Inference of the phenotypic resistance profile of Pseudomonas aeruginosa through an interpretative reading of the antibiogram in a pediatric hospital. 2006-2014.
Inferencia del perfil fenotípico de resistencia de Pseudomonas aeruginosa con la lectura interpretada del antibiograma en un hospital pediátrico entre los años 2006 y 2014
DOI:
https://doi.org/10.15446/revfacmed.v64n3.51770Palabras clave:
Pseudomonas Aeruginosa, Nosocomial Infections, Antibiotics, Antibiogram (en)Pseudomonas aeruginosa, Pruebas de sensibilidad microbiana, Antibióticos (es)
Descargas
Introduction: Pseudomonas aeruginosa behaves as an opportunistic pathogen involved in hospital infections, with high capacity to generate resistance to antibiotic treatment. The interpretative reading of the antibiogram makes possible inferring these resistance mechanisms and establishing appropriate antibiotic treatment.
Objective: The interpretative reading of the antibiogram seeks to infer the resistance phenotype of P. aeruginosa at Fundación Hospital de la Misericordia (HOMI, by its acronym in Spanish) between 2006 and 2014.
Materials and methods: Descriptive cross-sectional study where a search of positive antibiogram reports for P. aeruginosa was performed. The resistance phenotype was deduced based on the interpretative reading of the antibiogram.
Results: A sample of 463 positive antibiograms for P. aeruginosa was obtained; these samples were taken from children aged 0 to 17, showing a higher prevalence among infants and toddlers. The antibiograms mainly came from male subjects (62.2%). The most frequent hospitalization services were: PICU —pediatric intensive care unit— (30.2%) and general hospitalization (27.3%). The most common sources of isolation were: blood (24.4%) and urine (23.8%). 11 phenotypes were characterized, being the most common: natural phenotype (63.2%), loss of porin OprD (5.7%) and partial and full AmpC derepression (8.4% and 8.2%, respectively).
Conclusion: Isolation of P. aeruginosa at HOMI predominantly shows a natural phenotype. The interpretative reading of the antibiogram allowed inferring 11 phenotypes.
Introducción. Pseudomonas aeruginosa se comporta como un patógeno oportunista implicado en infecciones intrahospitalarias y tiene alta capacidad de generar resistencia al manejo antibiótico. La lectura interpretada del antibiograma permite inferir estos mecanismos de resistencia y establecer el antibiótico más apropiado.
Objetivo. La lectura interpretada del antibiograma busca inferir el fenotipo de resistencia de P. aeruginosa en la Fundación Hospital de la Misericordia (HOMI) entre 2006 y 2014.
Materiales y métodos. Estudio descriptivo de corte transversal donde se realizó la búsqueda de informes de antibiogramas positivos para P. aeruginosa y se dedujo el fenotipo de resistencia según la lectura interpretada del antibiograma.
Resultados. Se obtuvo una muestra de 463 antibiogramas positivos para P. aeruginosa aisladas de niños entre 0 y 17 años, con predominio en lactantes y preescolares de género masculino (62.2%). Los servicios de hospitalización más frecuentes fueron unidad de cuidado intensivo pediátrico (30.2%) y hospitalización general (27.3%). Los sitios de aislamiento más frecuentes fueron sangre (24.4%) y orina (23.8%). Se caracterizaron en total 11 fenotipos, los más frecuentes fueron natural (63.2%), pérdida de porina OprD (5.7%) y desrepresión parcial (8.4%) y total (8.2%) de AmpC.
Conclusión. Los aislamientos de P. aeruginosa en el HOMI tienen de manera predominante un fenotipo natural. La lectura interpretada del antibiograma permitió inferir 11 fenotipos.
ORIGINAL RESEARCH
DOI: https://doi.org/10.15446/revfacmed.v64n3.51770
Inference of the phenotypic resistance profile of Pseudomonas
aeruginosa through an interpretative reading of the
antibiogram in a pediatric hospital. 2006-2014
Inferencia del perfil fenotípico de resistencia de Pseudomonas aeruginosa con la lectura
interpretada del antibiograma en un hospital pediátrico entre los años 2006 y 2014
Received: 08/07/2015. Accepted: 24/11/2015.
Juan Jailer Arango1 • Aura Lucía Leal2 • María del Pilar Montilla1 • Germán Camacho-Moreno1,3
1 Universidad Nacional de Colombia - Bogotá Campus - Faculty of Medicine - Department of Pediatrics - Bogotá, D.C. - Colombia.
2 Universidad Nacional de Colombia - Bogotá Campus - Faculty of Medicine - Department of Microbiology - Bogotá, D.C. - Colombia.
3 Fundación Hospital de la Misericordia - Bogotá, D.C. - Colombia.
Corresponding author: Germán Camacho Moreno. Infections Committee, Fundación Hospital de la Misericordia. Avenida Caracas No. 1-13. Phone number: +57 1 3811970, ext.: 279. Bogotá, D.C., Colombia. Email: gcamachom@unal.edu.co.
| Abstract |
Introduction: Pseudomonas aeruginosa behaves as an opportunistic pathogen involved in hospital infections, with high capacity to generate resistance to antibiotic treatment. The interpretative reading of the antibiogram makes possible inferring these resistance mechanisms and establishing appropriate antibiotic treatment.
Objective: The interpretative reading of the antibiogram seeks to infer the resistance phenotype of P. aeruginosa at Fundación Hospital de la Misericordia (HOMI, by its acronym in Spanish) between 2006 and 2014.
Materials and methods: Descriptive cross-sectional study where a search of positive antibiogram reports for P. aeruginosa was performed. The resistance phenotype was deduced based on the interpretative reading of the antibiogram.
Results: A sample of 463 positive antibiograms for P. aeruginosa was obtained; these samples were taken from children aged 0 to 17, showing a higher prevalence among infants and toddlers. The antibiograms mainly came from male subjects (62.2%). The most frequent hospitalization services were: PICU —pediatric intensive care unit— (30.2%) and general hospitalization (27.3%). The most common sources of isolation were: blood (24.4%) and urine (23.8%). 11 phenotypes were characterized, being the most common: natural phenotype (63.2%), loss of porin OprD (5.7%) and partial and full AmpC derepression (8.4% and 8.2%, respectively).
