Cancer and mitochondrial function
El cáncer en la función mitocondrial
DOI:
https://doi.org/10.15446/revfacmed.v66n1.59898Palabras clave:
Cancer, Glycolysis, Mitochondria, Neovascularization pathologic (en)Cáncer, Mitocondria, Neovascularización patológica, Glucólisis (es)
Descargas
Se ha descrito que algunas alteraciones del metabolismo están asociadas con la pérdida de función mitocondrial en células tumorales. Aún se discute si tal pérdida se evidencia en la función o si la célula brinda máxima estabilidad a sus funciones, se requieren más estudios para conocer el comportamiento del cáncer en la mitocondria. Cuando tiene limitación de oxígeno y mutaciones en oncogenes, genes supresores de tumor y enzimas de la vía glucolítica o del metabolismo oxidativo mitocondrial, la célula tumoral permite la formación de un cáncer agresivo. Este artículo es producto de la revisión bibliográfica de la evidencia científica que se ha presentado en las últimas investigaciones respecto al cáncer y la función mitocondrial.
Metabolism alterations are associated with the loss of mitochondrial function in tumor cells. Current research discuss whether such loss is evident in function itself, or if cells can provide maximum stability to its functions. More studies are needed to determine the behavior of cancer in mitochondria. Tumor cells experience a limitation of oxygen and mutations in oncogenes, tumor suppressor genes and enzymes of the glycolytic pathway and/or mitochondrial oxidative metabolism, thus allowing the formation of aggressive cancer. This article is the result of a literature review of the scientific evidence that has been presented in the latest research on cancer and mitochondrial function.
artículo de reflexión
DOI: https://doi.org/10.15446/revfacmed.v66n1.59898
Cancer and mitochondrial function
El cáncer en la función mitocondrial
Recibido: 3/9/2016. Aceptado: 28/10/2016.
Sofía Isabel Freyre-Bernal1 • Jhan Sebastian Saavedra-Torres2,3 • Luisa Fernanda Zúñiga-Cerón2,3 • Wilmer Jair Díaz-Córdoba2,3 • María Virginia Pinzón-Fernández4
1 Universidad del Cauca - Faculty of Health Sciences - Department of Physiological Sciences - Popayán - Colombia.
2 Corporación Del Laboratorio al Campo - Research Seedling Unit - Popayán - Colombia.
3 Universidad del Cauca - Faculty of Health Sciences - Health Research Group - Popayán - Colombia.
4 Universidad del Cauca - Faculty of Health Sciences - Internal Medicine Department - Popayán - Colombia.
Corresponding author: Jhan Sebastian Saavedra-Torres. Health Research Group, Faculty of Health Sciences, Universidad del Cauca. Colombia, Cauca. Carrera 6 Nº 13N-50 de Popayán, sector de La Estancia. Telephone number: 8209870-8209800 ext. 2717
Email: jhansaavedra@unicauca.edu.co.
| Abstract |
Metabolism alterations are associated with the loss of mitochondrial function in tumor cells. Current research discuss whether such loss is evident in function itself, or if cells can provide maximum stability to its functions. More studies are needed to determine the behavior of cancer in mitochondria. Tumor cells experience a limitation of oxygen and mutations in oncogenes, tumor suppressor genes and enzymes of the glycolytic pathway and/or mitochondrial oxidative metabolism, thus allowing the formation of aggressive cancer. This article is the result of a literature review of the scientific evidence that has been presented in the latest research on cancer and mitochondrial function.
Keywords: Cancer; Glycolysis; Mitochondria; Neovascularization pathologic (MeSH).
