···H (see Figure 4b), that contribute with 15.6% and 7.9%, respectively. The π-π interactions contribute with 10.9% . Other contacts were also observed, however, their contribution is less than 5% each.
Molecular geometry optimization
Ab initio calculations yielded a planar structure with a dihedral angle equals to 0.001º between the quinoline and benzene planes and º torsion angles between the quinoline or benzene rings and the hydrazine group. As shown in Figure 5, these dihedral and torsional angles are not significantly deviated concerning to the results discussed in the crystallographic section.
Figure 5. Overlap of crystallographic and optimized (DFT:
B3LYP/6-311+G (d, p) structures of 1-E
. RMSD was 0.0985.
Linear regression analysis was also carried out to establish a systematic comparison between the optimized and crystallographic structures. The slope represents a ratio between the set of variations of calculated-experimental data. The intercept corresponds to the value of the dependent variable (calculated) when the independent variable (experimental) is equal to zero: meaning an over or underestimation value. Thus, perfect correlation is given when the slope and intercept values are 1 and 0, respectively (selected structural parameters and linear regression are shown in Table 2). The analysis reveals that theoretical calculations tend to a subtle overestimation respect to the crystallographic data set as shown in the intercept values (see Table 2). The highest deviation from experimental bond length measurements was observed in N1–H bond (17.17% error) since this group is highly influenced by hydrogen bond interaction (See Figure 3). However, other bond angles and length show less than 1.4% and 0.9% bond length and angle errors respectively.
* Bond length linear regression
exclude N1-H bond
Table 2. Theoretical-experimental comparison of selected molecular geometry
parameters.
Bond
Experimental
length (Å)
Calculated
length (Å)
Error (%)
C1-C2
1,388
1.4020
1.01
C2-C3
1.377
1.3897
0.92
C3-C4
1.378
1.3912
0.96
C4-C1
1.742
1.7605
1.06
C4-C5
1.391
1.3914
1.12
C5-C6
1.380
1.3904
0.75
C6-C1
1.391
1.4009
0.71
C1-N1
1.388
1.3938
0.42
N1-H*
0.867
1.0159
17.17
N1-N2
1.348
1.3388
0.68
N2-C7
1.279
1.2859
0.54
C7-C8
1.456
1.4622
0.43
C8-N3
1.323
1.3240
0.08
C8-C9
1.42
1.4266
0.46
C9-C10
1.352
1.3664
1.07
C10-C11
1.414
1.4202
0.44
C11-C16
1.417
1.4293
0.87
C11-C12
1.410
1.4155
0.39
C12-C13
1.359
1.3763
1.27
C13-C14
1.395
1.4144
1.39
C14-C15
1.366
1.3753
0.68
C15-C16
1.408
1.4186
0.75
C16-N3
1.372
1.3610
0.80
Linear
regression
Slope
1.0300
Intercept
0.0337
R2
0.99301
Bond
Crystallographic
angle (º)
Calculated
angle (º)
Error (%)
C3-C4-C5
120.51
120.49
0.02
C5-C4-C1
119.62
119.79
0.14
C6-C1-N1
121.84
121.95
0.09
N1-N2-C7
117.53
118.28
0.64
C7-C8-N3
116.41
115.38
0.89
C9-C10-C11
120.33
119.85
0.40
C11-C12-C13
120.72
120.47
0.20
C14-C15-C16
120.46
120.44
0.02
C16-N3-C8
118.19
118.68
0.42
Linear
regression
Slope
1.0242
Intercept
2.9178
R2
0.9223
UV-Vis Spectroscopy analysis
The electronic transitions of 1-E were studied by UV-Vis. As shown in Figure 6, the hydrazone derivative exhibits a high absorption band around 425 nm, which present a bathochromic shift with the increasing polarity of the solvent. Additionally, a new band around 380 nm corresponding to π-π* transitions related to the imine bond was observed using methanol as solvent ( 6 ). An optimized structure by DFT: B3LYP/6-311+G (d, p) calculation was used as input geometry for a second optimization at HF/6-31+G (d) level of theory including solvent effects through the SCI-PCM approximation to simulate chloroform solvation yielding an equivalent conformation with no differences in the electronic structure. From this optimized structure, TD-SCF method was employed to calculate excited states energy at HF/6-31+G (d) level of theory neglecting solvation. Besides, the theoretical UV-Visible spectrum presents a transition in the visible region and two transitions in the ultraviolet region (absorptions at 427, 319, and 278 nm respectively) with an absolute error percentage respect to experimental observations equal to 6.57, 10.34, and 11.15%, respectively. These results tend to an underestimation in a range between 28 to 33 nm.