Conclusion: Isolation of P. aeruginosa at HOMI predominantly shows a natural phenotype. The interpretative reading of the antibiogram allowed inferring 11 phenotypes.
Keywords: Pseudomonas Aeruginosa; Nosocomial Infections; Antibiotics; Antibiogram (MeSH).
Arango JJ, Leal AL, Montilla MP, Camacho-Moreno G. Inference of the phenotypic resistance profile of Pseudomonas aeruginosa through an interpretative reading of the antibiogram in a pediatric hospital. 2006-2014. Rev. Fac. Med. 2016;64(3):409-15. English. doi:
https://doi.org/10.15446/revfacmed.v64n3.51770.
| Resumen |
Introducción. Pseudomonas aeruginosa se comporta como un patógeno oportunista implicado en infecciones intrahospitalarias y tiene alta capacidad de generar resistencia al manejo antibiótico. La lectura interpretada del antibiograma permite inferir estos mecanismos de resistencia y establecer el antibiótico más apropiado.
Objetivo. La lectura interpretada del antibiograma busca inferir el fenotipo de resistencia de P. aeruginosa en la Fundación Hospital de la Misericordia (HOMI) entre 2006 y 2014.
Materiales y métodos. Estudio descriptivo de corte transversal donde se realizó la búsqueda de informes de antibiogramas positivos para P. aeruginosa y se dedujo el fenotipo de resistencia según la lectura interpretada del antibiograma.
Resultados. Se obtuvo una muestra de 463 antibiogramas positivos para P. aeruginosa aisladas de niños entre 0 y 17 años, con predominio en lactantes y preescolares de género masculino (62.2%). Los servicios de hospitalización más frecuentes fueron unidad de cuidado intensivo pediátrico (30.2%) y hospitalización general (27.3%). Los sitios de aislamiento más frecuentes fueron sangre (24.4%) y orina (23.8%). Se caracterizaron en total 11 fenotipos, los más frecuentes fueron natural (63.2%), pérdida de porina OprD (5.7%) y desrepresión parcial (8.4%) y total (8.2%) de AmpC.
Conclusión. Los aislamientos de P. aeruginosa en el HOMI tienen de manera predominante un fenotipo natural. La lectura interpretada del antibiograma permitió inferir 11 fenotipos.
Palabras clave: Pseudomonas aeruginosa; Pruebas de sensibilidad microbiana; Antibióticos (DeCS).
Arango JJ, Leal AL, Montilla MP, Camacho-Moreno G. [Inferencia del perfil fenotípico de resistencia de Pseudomonas aeruginosa con la lectura interpretada del antibiograma en un hospital pediátrico entre los años 2006 y 2014]. Rev. Fac. Med. 2016;64(3):409-15. English. doi:
https://doi.org/10.15446/revfacmed.v64n3.51770.
Introduction
Pseudomonas aeruginosa is a Gram negative, non-fermenting bacillus that has the ability to survive on inert surfaces and produce biofilms (1,2); this feature allows survival in hospital environments and growth in standard culture media, since their nutritional requirements are few (3). It has a genome with 6.3 million base pairs, which encodes 5 570 genes, and is rich in virulence factors (4).
In addition, P. aeruginosa is a pathogen involved in nosocomial infections associated with high morbidity and mortality rates, prolonged hospital stay and higher treatment costs. It also behaves as an opportunistic nosocomial agent, especially in patients with risk factors such as cystic fibrosis, prolonged hospitalization in critical care units and immunodeficiency (4).
Within the studied P. aeruginosa phenotypes, the wild or natural is characterized by being sensitive to carboxypenicillins, ureidopenicillins, ceftazidime, cefepime, cefoperazone, aztreonam and carbapenems (1), but it is also recognized by the intrinsic expression of resistance to a wide range of antibiotics and the development of mechanisms of antimicrobial resistance during operation via plasmids and integrons or mutations in the gene coding (5); this provides resistance to usually active compounds (6).
The intrinsic resistance of this bacteria is given by the low permeability of the outer membrane cell, the presence of inducible chromosomal β-lactamases (AmpC) and MexAB-OprM efflux system expression (7). Some strains can produce other β-lactamases such as oxacilinases (OXA), extended spectrum β-lactamases (ESBL) or carbapenemases (3). Based on the amino acid sequence, the used energy source and the substrate, efflux systems have been characterized in five superfamilies (5,8).
Another important mechanism is the aminoglycoside resistance, which occurs due to the enzymatic modification of antibiotics and affects the affinity of P. aeruginosa 30s ribosomal subunit. The involved enzymes are phosphoryltransferase (APH), adenyltransferases or nucleotidyltransferase (AADoANT) and acetyltransferase (AAC); methylation cases are also described in the 16s subunit of ribosomal RNA (9,10). Fluoroquinolone resistance is caused by changes in the DNA gyrase (affectation of genes gyrA and parC) and topoisomerase IV and active efflux systems (11).
This bacterium is inherently resistant to penicillin, aminopenicillins —β-lactamases inhibitors—, first and second generation cephalosporins, ceftriaxone, cefotaxime, oral third generation cephalosporins, chloramphenicol, nitrofurantoin, sulfonamides, trimethoprim, tetracyclines, novobiocin and nalidixic acid (1). Although there are cases of resistance to colistin, these mechanisms are still unknown and have been linked to alterations in the regulatory protein PmrA or outer membrane protein OprH; however, most strains remain sensitive (12).
In recent decades, there has been a global spread of bacterial resistance, considered as a growing and complex emergency, which was declared a public health problem in 1998 by the World Health Organization (WHO) (13).
The use of broad-spectrum antimicrobials has caused the onset of multidrug-resistant strains and, despite the importance of the mechanisms of resistance and their continuous identification, few antibacterial agents have been developed, leaving a limited number of therapeutic options for the management of patients with P. aeruginosa (14).