Freyre-Bernal SI, Saavedra-Torres JS, Zúñiga-Cerón LF, Díaz-Córdoba WJ, Pinzón-Fernández MV. Cancer and mitochondrial function. Rev. Fac. Med. 2018;66(1):83-6. English. doi:
https://doi.org/10.15446/revfacmed.v66n1.59898.
| Resumen |
Se ha descrito que algunas alteraciones del metabolismo están asociadas con la pérdida de función mitocondrial en células tumorales. Aún se discute si tal pérdida se evidencia en la función o si la célula brinda máxima estabilidad a sus funciones, se requieren más estudios para conocer el comportamiento del cáncer en la mitocondria. Cuando tiene limitación de oxígeno y mutaciones en oncogenes, genes supresores de tumor y enzimas de la vía glucolítica o del metabolismo oxidativo mitocondrial, la célula tumoral permite la formación de un cáncer agresivo. Este artículo es producto de la revisión bibliográfica de la evidencia científica que se ha presentado en las últimas investigaciones respecto al cáncer y la función mitocondrial.
Palabras clave: Cáncer; Mitocondria; Neovascularización patológica; Glucólisis (DeCS).
Freyre-Bernal SI, Saavedra-Torres JS, Zúñiga-Cerón LF, Díaz-Córdoba WJ, Pinzón-Fernández MV. [El cáncer en la función mitocondrial]. Rev. Fac. Med. 2018;66(1):83-6. English. doi:
https://doi.org/10.15446/revfacmed.v66n1.59898.
Introduction
The genes which encode the machinery that generates energy in the mitochondria are tumor suppressors; when they do not function properly, some processes and pathways that lead to cancer may be triggered. Mitochondria are organelles that have mitochondrial DNA (mtDNA), which is inherited only from the mother during the fertilization process. These organelles are the “energetic generators” of healthy cells, such as those that have their metabolisms encoded to be apoptosis inhibitors. (1,2)
Mitochondria regulate and coordinate apoptosis activation, hence their importance for the study and research on therapies against cancer. When mitochondria deregulate, the onset of diseases such as cancer is stimulated due to an increased catabolic process, not to mention their role in neurodegenerative diseases, which are associated with abnormal mitochondrial function and apoptosis. (1,2)
Mitochondria and cancer
mtDNA mutagenesis is involved in a wide arrange of tumor processes, including renal adenocarcinoma, colon cancer, head and neck tumors, astrocytic tumors, thyroid tumors, breast tumors, ovarian, prostate and bladder cancer tumors, neuroblastomas, and oncocytomas. Many mtDNA mutations in cancer cells clearly inhibit oxidative phosphorylation. Although some of these cancers have ancestral polymorphisms associations, others may be cancer cell mutations. (3-6) (table 1).
Cancer cells acquire enough ATP to support proliferation and to function endlessly, which has captivated scientists for nearly a century. Otto Warburg et al. conducted the first quantitative study on cancer cell metabolism in the 1920s. (7,8)
Table 1. Generalities of the role of cancer in mitochondrial function.
|
1 |
In cancer, the metabolism of mitochondria reinforces antioxidant defenses, in order to compensate for the activation of the anaerobic pathway and not to induce cells to apoptosis by damaging free radicals. |
|
2 |
Mitochondrial DNA encodes genes that may contribute to the development of cancer; such mutations provide metabolic adaptability to the cancer cell. |
|
3 |
Myc oncogene activation, tumor suppressor genes inactivation —such as p53—, and adaptation to hypoxia via the HIF-1 pathway are mechanisms involved in the Warburg effect. |
|
4 |
Myc activation allows mitochondria to have little depletion of the enzymes that metabolize to create energy from lipids and fatty acids. |
|
5 |
In cancer, the pyruvate dehydrogenase complex is regulated to adjust the flow of acetyl-CoA and the reduction equivalents in the mitochondria, and to sustain the aerobic glycolysis pathway as anaerobic without inducing apoptosis genes and processes. |
|
6 |
Mitochondria regulate genes and proteins through their endoplasmic reticulum to reduce the absorption of mitochondrial Ca+2, aiming to stabilize the transition pore of mitochondrial permeability, to not induce the pathways of apoptosis, and to increase cytosolic Ca+2. |
|
7 |
Mitochondrial DNA reduction is a cellular process of cancer that increases cytosolic Ca+2, which does not occur at mitochondrial level, in order to activate the signaling pathways that stimulate nuclear proteins and the membrane potential of the mitochondria. This allows invasive and tumor growth of the cell by synthesizing ATP at its maximum cellular function to create specific proteins and activate signaling pathways that allow metastasis. |
|
8 |
The energy metabolism of any proliferative cell, whether carcinogenic or not, must be based on active glycolysis. Stating that tumor cells consume more glucose than non-tumor cells is not recent. Tumor cells use and form embryonic iso-zygotes to have a very active metabolic pathway. |
|
9 |
There are several forms of regulatory glycolytic enzymes. The most predominant in the embryonic tissue are replaced by the "normal" versions at birth. It has been observed that, in cancer, the embryonic forms of hexokinase, phosphofructokinase, the N2 form of pyruvate kinase and the anaerobic (A) variety of LDH are predominant. |
Source: Own elaboration based on the data obtained in the study.