Figure 6. Theoretical and Experimental
UV-vis spectra of 1-E
(1.0 x
10-5 M) in different solvents.
GaussSum 3.0 software package was employed to deconvolute the computed electronic transitions using Gaussian 09 and to determine the contribution of the main electronic transitions (see Figure 7). Higher oscillator strength was observed at 427 nm signal (0.3465). This absorption maximum was characterized by a main electronic transition from HOMO to LUMO+1 energy levels (34%), where a homogeneous electronic distribution is displaced to a MO over the hydrazone and phenyl groups. A second main electronic transition from HOMO-2 to LUMO+1 (5%) was also observed. Second higher oscillator strength was observed at 278 nm (0.1705), where the main electronic transition (HOMO-1®LUMO+5, 21%) is characterized by a partial electronic displacement from the quinoline to hydrazone and chlorophenyl moieties.
Figure 7. Main electronic transitions in ultraviolet-visible and contribution
percentage.
NMR and FT-IR spectroscopy analysis
Theoretical-experimental comparison of FT-IR and NMR spectra of 1-E was also carried out. The four most prominent bands are C=N stretching (1579 cm-1), N-H rocking (1508 cm-1), N-N stretching (1142 cm-1), and C-H bending (1241cm-1). Displacement vectors for these vibrations are shown in Figure 8. These vibrational modes are degenerated except N-H bending since displacement vectors clearly showed other vibrational modes coexisting simultaneously in each frequency.
Figure 8. Displacement
vectors for selected vibrational modes. A) C-H bending. B) N-N stretching. C)
C=N stretching. D) N-H bending.
The absence of imaginary frequencies confirms that the optimized structure is a global minimum. A comparison of selected computed vibrational frequencies and assigned values experimentally is shown in Table 3 from supporting information. Calculated values tend to overestimation even though the scale factor used is less than 1.00 ( 30 ). The maximum error rate was observed in C-Cl frequency (5.45%), C=N and N-H frequencies are the second and third biggest deviation respect to experimental observations. C-Cl and N-H errors may be explained because these moieties are typically involved in hydrogen bonding interactions in the solid state and those were neglected in theoretical calculations.
Vibrational mode
Theoretical (cm-1)
Scaled value
(cm-1)
Experimental frequency
(cm-1)
Error rate (%)
N-H stretching
3499
3387
3209
5,26
N=C-H stretching
3062
2964
2985
-0,71
C=N stretching
1631
1579
1495
5,32
N-N stretching
1180
1142
1091
4,47
C-Cl stretching
1100
1065
1007
5,45
C=C stretch. Aryl
1640
1587
1587
0.02
N-H rocking
1558
1508
1555
-3,12
C-H bending
1282
1241
1236
0,40
The correlation between the computed and experimental 1H-NMR chemical shifts was unacceptable as the signal of N-H, showed the biggest deviation (see Table 4), mainly due to its high influence by solvation ( 31 ). Therefore, the neglected solvation effect should help to improve the quality of 1H-NMR chemical shift calculations. The 13C-NMR chemical shifts showed acceptable correlation respect to experimental values yielding an average rate of error equal to 2.61%.