The techniques used for identification of resistance mechanisms include phenotypic and genotypic or geneticoes. The first is based on the sensitivity to antibiotics of different bacteria and its main objective is to guide the physician when deciding the ideal antibiotic treatment according to patient clinic and history. This technique is performed using the antibiogram, where the sensitivity of bacteria to different antibiotics is obtained in vitro, thus predicting efficacy in vivo through a qualitative or quantitative result, which will suggest whether the bacteria is sensitive or resistant to an antibiotic and will also determine minimum inhibitory concentration (MIC).
Based on MIC values established by various committees that take into account the microbiological, pharmaceutical and clinical efficacy properties, some breakpoints are set on susceptibility tests and interpretation is defined as suceptible, intermediate or resistant. These committees include the Clinical & Laboratory Standards Institute in the U.S., which is the basis for this study.
Based on the results of the sensitivity tests, the interpretative reading of the antibiogram was performed; this tool was described by Patrice Courvaline in 1992, and takes into account three foundations: a) phenotypic characterization of resistance according to the study of sensitivity regarding antibiotic groups of the same family, b) deduction based on the corresponding resistance phenotype of the involved biochemical mechanism, and c) inference of the phenotype previously established from the resistance mechanism deduced (1,15)
The Fundación HOMI is an exclusively pediatric institution with 305 beds: 15 beds for the pediatric intensive care unit (PICU), 24 for pediatric intermediate care, 12 for the neonatal intensive care unit (NICU), 49 for the oncohematology service, 6 for hematopoietic stem cell transplantation, 12 for the burn unit and the rest for general pediatrics. This capability makes the institution a center of national reference that treats patients at high risk of infection with P. aeruginosa.
The institutional report of 2013, provided by the Group for the Control of Antimicrobial Resistance in Bogotá (GREBO, by its acronym in Spanish), reported a frequency of infection by P. aeruginosa in PICU of 7% in a total of 250 isolates and ranking sixth among other germs; in the non-ICU area, the infection rate was also 7% for a total of 804 isolates, ranking fifth. The same report showed antibiotic resistance to piperacillin tazobactam of 8.2%, cefepime of 6.8%, imipenem of 14.1% and meropenem 11.4%.
This paper seeks to understand the resistance profiles of isolates of P. aeruginosa and to infer the resistance mechanisms prevalent in the institution in order to design control measures to expand and optimize the antimicrobial management through knowledge of institutional susceptibility to this germ.
Materials and methods
This was a descriptive cross-sectional study.
Definition of study subjects
Population: all patients hospitalized in HOMI during 2006 and 2014 with antibiotic susceptibility reports testing positive for P. aeruginosa.
Sample: all antibiograms obtained.
Unit of analysis: antibiograms report.
Inclusion criteria: antibiograms reports testing positive for P. aeruginosa in patients hospitalized in HOMI during 2006 and 2014, identified through the Vitek (Biomereux, France) automated system.
Exclusion criteria: incomplete antibiograms reports testing positive for P. aeruginosa in hospitalized patients and reports of patients evaluated by outpatient consultation in HOMI.
Description of the interventions
Susceptibility testing reports were identified, and the variables sex, age, date and place of hospitalization of patients at the time of sampling were considered.
When susceptibility testing reports were identified, an interpretive reading was made and possible mechanisms of bacterial resistance were suggested.
Procedures
1. Identification of antibiograms: positive reports were sought in the WHONET 5.6® database.
2. Deduction of the resistance mechanism: through the antibiogram, the analysis of bacterial phenotype and the identification of possible mechanisms of resistance were conducted; for analyzing the resistance profiles, the WHONET 5.6® software was used, and for interpretation of the criteria, the CLSI 2012 standards were taken into account (16).
Sensitivity tests were analyzed for five antibiotics: piperacillin tazobactam (PTZ), cefepime (FEP), ceftazidime (CAZ), imipenem (IPM) and meropenem (MEM).The interpreted reading was based on the paper by Vila et al. (1), which was modified taking into account the antibiogram report obtained at HOMI (Table 1 and 2).
Table 1. Enzymatic resistance mechanisms.
|
Resistance phenotypes |
|||||
|
PTZ |
CAZ |
CEF |
IMP |
MER |
Inferred resistance mechanism |
|
S |
S |
S |
S |
S |
Natural |
|
I |
I |
S/I |
S |
S |
Partial AmpC derepression |
|
R |
R |
I/R |
S |
S |
Total AmpC derepression |
|
S |
S |
S/I |
S |
S |
Class A β-lactamase- Non-ESBL |
|
S/I |
R |
R |
S |
S |
Class A β-lactamase- ESBL |
|
S |
R |
R |
I/R |
I/R |
GES-2 |
|
I/R |
S |
R |
S |
S |
OXA-1, OXA-31 |
|
R |
R |
I/R |
S |
S |
OXAs- ESBL |
|
R |
R |
R |
I/R |
I/R |
Metallo-β- lactamase |
PTZ: piperacillin tazobactam; CAZ: ceftazidime; CEF: cefepime; IMP: imipenem; MER: meropenem; R: resistant; S: suceptible; I: intermediate. Source: (1).
Table 2. Deficiency of porins and active efflux systems.
|
Resistance phenotypes |
|||||
|
PTZ |
CAZ |
CEF |
IMP |
MER |
Resistance mechanism |
|
S |
S |
S |
R |
I |
Porin OprD Loss |
|
I/R |
I/R |
I/R |
S |
I |
MexAB-OprM system |
|
I/R |
I/R |
R |
S |
S |
MexCD-OprJ system |
|
I/R |
I/R |
I/R |
R |
I |
MexEF-OprN system |
|
I/R |
I/R |
I/R |
S |
S |
MexXY-OprM system |
PTZ: piperacillin tazobactam; CAZ: ceftazidime; CEF: cefepime; IMP: imipenem; MER: meropenem R: resistant; S: suceptible; I: intermediate. Source: (1).