Cells can obtain energy through a process called glycolysis that consists of anaerobic fermentation, in which the waste products of that fermentative process are pyruvate and lactic acid. (9,10) Tumor tissues metabolize approximately ten times more glucose into lactate at a given time than normal tissues. (6,8) Warburg hypothesized that effective cellular respiration caused by mitochondrial damage leads to carcinogenesis. (9,10)
Warburg’s effect describes that cancer cells use glycolysis followed by lactic fermentation as an energy source, even if there is an appropriate amount of oxygen for respiration. (1,11) In other words, instead of developing a complete respiration process in the presence of adequate amounts of oxygen, cancer cells ferment and continue to mutate to preserve their tumoral domain. (12)
At the cellular level, tumors have survival advantages due to lactate secretion. (4,13,14) Lactic acid confers invasive properties to tumor cells, affecting the normal structure of tissues. (15) Additionally, the expression of vascular endothelial growth factor and its receptor (VEGF and VEGFR, respectively) responds to different stimuli to generate new blood vessels from preexisting ones. (1,16-18) VEGFα stimulates vascular endothelial cell growth, cell survival and proliferation regulated by the nuclear and mitochondrial action of the cell. Furthermore, VEGF and gene mutations leading to activate metalloproteinases to degrade the extracellular matrix allow greater metastatic action. (19) Recent studies suggest that VEGF can protect cells from apoptosis and increase their resistance to conventional chemotherapy and radiotherapy. (20,21)
Similarly, metastasis is of great importance since most of cancer deaths occur because primary cancer spreads to distant sites. In most cases, cancer patients with localized tumors have a better survival rate than patients with cancer and metastatic tumors. (22-25) It is also suggested that the increase of oncogene mutations, tumor suppressor genes and enzymes of the glycolytic pathway and/or mitochondrial oxidative metabolism (Myc, Akt, p53, HIF1-α) allow to turn cancer cells into efficient metastatic cells. (3)
The hypoxia-inducible factor (HIF-1) protein is normally activated in response to certain cellular crises, such as lack of oxygen. However, in the case of mitochondria with abnormal and tumor mechanisms, the expression of HIF-1 that is perpetuated in the presence of damage signs in the SDH gene is stimulated. This is caused by the cell following already established oncogenes guidelines to carry out a unique type of homeostasis for cancer and to supply high levels of glucose and oxygen to replicate itself without control. (1,26,27)
Variety of metabolic fuels for tumor cells
A wide variety of metabolic fuels can be observed in tumor pathways, in which tumor cells are able to utilize different bioenergetic substrates, including glutamine, glucose, fatty acids, ketone bodies, and acetate. These substrates can be provided by the stromal cells in the microenvironment. (28) Particularly, glutamine and glucose can provide building blocks for the synthesis of many biomolecules that allow the regulation of oncogenesis processes. It is worth mentioning that metabolic enzymes with mutations are found in several tumors and in the oncometabolites accumulated in different types of tumors. (28,29)
Cancer cells also show increased demand for fatty acids other than glutamine. (6,30) Fatty acids can be synthesized endogenously or taken from exogenous sources. For example, prostate tumors import less glucose than other tumors, (31) therefore, β-oxidation of fatty acids is an important source of energy. (32,33)
Moreover, two recent studies showed that acetate is a bioenergetic substrate for glioblastoma and brain metastases. (34) Catabolism in stromal and adipocyte cells provides fuel and building blocks for the anabolic growth of cancer cells through metabolic coupling. (35)
Cancer cells and independence
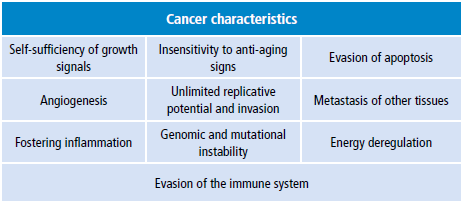
In 2000 and 2011, Hanahan and Weinberg summarized an extensive research on cancer and the top 10 characteristics of cancer and its correlation with mitochondria (table 2). (36-39) These authors describe how cancer cells can be stimulated by infectious phenomena, inflammation, viruses, toxic substances and other actions that allow the proliferation of anomalous cells.