1H-NMR Chemical shift (ppm)
13C-NMR Chemical shift (ppm)
Experimental
Calculated
Error
Experimental
Calculated
Error
7.38
7.00
-5.43
115.8
113.81
1.72
7.38
7.73
4.53
118.4
114.54
3.26
7.38
7.94
7.05
122.6
129.09
-5.29
7.38
8.26
10.65
125.9
129.41
-2.79
7.75
8.10
4.32
126.6
130.46
-3.05
7.97
8.35
4.55
127.4
131.88
-3.52
8.16
8.25
1.09
128.4
132.08
-2.87
8.25
8.55
3.51
128.8
134.87
-4.71
8.35
8.82
5.33
129.1
137.89
-6.81
8.72
8.71
-0.11
129.7
136.57
-5.30
12.43
7.47
-66.40
133.2
138.54
-4.01
-
-
-
142.03
139.14
2.03
Electrochemical studies
The electrochemical properties of compounds 1-E and 1-Z were studied by cyclic voltammetry (CV) and Osteryoung Square Wave Voltammetry (OSWV) (See Experimental Section).
Figure 9. Cyclic voltammogram of hydrazone derivatives at 100
mVs-1. Glassy carbon as working electrode and using ferrocene as an
internal reference.
When analyzing the cathodic potentials (see Figure 9), three irreversible reduction potentials for the 1-E isomer are observed. These potentials remained irreversible upon increasing scan rates. OSWV revealed two additional reductions (see Figure 10) which are located very close to those observed in CV. Presumably, the closed peak potentials correspond to the processes occurring on the surface of the electrode and the corresponding cathodically shifted potential to the process occurring in the solution. The first reduction in compound 1-E is located at -1.09 V and corresponds to the imine reduction whereas for 1-Z is cathodically shifted towards -2.54 V (see Table 5). These results indicate that upon reduction the [1-E ]· - radical anion is stabilized by π delocalization in the quinoline system. However, in the Z configuration the nitrogen from the quinoline is acting as a Lewis base (forming the intramolecular hydrogen bond) and, therefore, its participation in the radical anion stabilization is restricted thus shifting cathodically the reduction potential. Likewise, the differences in the current intensity for the cathodic/anodic potentials suggest different reduction mechanism for both configurational isomers.
Figure 10. OSW of hydrazone derivatives 1-E
and 1-Z
at 100 mVs-1. Glassy
carbon as working electrode and using ferrocene as an internal reference.
aWorking
electrode: glassy carbon; counter electrode: Pt wire; Pseudoreference
electrode: Ag wire. Supporting electrolyte: TBAPF6.Scan rate: 0.1 V
s−1.
Table 5. Electrochemical Potentials of hydrazone derivatives vs Ferrocene
in DMF (V)a.
Compound
Ep,oxd(1)
Ep,oxd(2)
Ep,red(1)
Ep,red(2)
Ep,red(3)
Ep,red(4)
Ep
,red(5)
1-E
0.42
0.73
-1.09
-1.24
-2.10
-2.28
-2.62
1-Z
0.31
0.76
-2.54
-2.75
Compounds 1-E and 1-Z exhibited two irreversible oxidation potentials which are attributed to the –NH groups of the hydrazone framework. The first reduction potential of 1-E is anodically shifted, when compared to its 1-Z counterpart. This behavior indicates a larger acidity of 1-Z gained by the intramolecular hydrogen bond formation.
Conclusions
The solid-state structural investigation trough single-crystal X-ray diffraction reveals a crystal packaging governed by N1–H1···N3 hydrogen bond, forming inverted dimers along [010] direction. The theoretical calculations carried out at DFT: B3LYP/6-311+G (d, p) level of theory showed good correlation with experimental data, yielding error rates less than 1.4% and 0.9% error for bond length and angle. Also, spectroscopic properties were successfully calculated employing ab initio calculations. The UV-Vis spectrum was deconvoluted giving the main electronic transitions from HOMO to LUMO+1 and HOMO-1 to LUMO+5.
In solution, this novel compound exhibited UV radiation-mediated configurational isomerism conversion from 1-E to 1-Z confirmed by 1H-NMR evidence: chemical shift change and new signals appearance owing to system asymmetry. Finally, modified electrochemical behavior induced by configurational changes was observed, supporting the applicability of these type of molecular systems as an electrochemical template.