Statistical analysis
A descriptive analysis of absolute and relative frequencies for qualitative and quantitative variables was performed. For the latter, central tendency and dispersion measures were calculated.
Ethical considerations
Since the study is observational, it was classified as an investigation without risk as stipulated in Resolution 8430 of 1993 by the Ministry of Health (17). The protocol was taken to the Scientific Research and Teaching Unit of the Department of Pediatrics at Universidad Nacional de Colombia and to the Ethics Committee of Fundación HOMI, where it was evaluated and approved.
Results
During the six years of the study, 463 reports were obtained for positive antibiograms of P. aeruginosa strains, mostly in male patients (61.6%). 62.9% of the samples were obtained from lactating patients, 15.1% from preschool children, 14.5% from schoolchildren and 7.6% from adolescents. The most frequent isolation sites were blood (25.5%), urine (20.7%) and tracheobronchial secretion (13.8%) (Table 3). The most frequent isolation area in the hospital was the intensive care unit (29.4%), followed by general hospitalization (28.1%) and emergency room (16.6%). The most common phenotype was natural (66.5%), followed by partial and full AmpC derepression (8.4% and 8.2%) and OprD porin loss (6%).
The resistance rates found for each antibiotic by hospital area were: meropenem in PICU, 7%; in NICU, 3.5%, and in ICU, 8.4%. Cefepime in PICU was 11.9%; in NICU, 3.5%, and non-ICU, 1.4%. Piperacillin tazobactam in PICU was 12.1%; no resistance was found in NICU, and in ICU, 14.3%.
Table 4 shows that the most common phenotype in critical care areas was natural (57%), followed by porin OprD loss (12%) and partial and total AmpC derepression (9.5% and 8.9%, respectively). In non-critical hospital areas, natural phenotype was the most frequent (71.5%), followed by partial and total AmpC derepression (7.9% each) and metallo-β-lactamase (6.6%).
Table 5 shows that the most common phenotype was natural in all critical units, being predominant in pediatric intensive care (56.6%). In general, the most common phenotypes were porin OprD loss and AmpC derepression in the PICU; partial derepression of AmpC and Class A β-lactamase non-ESBLs in NICU; total and partial AmpC derepression in the oncology unit; metallo-β-lactamase, followed by total or partial AmpC derepression and porin OprD loss in general hospitalization; metallo-β-lactamase and partial AmpC derepression in hospitalization for surgery; total AmpC derepression and OXA-1, OXA-31 in the burn unit, and total and partial AmpC derepression and porin OprD loss in emergency room.
Table 3. Distribution of isolates of Pseudomonas aeruginosa, according to sample type, service and resistance phenotype, 2006-2014.
|
Variables |
Categories |
n |
% |
|
Type of sample |
Blood |
118 |
25.5 |
|
Urine |
96 |
20.7 |
|
|
Tracheobronchial |
80 |
17.3 |
|
|
Skin |
64 |
13.8 |
|
|
Stool |
30 |
6.5 |
|
|
Peritoneum |
26 |
5.6 |
|
|
Catheter |
25 |
5.4 |
|
|
Cerebrospinal fluid |
9 |
1.9 |
|
|
Bone |
9 |
1.9 |
|
|
Others |
4 |
0.9 |
|
|
Pleural fluid |
2 |
0.4 |
|
|
Hospital isolation area |
Pediatric critical care units |
136 |
29.4 |
|
General hospitalization |
130 |
28.1 |
|
|
Emergency room |
77 |
16.6 |
|
|
Surgery |
57 |
12.3 |
|
|
Oncology |
25 |
5.4 |
|
|
Neonatal critical care units |
22 |
4.8 |
|
|
Burn unit |
15 |
3.2 |
|
|
Hematopoietic transplant unit |
1 |
0.2 |
|
|
Phenotype |
Natural |
308 |
66.5 |
|
Partial AmpC derepression |
39 |
8.4 |
|
|
Total AmpC derepression |
38 |
8.2 |
|
|
Porin OprD loss |
28 |
6.0 |
|
|
Metallo-β-lactamase |
26 |
5.6 |
|
|
Class A β-lactamase- No ESBL |
15 |
3.2 |
|
|
Class A β-lactamase- ESBL |
3 |
0.6 |
|
|
OXA-1, OXA-31 |
2 |
0.4 |
|
|
MexEF-OprN system |
2 |
0.4 |
|
|
GES-2 |
1 |
0.2 |
|
|
MexXY-OprM system |
1 |
0.2 |
|
|
Total |
463 |
100 |
|
Source: Own elaboration based on the data obtained in the study.
Table 4. Phenotype frequency distribution according to hospital isolation of Pseudomonas aeruginosa, 2006-2014.
|
Variable |
Categories |
Hospital area |
Total |
||||
|
Critical care |
Non-critical care |
||||||
|
n |
% |
n |
% |
n |
% |
||
|
Phenotype |
Natural |
90 |
57.0 |
218 |
71.5 |
308 |
66.5 |
|
Porin OprD loss |
19 |
12.0 |
9 |
3.0 |
28 |
6.0 |
|
|
Partial AmpC derepression |
15 |
9.5 |
24 |
7.9 |
39 |
8.4 |
|
|
Total AmpC derepression |
14 |
8.9 |
24 |
7.9 |
38 |
8.2 |
|
|
Metallo-β-lactamase |
10 |
6.3 |
16 |
5.2 |
26 |
5.6 |
|
|
Class A β-lactamase- No ESBL |
7 |
4.4 |
8 |
2.6 |
15 |
3.2 |
|
|
Class A β-lactamase- ESBL |
1 |
0.6 |
2 |
0.7 |
3 |
0.6 |
|
|
GES-2 |
1 |
0.6 |
0 |
0.0 |
1 |
0.2 |
|
|
MexEF-OprN system |
1 |
0.6 |
1 |
0.3 |
2 |
0.4 |
|
|
OXA-1, OXA-31 |
0 |
0.0 |
2 |
0.7 |
2 |
0.4 |
|
|
MexXY-OprM system |
0 |
0.0 |
1 |
.3 |
1 |
0.2 |
|
|
Total |
158 |
100 |
305 |
100 |
463 |
100 |
|
Source: Own elaboration based on the data obtained in the study.