In these works, they emphasize that the centralist vision of cancer has transcended the anomalous production of cellular mutations, and also consider that tumor evolution is based on the appearance of changes and the stress in the cellular ecosystem that induces the genome to instability and produces mutations, signal activations with erroneous sequences and mechanisms of evasion in the immune system. (23,40)
With this in mind, tumors develop when normal cells undergo genetic alterations that affect growth points. This results in a disproportionate growth that eventually leads to the onset of the disease. As pre-malignant cells evolve to cancer cells, the environment surrounding the tumor coevolves as well, creating a dynamic circuit of tumor-microenvironment interaction. (23,40)
Approximately 80% of cancers are carcinomas, that is, cancers that originate in the epithelial tissue, and their main support is the stroma, which nourishes, protects and supports the epithelial tissue. It could be said that stroma is the connective tissue that forms the framework of an organ, and includes the extracellular matrix and the cells that synthesize it (fibroblasts, endothelial cells, etc.). (23)
Table 2. Top 10 characteristics of cancer and their correlation to mitochondria.
|
Cancer characteristics |
||
|
Self-sufficiency of growth signals |
Insensitivity to anti-aging signs |
Evasion of apoptosis |
|
Angiogenesis |
Unlimited replicative potential and invasion |
Metastasis of other tissues |
|
Fostering inflammation |
Genomic and mutational instability |
Energy deregulation |
|
Evasion of the immune system |
||
Source: Own elaboration based on (23).
Cancer cells communicate with their environment while exchanging soluble molecules with the paracrine stroma, which turns stroma into the support of the tumor and, therefore, facilitates its progression. It is worth mentioning that the success of the tumor depends on its ability to survive in an inhospitable microenvironment. Besides stromal cells, inflammatory cells may also be found in this microenvironment. (39,40)
Why to continue research on mitochondria?
Currently, cancer studies focus their efforts on finding a molecular mechanism that links mitochondrial mutations to tumor formation. Research seeks to increase the understanding of the molecular basis of cancer, with the purpose of finding new prevention, diagnosis and treatment methods for the disease.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgement
None stated by the authors.
References
1.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12(10):685-98. http://doi.org/f376vb.
2.Desagher. S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10(9):369-77. http://doi.org/cnbkgg.
3.Jäättelä M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene. 2004;23(16):2746-56. http://doi.org/dzmrm7.
4.Cuezva JM, Krajewska M, de Heredia ML, Krajewski S, Santamaría G, Kim H, et al. The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 2002;62(22):6674-81.
5.Polyak K, Li Y, Zhu H, Lengauer C, Wilson JK, Markowitz SD, et al. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet. 1998;20(3):291-3. http://doi.org/fp74fd.
6.Alam MM, Lal S, FitzGerald KE, Zhang L. A holistic view of cancer bioenergetics: mitochondrial function and respiration play fundamental roles in the development and progression of diverse tumors. Clin Transl Med. 2016;5(1):3. http://doi.org/chtw.
7.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519-30. http://doi.org/bjmdt2.
8.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-14. http://doi.org/fj737q.