Acknowledgements
The authors are grateful to the Vicerrectoria de Investigaciones and Centro de Excelencia en Nuevos Materiales (CENM) from the Universidad del Valle (Colombia), as well as the Departamento Administrativo de Ciencia, Tecnología e Innovación (COLCIENCIAS) for the financial support of this work. M.S.-M. and R. D. acknowledges Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Conselho Nacional de Desenvolvimento Cientfico y Tecnológico for the CNPq and CAPES/PNPD scholarships from Brazilian Ministry of Education, and R. de Almeida Santos to facilitate the measurements and FAPESP (2009/54011-8) for providing Apex-II equipment. R.F.D. acknowledges to the Dirección General de Investigaciones (DGI) of the Universidad Santiago de Cali. G.G. thanks to the Laboratorio de Computación Científica from Facultad de Ciencias Naturales (Icesi University) for the computational resources support as well as Prof. Carlos A. Arango for his invaluable guidance.
References
References
Lehn, J.-M. Perspectives in Chemistry—Aspects of Adaptive Chemistry and Materials. Angew. Chemie Int. Ed. 2015, 54 (11), 3276–3289. https://doi.org/10.1002/anie.201409399.
Li, J.; Nowak, P.; Otto, S. Dynamic Combinatorial Libraries: From Exploring Molecular Recognition to Systems Chemistry. J. Am. Chem. Soc. 2013, 135 (25), 9222–9239. https://doi.org/10.1021/ja402586c.
Tatum, L. A.; Su, X.; Aprahamian, I. Simple Hydrazone Building Blocks for Complicated Functional Materials. Acc. Chem. Res. 2014, 47 (7), 2141–2149. https://doi.org/10.1021/ar500111f.
Su, X.; Aprahamian, I. Hydrazone-Based Switches, Metallo-Assemblies and Sensors. Chem. Soc. Rev. 2014, 43 (6), 1963–1981. https://doi.org/10.1039/C3CS60385G.
Lehn, J.-M. Conjecture: Imines as Unidirectional Photodriven Molecular Motors -Motional and Constitutional Dynamic Devices. Chemistry. 2006, 12 (23), 5910–5915. https://doi.org/10.1002/chem.200600489.
Chaur, M. N.; Collado, D.; Lehn, J.-M. Configurational and Constitutional Information Storage: Multiple Dynamics in Systems Based on Pyridyl and Acyl Hydrazones. Chem. A Eur. J. 2011, 17 (1), 248–258. https://doi.org/10.1002/chem.201002308.
Romero, E. L.; D´Vries, R. F.; Zuluaga, F.; Chaur, M. N. Multiple Dynamics of Hydrazone Based Compounds. J. Brazilian Chem. Soc. 2015, 26 (6), 1265–1273. http://dx.doi.org/10.5935/0103-5053.20150092.
Gordillo, M. A.; Soto-Monsalve, M.; Gutiérrez, G.; D’Vries, R. F.; Chaur, M. N. Theoretical and Experimental Comparative Study of a Derivative from 2-Pyridinecarboxaldehyde Which Exhibits Configurational Dynamics. J. Mol. Struct. 2016, 1119. https://doi.org/10.1016/j.molstruc.2016.04.055.
Chaur, M. N. Aroylhydrazones as potential systems for information storage: photoisomerization and metal complexation Rev. Colomb. Quim. 2012, 41 (3), 349-358. Doi: https://doi.org/10.15446/rev.colomb.quim.v45n3.57351
Ulrich, S.; Buhler, E.; Lehn, J.-M. Reversible Constitutional Switching between Macrocycles and Polymers Induced by Shape Change in a Dynamic Covalent System. New J. Chem. 2009, 33 (2), 271–292. https://doi.org/ 10.1039/B817261G.
Carmona-Vargas, C. C.; Váquiro, I. Y.; Jaramillo-Gómez, L. M.; Lehn, J.-M.; Chaur, M. N. Grid-Type Complexes of M2+ (M=Co, Ni, and Zn) with Highly Soluble Bis(hydrazone)thiopyrimidine-Based Ligands: Spectroscopy and Electrochemical Properties. Inorganica Chim. Acta. 2017, In Press. https://doi.org/10.1016/j.ica.2017.05.002.