Table 6 shows that the natural phenotype predominated in all samples except in pleural fluid, where derepression of AmpC and metallo-β-lactamase phenotypes were predominant. Skin, urine and blood presented natural phenotype, followed by AmpC derepression and metallo-β-lactamases in the two last tissues. In tracheobronchial secretion and blood, after the natural phenotype, the most frequent was porin OprD loss, and in catheter, natural phenotype was followed by class A β-lactamase non-ESBL phenotype.
Discussion
P. aeruginosa is found repeatedly in patients within a specific context —hospitalized, immunocompromised, treated with broad-spectrum antibiotics, carriers of instrumented or prosthetic materials, patients with severe infection, prolonged hospitalization and presence of cross infections— so it constitutes the cause of hospital-acquired infections (HAI) (6). The prognosis of infection, compared with other agents, is reserved because the antibiotic treatment may not be effective in many cases, even in patients treated properly and early (3).
This bacterium is a lethal pathogen that is credited with 35% mortality in bacteremia and 69% in ventilator-associated pneumonia (18). This high mortality rate represents the potential to improve therapies and interventions.
In this study, the collected sample is important since it found that male infants are the patients with greater isolation of the pathogen; such isolation was seen in blood, urine and tracheobronchial secretion, and the most common hospital areas were PICU, general hospitalization and emergency department.
Table 5. Frequency distribution of the resistance phenotype of Pseudomonas aeruginosa according to the isolation area, 2006-2014.
|
Variable |
Categories |
Hospital isolation area |
Total |
||||||||||||||||
|
Pediatric critical care units |
Neonatal critical care units |
Oncology |
Hematopoietic transplant unit |
General hospitalization |
Surgery |
Burn unit |
Emergency Room |
||||||||||||
|
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
||
|
Phenotype |
Natural |
77 |
56.6 |
13 |
59.1 |
19 |
76 |
1 |
100 |
93 |
71.5 |
36 |
63.2 |
11 |
73.3 |
58 |
75.30 |
308 |
66.50 |
|
Porin OprD loss |
19 |
14 |
0 |
0 |
1 |
4 |
0 |
0 |
2 |
1.5 |
3 |
5.3 |
0 |
0 |
3 |
3.90 |
28 |
6.00 |
|
|
Total AmpC derepression |
13 |
9.6 |
1 |
4.5 |
3 |
12 |
0 |
0 |
8 |
6.2 |
3 |
5.3 |
3 |
20 |
7 |
9.10 |
38 |
8.20 |
|
|
Partial AmpC derepression |
11 |
8.1 |
4 |
18.2 |
2 |
8 |
0 |
0 |
12 |
9.2 |
6 |
10.5 |
0 |
0 |
4 |
5.20 |
39 |
8.40 |
|
|
Metallo-β-lactamase |
9 |
6.6 |
1 |
4.5 |
0 |
0 |
0 |
0 |
6 |
4.6 |
8 |
14 |
0 |
0 |
2 |
2.60 |
26 |
5.60 |
|
|
Class A β-lactamase- Non- ESBL |
4 |
2.9 |
3 |
13.6 |
0 |
0 |
0 |
0 |
5 |
3.8 |
1 |
1.8 |
0 |
0 |
2 |
2.60 |
15 |
3.20 |
|
|
Class A β-lactamase- ESBL |
1 |
0.7 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
1.5 |
0 |
0 |
0 |
0 |
0 |
0.00 |
3 |
0.60 |
|
|
GES-2 |
1 |
0.7 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0.00 |
1 |
0.20 |
|
|
MexEF-OprN system |
1 |
0.7 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0.8 |
0 |
0 |
0 |
0 |
0 |
0.00 |
2 |
0.40 |
|
|
OXA-1, OXA-31 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
6.7 |
1 |
1.30 |
2 |
0.40 |
|
|
MexXY-OprM system |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0.8 |
0 |
0 |
0 |
0 |
0 |
0.00 |
1 |
0.20 |
|
|
Total |
136 |
100 |
22 |
100 |
25 |
100 |
1 |
100 |
130 |
100 |
57 |
100 |
15 |
100 |
77 |
100 |
463 |
100 |
|
Source: Own elaboration based on the data obtained in the study.
Table 6. Frequency distribution of Pseudomonas aeruginosa strains according to tissue insulation, 2006-2014.
|
Variable |
Category |
Isolation tissue |
Total |
||||||||||||||||||||||
|
Skin |
Tracheo bronchial |
Cerebrospinal fluid |
Catheter |
Peritoneum |
Pleural fluid |
Bone |
Blood |
Urine |
Stool |
Others |
|||||||||||||||
|
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
n |
% |
||
|
Phenotype |
Natural |
40 |
62.5 |
45 |
56.3 |
7 |
77.8 |
18 |
72 |
23 |
88.5 |
|
|
4 |
44.4 |
74 |
62.7 |
76 |
79.2 |
19 |
63.3 |
2 |
50 |
308 |
66.50 |
|
Partial AmpC derepression |
8 |
12.5 |
8 |
10 |
1 |
11.1 |
1 |
4 |
|
|
1 |
50 |
2 |
22.2 |
8 |
6.8 |
7 |
7.3 |
3 |
10 |
|
|
39 |
8.40 |
|
|
Total AmpC derepression |
3 |
4.7 |
7 |
8.8 |
1 |
11.1 |
4 |
16 |
2 |
7.6 |
|
|
|
|
9 |
7.6 |
5 |
5.2 |
5 |
16.7 |
2 |
50 |
38 |
8.20 |
|
|
Class A β-lactamase- Non- ESBL |
2 |
3.1 |
4 |
5 |
|
|
1 |
4 |
1 |
3.8 |
|
|
|
|
7 |
5.9 |
|
|
|
|
|
|
15 |
3.20 |
|
|
Class A β-lactamase- ESBL |
|
|
1 |
1.3 |
|
|
|
|
|
|
|
|
|
|
2 |
1.7 |
|
|
|
|
|
|
3 |
0.60 |
|
|
GES-2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
0.8 |
|
|
|
|
|
|
1 |
0.20 |
|
|
OXA-1, OXA-31 |
1 |
1.6 |
1 |
1.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
0.40 |
|
|
Metallo-β-lactamase |
5 |
7.8 |
4 |
5 |
|
|
|
|
|
|
1 |
50 |
3 |
33.3 |
7 |
5.9 |
5 |
5.2 |
1 |
3.3 |
|
|
26 |
5.60 |
|
|
Porin OprD loss |
5 |
7.8 |
9 |
11.3 |
|
|
1 |
4 |
|
|
|
|
|
|
10 |
8.5 |
2 |
2.1 |
1 |
3.3 |
|
|
28 |
6.00 |
|
|
MexEF-OprN system |
|
|
1 |
1.3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
3.3 |
|
|
2 |
0.40 |
|
|
MexXY-OprM system |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
1 |
|
|
|
|
1 |
0.20 |
|
|
Total |
64 |
100 |
80 |
100 |
9 |
100 |
25 |
100 |
26 |
100 |
2 |
100 |
9 |
100 |
118 |
100 |
96 |
100 |
30 |
100 |
4 |
100 |
463 |
100 |
|
Source: Own elaboration based on the data obtained in the study.