9.Simmons J.G. Doctors & Discoveries. Boston: Houghton Mifflin Com; 2002.
10.Warburg O, Psener K, Negelein E. Üeber den Stoffwechsel der Tumoren. Biochem Z. 1924;152(1):319-44.
11.Vazquez A, Liu J, Zhu Y, Oltvai ZN. Catabolic efficiency of aerobic glycolysis: the Warburg effect revisited. BMC Syst Biol. 2010;4:58. http://doi.org/bvqgqr.
12.Brunelle JK, Bell EL, Quesada NM, Veercauteren K, Tiranti V, Zeviani M, et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1(6):409-14. http://doi.org/d2d9hx.
13.Bertram JS. The molecular biology of cancer. Mol Asp Med. 2000;21(6):167-223. http://doi.org/bh3qhx.
14.Kyle RA, Shampo MA. Otto Heinrich Warburg. Mayo Clin Proc. 1988;63(1):79. http://doi.org/cmtd.
15.Ramírez-Agudelo ME, Rojas-López M. La necrosis, un mecanismo regulado de muerte celular. IATREIA. 2010;23(2):166-77.
16.Cuezva JM, Ortega AD, Willers I, Sanchez-Cenizo L, Aldea M, Sánchez-Aragó M. The tumor suppressor function of mitochondria: translation into the clinics. Biochim Biophys Acta. 2009;1792(12):1145-58. http://doi.org/bgzqtz.
17.Devita VT, Hellman S, Roseenberg SA. Cáncer: Principios y Práctica de Oncología . Vol 2. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
18.Martínez-Hezquerro JD, Herrera LA. Angiogénesis: VEGF/VEGFRs como blancos terapéuticos en el tratamiento contra el cáncer. Cancerología. 2006;1(1):83-96.
19.Saavedra-Torres JS, Zuñiga-Cerón LF, Freyre-Bernal SI, Muñoz-Ordoñez GW, Salguero C. El rol de vegf en la angiogénesis fisiológica y tumoral. Medicina. 2017;39(3):190-209.
20.Duffy AM, Bouchier-Hayes DJ, Harmey JH. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF. Firts. Austin: Bioscience; 200-2013.
21.Reichardt LF, Tomaselli KJ. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531-70. http://doi.org/frrkc4.
22.Mandal A. ¿Cuál es Metástasis? London: News Medica Life Sciences & Medicine; 2014.
23.Saavedra-Torres JS, Zúñiga-Cerón LF, Vásquez-López JA, Navia-Amézquita CA, Mosquera-Sánchez LP, Freyre-Bernal SI. La matriz extracelular: un ecosistema influyente en la forma y comportamiento de las células. Morfolia. 2015;7(1):12-35.
24.Kuo TH, Kubota T, Watanabe M, Furukawa T, Teramoto T, Ishibiki K, et al. Liver colonization competence governs colon cancer matastasis. Proc Natl Acad Sci U S A. 1995;92(26):12085-9.
25.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298-307. http://doi.org/b95kmv.
26.Marín-Hernández A. El factor inducido por la hipoxia-1 (HIF-1) y la glucólisis en las células tumorales. REB. 2009;28(2):42-51.
27.Simonnet H, Alazard N, Pfeiffer K, Gallou C, Bérud C, Demont J, et al. Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis. 2002;23(5):759-68.
28.Bergström J, Fürst P, Norée LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974;36(6):693-7. http://doi.org/chtx.
29.Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108(49):19611-6. http://doi.org/ddpjzn.
30.Zaidi N, Lupien L, Kuemmerle NB, Kinlaw WB, Swinne JV, Smans K. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52(4):585-9. http://doi.org/f5kv3t.
31.Price DT, Coleman RE, Liao RP, Robertsn CN, Polascik TJ, DeGrad TR. Comparison of [18 F] fluorocholine and [18 F] fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J Urol. 2002;168(1):273-80. http://doi.org/cx77j9.
32.Liu Y, Zuckier LS, Ghesani NV. Dominant uptake of fatty acid over glucose by prostate cells: a potential new diagnostic and therapeutic approach. Anticancer Res. 2010;30(2):369-74.