Su, X.; Robbins, T. F.; Aprahamian, I. Switching through Coordination-Coupled Proton Transfer. Angew. Chemie Int. Ed. 2011, 50 (8), 1841–1844. https://doi.org/ 10.1002/anie.201006982.
Romero, E. L.; Cabrera-Espinoza, A.; Ortiz-Peña, N.; Soto-Monsalve, M.; Zuluaga, F.; D’Vries, R. F.; Chaur, M. N. New Pyrazolino and pyrrolidino[60]fullerenes: The Introduction of the Hydrazone Moiety for the Formation of Metal Complexes. J. Phys. Org. Chem. 2017, 30 (2), e3601–n/a. https://doi.org/10.1002/poc.3601.
Gutiérrez, G; Gordillo, M.A.;Chaur, M. N. A DFT study on Dichloro {(E)-4-dimethylamino-N'-[(pyridin-2-yl) methylideneKN] benzohydrazide-k0}M2+ (M = Zn, Cu, Ni, Fe, Mn, Ca and Co) complexes: Effect of the metal over association energy and complex geometry. Rev. colomb. quim. 2016, 45, 3, 28-32. DOI: https://doi.org/10.15446/rev.colomb.quim.v45n3.57351
Landge, S. M.; Tkatchouk, E.; Benítez, D.; Lanfranchi, D. A.; Elhabiri, M.; Goddard, W. A.; Aprahamian, I. Isomerization Mechanism in Hydrazone-Based Rotary Switches: Lateral Shift, Rotation, or Tautomerization? J. Am. Chem. Soc. 2011, 133 (25), 9812–9823. https://doi.org/10.1021/ja200699v.
Castro Agudelo, B.; Ochoa-Puentes, C.; Rodríguez-Córdoba, W.; Reiber, A.; Sierra, C. A. Synthesis, characterization, X-ray crystal structure and DFT calculations of 4-([2,2':6',2"-terpyridin]-4'-yl)phenol Rev. Colomb. Quím. 2018, 47 (1). In press. DOI: https://doi.org/10.15446/rev.colomb.quim.v47n1.66281.
Parada, G.; Fernández, D.; Reyes, A.; Suárez, M.F.; Fadini, L. Sinteis y Estudio Teorico de Compuesto de Ru(II) con Ligantes Ferrocennílicos para Aplicaciones Electroquímicas. Rev. Colomb. Quim. 2007, 36 (2), 199-211. In press. DOI: https://doi.org/ 10.15446/rev.colomb.quim
Fernández, M. A.; Barona, J. C.; Polo-Cerón, D.; Chaur, M. N. Photochemical and Electrochemical Studies on Lanthanide Complexes of 6-(Hydroxymethyl)pyridine- 2-carboxaldehyde[2- Methyl-Pyrimidine-4,6-Diyl] Bis-Hydrazone. Rev. Colomb. Quím. 2014, 43 (1), 5–11. https://doi.org/10.15446/rev.colomb.quim.v43n1.50540.
Puskullu, M. O.; Shirinzadeh, H.: Nenni, M.; Gurer-Orhan, H.; Suzen, S. Synthesis and evaluation of antioxidant activity of new quinoline-2-carbaldehyde hydrazone derivatives: bioisosteric melatonin analogues. J. Enzyme Inhib. Med. Chem. 2016, 31(1): 121–125. https://doi.org/10.3109/14756366.2015.1005012.
Bruker-AXS. SAINT. Bruker-Siemens: Madison, Wisconsin,USA 2006.
Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71 (1), 3–8. https://doi.org/10.1107/S2053229614024218.
Farrugia, L. J. WinGX and ORTEP for Windows : An Update. J. Appl. Crystallogr. 2012, 45 (4), 849–854. https://doi.org/10.1107/S0021889812029111.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2 : A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42 (2), 339–341. https://doi.org/10.1107/S0021889808042726.
Macrae, C. F.; Bruno, I. J.; Chisholm, J. A.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. A. Mercury CSD 2.0 – New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41 (2), 466–470. https://doi.org/10.1107/S0021889807067908.