Discussion
P. aeruginosa is found repeatedly in patients within a specific context —hospitalized, immunocompromised, treated with broad-spectrum antibiotics, carriers of instrumented or prosthetic materials, patients with severe infection, prolonged hospitalization and presence of cross infections— so it constitutes the cause of hospital-acquired infections (HAI) (6). The prognosis of infection, compared with other agents, is reserved because the antibiotic treatment may not be effective in many cases, even in patients treated properly and early (3).
This bacterium is a lethal pathogen that is credited with 35% mortality in bacteremia and 69% in ventilator-associated pneumonia (18). This high mortality rate represents the potential to improve therapies and interventions.
In this study, the collected sample is important since it found that male infants are the patients with greater isolation of the pathogen; such isolation was seen in blood, urine and tracheobronchial secretion, and the most common hospital areas were PICU, general hospitalization and emergency department.
The most common phenotype in all areas and isolates was natural, being more frequent in non-critical areas. However, other 10 phenotypes, including partial or complete AmpC derepression (resistance to narrow spectrum aminopenicillins and cephalosporins inducible by cefoxitin and imipenem, sensitive only to carbapenems, resistance to piperacillin tazobactam) porin OprD loss (resistance to carbapenems), metallo-β-lactamase (carbapenemases), class a β-lactamase non-ESBL and ESBL (resistance to carboxypenicillins, ureidopenicillins, ceftazidime, cefepime, cefpirome and aztreonam), OXA-1, OXA-31 (no inhibition by clavulanic acid, sulbactam or tazobactam, with hydrolytic activity in ceftazidime, cefepime, cefpirome and aztreonam), GES-2, and MexEF-OprN and MexXY-OprM systems (possible involvement in the activity of beta-lactams, carbapenems, fluoroquinolones, macrolides, tetracyclines, chloramphenicol, novobiocin and lincomycin) (8).
It is noteworthy that PICU, a place where the use of carbapenems is high, the loss of porin OprD and AmpC derepression were the most frequent resistance mechanisms. In the oncology unit, the service in which the use of carbapenems is restricted to unstable patients, total and partial derepression of AmpC were more frequent.
In general hospitalization services, the most common phenotype was metallo-β-lactamase, followed by partial AmpC derepression and OprD porin loss, which can be related to the pressure for selection to which patients treated there are subjected, including oncohematology and rheumatology undergoing immunosuppression. In surgery hospitalization, metallo-β-lactamase and partial AmpC derepression were more frequent, which can be correlated with the arrival of critically ill patients who require a surgical procedure for improvement, prior to the empirical use of broad-spectrum antibiotics, leading to selection pressure.
In the burn unit, the phenotype AmpC derepression and OXA-1, OXA-31 were more frequent due to the high probability of colonization and infection in these patients. In the emergency room, the AmpC derepression phenotype was more frequent, which can be correlated with the acquisition of the infection in non-associated health care areas, that is, this is a community phenotype.
This study shows that multiple resistance phenotypes in a non-negligible percentage can occur simultaneously and confirms that infection with P. aeruginosa is polyclonal; one of the main causes is the indiscriminate use of antibiotics at hospital level since there is a selection pressure. Therefore, knowing the rates of resistance and the institutional phenotype profile allows optimizing the selection of antimicrobials in the institution and establishing which antibiotic of choice is in each of the services, especially in an empirical manner.
HOMI is a pediatric referral institution in Colombia that handles a wide range of highly complex pathologies; there, the characterization of P. aeruginosa is essential in the context of rational use of antibiotics and to reduce the resistant strains of the institution.
This work has biases derived from secondary sources originated by bacterial isolates and, as only single records of the antibiograms were analyzed, it is not possible to differentiate between colonization and infection. Since the inference is interpreted according to the reading of the antibiogram, molecular techniques should be applied to confirm the findings.
This study is derived from a thesis that is in the repository of Universidad Nacional de Colombia (19).
Funding
This study was funded by the national program of projects to strengthen research, development and innovation in graduate programs at Universidad Nacional de Colombia (Project Code 19325) and the Program for Pediatric Research María Josefa Cualla from Fundación HOMI.
Conflict of interests
None stated by the authors.
Acknowledgements
The research team would like to express their gratitude to Universidad Nacional de Colombia, the Fundación HOMI and the Group for the Control of Antimicrobial Resistance in Bogotá for their support during the activities that allowed the production and distribution of this work. Similarly, to Chief Sandra Liliana Romero, nurse coordinator of the infections committee, and Dr. Yuli Andrea Olarte, bacteriologist of the Fundanción HOMI.