33.Zha S, Ferdinandusse S, Hicks JL, Denis S, Dunn TA, Wanders RJ, et al. Peroxisomal branched chain fatty acid beta-oxidation pathway is upregulated in prostate cancer. Prostate. 2005;63(4):316-23. http://doi.org/ftgnnb.
34.Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK, et al. Acetate dependence of tumors. Cell. 2014;159(7):1591-602. http://doi.org/f6wrwh.
35.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47-60. http://doi.org/f52594.
36.Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100(1):57-70. http://doi.org/bm35gq.
37.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-74. http://doi.org/b6snj3.
38.Cascales-Angosto M, Álvarez-Gómez JA. Metaloproteinasas, matriz extracelular y cáncer. An. R. Acad. Nac. Farm. 2010;76(1):59-84.
39.González-Ávila G, González A, Delgado J, Gutiérrez-González LH. Participación de las metaloproteasas de matriz en la progresión del cáncer. Rev Inst Nal Enf Resp Mex. 2009;22(4):328-36.
40.Vivas D, Inga R, Yarlequé A. Uso potencial de componentes del veneno de serpiente en el tratamiento del cáncer. Rev Peru Med Exp Salud Publica. 2012;29(3):396-401.
Recibido: 3 de septiembre de 2016; Aceptado: 28 de octubre de 2016
Abstract
Metabolism alterations are associated with the loss of mitochondrial function in tumor cells. Current research discuss whether such loss is evident in function itself, or if cells can provide maximum stability to its functions. More studies are needed to determine the behavior of cancer in mitochondria. Tumor cells experience a limitation of oxygen and mutations in oncogenes, tumor suppressor genes and enzymes of the glycolytic pathway and/or mitochondrial oxidative metabolism, thus allowing the formation of aggressive cancer. This article is the result of a literature review of the scientific evidence that has been presented in the latest research on cancer and mitochondrial function.
Keywords:
Cancer, Glycolysis, Mitochondria, Neovascularization pathologic (MeSH).Resumen
Se ha descrito que algunas alteraciones del metabolismo están asociadas con la pérdida de función mitocondrial en células tumorales. Aún se discute si tal pérdida se evidencia en la función o si la célula brinda máxima estabilidad a sus funciones, se requieren más estudios para conocer el comportamiento del cáncer en la mitocondria. Cuando tiene limitación de oxígeno y mutaciones en oncogenes, genes supresores de tumor y enzimas de la vía glucolítica o del metabolismo oxidativo mitocondrial, la célula tumoral permite la formación de un cáncer agresivo. Este artículo es producto de la revisión bibliográfica de la evidencia científica que se ha presentado en las últimas investigaciones respecto al cáncer y la función mitocondrial.
Palabras clave:
Cáncer, Mitocondria, Neovascularización patológica, Glucólisis (DeCS).Introduction
The genes which encode the machinery that generates energy in the mitochondria are tumor suppressors; when they do not function properly, some processes and pathways that lead to cancer may be triggered. Mitochondria are organelles that have mitochondrial DNA (mtDNA), which is inherited only from the mother during the fertilization process. These organelles are the "energetic generators" of healthy cells, such as those that have their metabolisms encoded to be apoptosis inhibitors. 1,2
Mitochondria regulate and coordinate apoptosis activation, hence their importance for the study and research on therapies against cancer. When mitochondria deregulate, the onset of diseases such as cancer is stimulated due to an increased catabolic process, not to mention their role in neurodegenerative diseases, which are associated with abnormal mitochondrial function and apoptosis. 1,2
Mitochondria and cancer
mtDNA mutagenesis is involved in a wide arrange of tumor processes, including renal adenocarcinoma, colon cancer, head and neck tumors, astrocytic tumors, thyroid tumors, breast tumors, ovarian, prostate and bladder cancer tumors, neuroblastomas, and oncocytomas. Many mtDNA mutations in cancer cells clearly inhibit oxidative phosphorylation. Although some of these cancers have ancestral polymorphisms associations, others may be cancer cell mutations. 3-6 (table 1).