Dennington, R.; Keith, T.; Millam, J. GaussView. Semichem Inc.: Shawnee Mission 2009.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; et al. Gaussian 09. Gaussian, Inc.: Wallingford CT 2009.
O’Boyle, N. M.; Tenderholt, A. L.; Langner, K. M. Cclib: A Library for Package-Independent Computational Chemistry Algorithms. J. Comput. Chem. 2008, 29 (5), 839–845. https://doi.org/ 10.1002/jcc.20823.
Bruno, I. J.; Cole, J. C.; Kessler, M.; Luo, J.; Motherwell, W. D. S.; Purkis, L. H.; Smith, B. R.; Taylor, R.; Cooper, R. I.; Harris, S. E.; et al. Retrieval of Crystallographically-Derived Molecular Geometry Information. J. Chem. Inf. Comput. Sci. 2004, 44 (6), 2133–2144. https://doi.org/10.1021/ci049780b.
B. Aakeroy, C.; A. Evans, T.; R. Seddon, K.; Palinko, I. The C-H···Cl Hydrogen Bond: Does It Exist? New J. Chem. 1999, 23 (2), 145–152. https://doi.org/10.1039/A809309A.
Andersson, M. P.; Uvdal, P. New Scale Factors for Harmonic Vibrational Frequencies Using the B3LYP Density Functional Method with the Triple-ζ Basis Set 6-311+G(d,p). J. Phys. Chem. A. 2005, 109 (12), 2937–2941. https://doi.org/10.1021/jp045733a.
Friebolin, H. Basic One-and Two-Dimensional NMR Spectroscopy; John Wiley & Sons, Inc: Weinheim, 1993.
How to Cite
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Soufiane Akhramez, Abderrafia Hafid, Mostafa Khouili, Mohamed Saadi, Lahcen El Ammari, El Mostafa Ketatni. (2019). Synthesis, crystal structure and Hirshfeld surface analysis of 2-chloro-3-[(E)-(2-phenylhydrazinylidene)methyl]quinoline. Acta Crystallographica Section E Crystallographic Communications, 75(7), p.964. https://doi.org/10.1107/S2056989019007692.
2. Sanjay Kumar, Purvi Shah, Siddharth K. Tripathi, Shabana I. Khan, Inder Pal Singh. (2022). Synthesis and In vitro Evaluation of Hydrazonomethyl-Quinolin–8–ol and Pyrazol–3–yl-Quinolin–8–ol Derivatives for Antimicrobial and Antimalarial Potential. Medicinal Chemistry, 18(9), p.949. https://doi.org/10.2174/1573406418666220303144929.
3. Alejandra M. Arango, Julien Wist, Javier Ellena, Richard D'Vries, Manuel N. Chaur. (2020). Multiple Reversible Dynamics of Pyrimidine Based Acylhydrazones. European Journal of Organic Chemistry, 2020(26), p.4009. https://doi.org/10.1002/ejoc.202000553.
4. Gamal A. E. Mostafa, Ahmed H. Bakheit, Mohamed H. Al-Agamy, Rashad Al-Salahi, Essam A. Ali, Haitham Alrabiah. (2023). Synthesis of 4-Amino-N-[2 (diethylamino)Ethyl]Benzamide Tetraphenylborate Ion-Associate Complex: Characterization, Antibacterial and Computational Study. Molecules, 28(5), p.2256. https://doi.org/10.3390/molecules28052256.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2018 Revista Colombiana de Química

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors will retain their copyright and guarantee the journal the right of first publication of their work, which will be simultaneously subject to the Creative Commons Recognition License (CC. Attribution 4.0) that allows third parties to always share the work as the authors and their publication in this journal be indicated.
Authors may adopt other non-exclusive license agreements for the distribution of the version of the published work (eg: deposit it in an institutional archive or publish it in a monographic volume) provideding always the first publication in this Journal.
Authors are allowed and encouraged to disseminate their work through the Internet (eg, in institutional files or on their website) before and during the submission process, which can lead to interesting exchanges and increase citations of the published work (See The effect of open access).