References
1.Vila J, Marco F. Lectura interpretada del antibiograma de bacilos gramnegativos no fermentadores. Enferm. Infecc. Microbiol. Clin. 2010;28(10):726-36. http://doi.org/b544h4.
2.Sigurdsson G, Fleming RM, Heinken A, Thiele I. A Systems Biology Approach to Drug Targets in Pseudomonas aeruginosa Biofilm. PLoS ONE. 2012;7(4):e34337. http://doi.org/bp8d.
3.Fariñas MC, Martínez-Martínez L. Infecciones causadas por bacterias gramnegativas multirresistentes: enterobacterias, Pseudomonas aeruginosa, Acinetobacter baumannii y otros bacilos gramnegativos no fermentadores. Enferm. Infecc. Microbiol. Clin. 2013;31(6):402-9. http://doi.org/f2j56t.
4.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959-64. http://doi.org/cr53sh.
5.Lister PD, Wolter DJ, Hanson ND. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009;22(4):582-610. http://doi.org/fq5fp5.
6.Martínez-Martínez L, Calvo J. El problema creciente de la resistencia antibiótica en bacilos gramnegativos: situación actual. Enferm. Infecc. Microbiol. Clin. 2010;28(Suppl 2):25-31. http://doi.org/cb4cgr.
7.Mesaros N, Nordmann P, Plésiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, et al. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect. 2007;13(6):560-78. http://doi.org/c2w6z8.
8.Strateva T, Yordanov D. Pseudomonas aeruginosa - a phenomenon of bacterial resistance. J. Med. Microbiol. 2009;58(Pt 9):1133-48.
http://doi.org/dxrqbz.
9.Doi Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 2007;45(1):88-94. http://doi.org/fm3q6k.
10.Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. Substrate Specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM Efflux Pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2000;44(12):3322-7. http://doi.org/d6x6vr.
11.Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis. 2001;7(2):337-41. http://doi.org/d553xg.
12.Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin. Microbiol. Rev. 2008;21(3):449-65. http://doi.org/c8jmtn.
13.Leal A. Boletin informativo, GREBO. Suplemento 2. Bogotá, D.C.: Grupo para el Control de la Resistencia Bacteriana de Bogotá; 2010.
14.Fenner L, Richet H, Raoult D, Papazian L, Martin C, La Scola B. Are clinical isolates of Pseudomonas aeruginosa more virulent than hospital environmental isolates in amebal co-culture test? Crit. Care. Med. 2006;34(3):823-8. http://doi.org/cmzcd7.
15.Cantón R. Lectura interpretada del antibiograma: una necesidad clínica. Enferm. Infecc. Microbiol. Clin. 2010;28(6):375-85. http://doi.org/dbw48z.
16.Clinical and Laboratory Standards Institute. Performance Standards for antimicrobial susceptibility Testing; Twenty-Second Informational Supplement. Wayne: M100-S22. Vol 31 No. 1; 2012.
17.Colombia. Ministerio de Salud. Resolución 8430 de 1993 (octubre 4): Por el cual se establecen normas científicas, técnicas y administrativas para la investigación en salud. Bogotá, D.C. octubre 4 de 1993 [cited 2016 Sep 5]. Available from: https://goo.gl/VxO6Zu.
18.Rello J, Kollef MH, Díaz E, Rodríguez A. Infectious Diseases in Critical Care. New York: Springer; 2007.
19.Arango-Alvarado JJ. Perfil epidemiológico, clínico, fenotípico, y genético de Pseudomonas aeruginosa en la Fundación Hospital de la Misericordia durante los años 2013-2014. [Tesis]. Bogotá, D.C.: Universidad Nacional de Colombia; 2014 [cited 2016 Sep 27]. Available from: https://goo.gl/WNUX4Z.
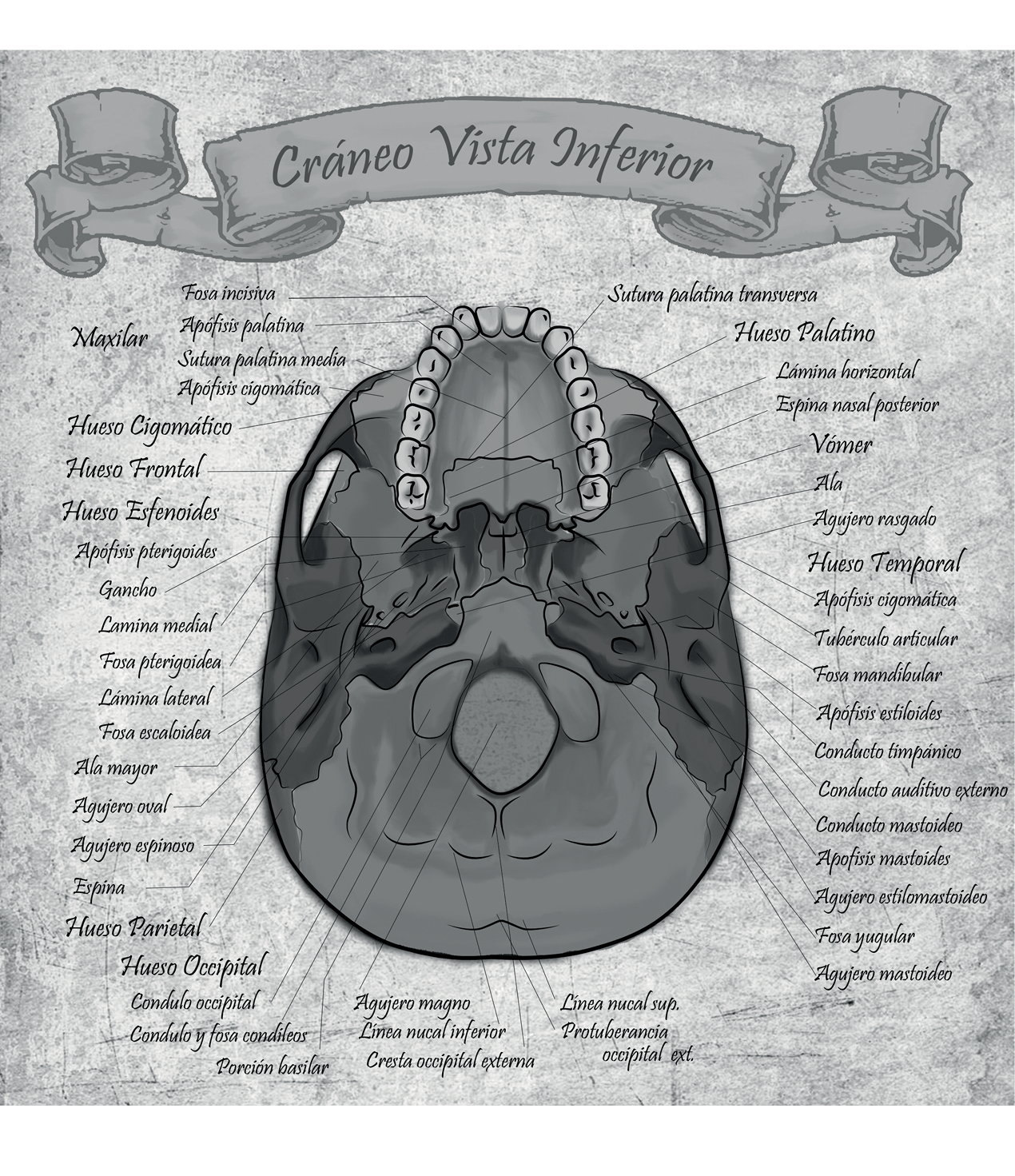
César Alexander Eslava Franco
“Mapas anatómicos”
Universidad Nacional de Colombia
Referencias
Vila J, Marco F. Lectura interpretada del antibiograma de bacilos gramnegativos no fermentadores. Enferm. Infecc. Microbiol. Clin. 2010;28(10):726-36. http://doi. org/b544h4.