Source: Own elaboration based on the data obtained in the study.Table 1: Generalities of the role of cancer in mitochondrial function. cases, cancer patients with localized tumors have a better survival

Cancer cells acquire enough ATP to support proliferation and to function endlessly, which has captivated scientists for nearly a century. Otto Warburg et al. conducted the first quantitative study on cancer cell metabolism in the 1920s. 7,8
Cells can obtain energy through a process called glycolysis that consists of anaerobic fermentation, in which the waste products of that fermentative process are pyruvate and lactic acid. 9,10 Tumor tissues metabolize approximately ten times more glucose into lactate at a given time than normal tissues. 6,8 Warburg hypothesized that effective cellular respiration caused by mitochondrial damage leads to carcinogenesis. 9,10
Warburg’s effect describes that cancer cells use glycolysis followed by lactic fermentation as an energy source, even if there is an appropriate amount of oxygen for respiration. 1,11 In other words, instead of developing a complete respiration process in the presence of adequate amounts of oxygen, cancer cells ferment and continue to mutate to preserve their tumoral domain. 12
At the cellular level, tumors have survival advantages due to lactate secretion. 4,13,14 Lactic acid confers invasive properties to tumor cells, affecting the normal structure of tissues. 15 Additionally, the expression of vascular endothelial growth factor and its receptor (VEGF and VEGFR, respectively) responds to different stimuli to generate new blood vessels from preexisting ones. 1,16-18 VEGFα stimulates vascular endothelial cell growth, cell survival and proliferation regulated by the nuclear and mitochondrial action of the cell. Furthermore, VEGF and gene mutations leading to actívate metalloproteinases to degrade the extracellular matrix allow greater metastatic action. 19 Recent studies suggest that VEGF can protect cells from apoptosis and increase their resistance to conventional chemotherapy and radiotherapy. 20,21
Similarly, metastasis is of great importance since most of cáncer deaths occur because primary cancer spreads to distant sites. In most cases, cancer patients with localized tumors have a better survival rate than patients with cancer and metastatic tumors. 22-25 It is also suggested that the increase of oncogene mutations, tumor suppressor genes and enzymes of the glycolytic pathway and/or mitocondrial oxidative metabolism (Myc, Akt, p53, HIF1-α) allow to turn cáncer cells into efficient metastatic cells. 3
The hypoxia-inducible factor (HIF-1) protein is normally activated in response to certain cellular crises, such as lack of oxygen. However, in the case of mitochondria with abnormal and tumor mechanisms, the expression of HIF-1 that is perpetuated in the presence of damage signs in the SDH gene is stimulated. This is caused by the cell following already established oncogenes guidelines to carry out a unique type of homeostasis for cancer and to supply high levels of glucose and oxygen to replicate itself without control. 1,26,27
Variety of metabolic fuels for tumor cells
A wide variety of metabolic fuels can be observed in tumor pathways, in which tumor cells are able to utilize different bioenergetic substrates, including glutamine, glucose, fatty acids, ketone bodies, and acetate. These substrates can be provided by the stromal cells in the microenvironment. 28 Particularly, glutamine and glucose can provide building blocks for the synthesis of many biomolecules that allow the regulation of oncogenesis processes. It is worth mentioning that metabolic enzymes with mutations are found in several tumors and in the oncometabolites accumulated in different types of tumors. 28,29
Cancer cells also show increased demand for fatty acids other tan glutamine. 6,30 Fatty acids can be synthesized endogenously or taken from exogenous sources. For example, prostate tumors import less glucose than other tumors, 31 therefore, β-oxidation of fatty acids is an important source of energy. 32,33
Moreover, two recent studies showed that acetate is a bioenergetic substrate for glioblastoma and brain metastases. 34 Catabolism in stromal and adipocyte cells provides fuel and building blocks for the anabolic growth of cancer cells through metabolic coupling. 35
Cancer cells and independence
In 2000 and 2011, Hanahan and Weinberg summarized an extensive research on cancer and the top 10 characteristics of cancer and its correlation with mitochondria (table 2). 36-39 These authors describe how cancer cells can be stimulated by infectious phenomena, inflammation, viruses, toxic substances and other actions that allow the proliferation of anomalous cells.