Sigurdsson G, Fleming RM, Heinken A, Thiele I. A Systems Biology Approach to Drug Targets in Pseudomonas aeruginosa Biofilm. PLoS ONE. 2012;7(4):e34337. http://doi. org/bp8d.
Fariñas MC, Martínez-Martínez L. Infecciones causadas por bacterias gramnegativas multirresistentes: enterobacterias, Pseudomonas aeruginosa, Acinetobacter baumannii y otros bacilos gramnegativos no fermentadores. Enferm. Infecc. Microbiol. Clin. 2013;31(6):402-9. http://doi. org/f2j56t.
Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959-64. http://doi. org/cr53sh.
Lister PD, Wolter DJ, Hanson ND. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009;22(4):582-610. http://doi. org/fq5fp5.
Martínez-Martínez L, Calvo J. El problema creciente de la resistencia antibiótica en bacilos gramnegativos: situación actual. Enferm. Infecc. Microbiol. Clin. 2010;28(Suppl 2):25-31. http://doi. org/cb4cgr.
Mesaros N, Nordmann P, Plésiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, et al. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect. 2007;13(6):560-78. http://doi. org/c2w6z8.
Strateva T, Yordanov D. Pseudomonas aeruginosa - a phenomenon of bacterial resistance. J. Med. Microbiol. 2009;58(Pt 9):1133-48.
http://doi. org/dxrqbz.
Doi Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 2007;45(1):88-94. http://doi. org/fm3q6k.
Masuda N, Sakagawa E, Ohya S, Gotoh N, Tsujimoto H, Nishino T. Substrate Specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM Efflux Pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2000;44(12):3322-7. http://doi. org/d6x6vr.
Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis. 2001;7(2):337-41. http://doi. org/d553xg.
Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin. Microbiol. Rev. 2008;21(3):449-65. http://doi. org/c8jmtn.
Leal A. Boletin informativo, GREBO. Suplemento 2. Bogotá, D. C. : Grupo para el Control de la Resistencia Bacteriana de Bogotá; 2010.
Fenner L, Richet H, Raoult D, Papazian L, Martin C, La Scola B. Are clinical isolates of Pseudomonas aeruginosa more virulent than hospital environmental isolates in amebal co-culture test? Crit. Care. Med. 2006;34(3):823-8. http://doi. org/cmzcd7.
Cantón R. Lectura interpretada del antibiograma: una necesidad clínica. Enferm. Infecc. Microbiol. Clin. 2010;28(6):375-85. http://doi. org/dbw48z.
Clinical and Laboratory Standards Institute. Performance Standards for antimicrobial susceptibility Testing; Twenty-Second Informational Supplement. Wayne: M100-S22. Vol 31 No. 1; 2012.
Colombia. Ministerio de Salud. Resolución 8430 de 1993 (octubre 4): Por el cual se establecen normas científicas, técnicas y administrativas para la investigación en salud. Bogotá, D. C. octubre 4 de 1993 [cited 2016 Sep 5]. Available from: https://goo. gl/VxO6Zu.
Rello J, Kollef MH, Díaz E, Rodríguez A. Infectious Diseases in Critical Care. New York: Springer; 2007.
Arango-Alvarado JJ. Perfil epidemiológico, clínico, fenotípico, y genético de Pseudomonas aeruginosa en la Fundación Hospital de la Misericordia durante los años 2013-2014. [Tesis]. Bogotá, D. C. : Universidad Nacional de Colombia; 2014 [cited 2016 Sep 27]. Available from: https://goo. gl/WNUX4Z.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Valmir Nascimento Rastely-Junior, Hosanea Santos Nascimento Rocha, Mitermayer Galvão Reis. (2025). AmpC β-Lactamase-Producing Microorganisms in South American Hospitals: A Meta-Regression Analysis, Meta-Analysis, and Review of Prevalence. Tropical Medicine and Infectious Disease, 10(10), p.280. https://doi.org/10.3390/tropicalmed10100280.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Revista de la Facultad de Medicina

Esta obra está bajo una licencia Creative Commons Reconocimiento 3.0 Unported.
-