Source: Own elaboration based on 23.Table 2: Top 10 characteristics of cancer and their correlation to mitochondria.
In these works, they emphasize that the centralist vision of cáncer has transcended the anomalous production of cellular mutations, and also consider that tumor evolution is based on the appearance of changes and the stress in the cellular ecosystem that induces the genome to instability and produces mutations, signal activations with erroneous sequences and mechanisms of evasion in the immune system. 23,40
With this in mind, tumors develop when normal cells undergo genetic alterations that affect growth points. This results in a disproportionate growth that eventually leads to the onset of the disease. As premalignant cells evolve to cancer cells, the environment surrounding the tumor coevolves as well, creating a dynamic circuit of tumormicroenvironment interaction. 23,40
Approximately 80% of cancers are carcinomas, that is, cancers that originate in the epithelial tissue, and their main support is the stroma, which nourishes, protects and supports the epithelial tissue. It could be said that stroma is the connective tissue that forms the framework of an organ, and includes the extracellular matrix and the cells that synthesize it (fibroblasts, endothelial cells, etc.). 23
Cancer cells communicate with their environment while exchanging soluble molecules with the paracrine stroma, which turns stroma into the support of the tumor and, therefore, facilitates its progression. It is worth mentioning that the success of the tumor depends on its ability to survive in an inhospitable microenvironment. Besides stromal cells, inflammatory cells may also be found in this microenvironment. 39,40
Why to continue research on mitochondria?
Currently, cancer studies focus their efforts on finding a molecular mechanism that links mitochondrial mutations to tumor formation. Research seeks to increase the understanding of the molecular basis of cancer, with the purpose of finding new prevention, diagnosis and treatment methods for the disease.
Acknowledgement
None stated by the authors.
References
Referencias
Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12(10):685- 98. http://doi.org/f376vb.
Desagher. S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10(9):369-77. http://doi.org/cnbkgg.
Jäättelä M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene. 2004;23(16):2746-56. http://doi.org/dzmrm7.
Cuezva JM, Krajewska M, de Heredia ML, Krajewski S, Santamaría G, Kim H, et al. The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 2002;62(22):6674-81.
Polyak K, Li Y, Zhu H, Lengauer C, Wilson JK, Markowitz SD, et al. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet. 1998;20(3):291-3. http://doi.org/fp74fd.
Alam MM, Lal S, FitzGerald KE, Zhang L. A holistic view of cancer bioenergetics: mitochondrial function and respiration play fundamental roles in the development and progression of diverse tumors. Clin Transl Med. 2016;5(1):3. http://doi.org/chtw.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519-30. http://doi.org/bjmdt2.
Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309- 14. http://doi.org/fj737q.
Simmons J.G. Doctors & Discoveries. Boston: Houghton Mifflin Com; 2002.
Warburg O, Psener K, Negelein E. Üeber den Stoffwechsel der Tumoren. Biochem Z. 1924;152(1):319-44.
Vazquez A, Liu J, Zhu Y, Oltvai ZN. Catabolic efficiency of aerobic glycolysis: the Warburg effect revisited. BMC Syst Biol. 2010;4:58. http://doi.org/bvqgqr.
Brunelle JK, Bell EL, Quesada NM, Veercauteren K, Tiranti V, Zeviani M, et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1(6):409-14. http://doi.org/d2d9hx.
Bertram JS. The molecular biology of cancer. Mol Asp Med. 2000;21(6):167-223. http://doi.org/bh3qhx.
Kyle RA, Shampo MA. Otto Heinrich Warburg. Mayo Clin Proc. 1988;63(1):79. http://doi.org/cmtd.
Ramírez-Agudelo ME, Rojas-López M. La necrosis, un mecanismo regulado de muerte celular. IATREIA. 2010;23(2):166-77.
Cuezva JM, Ortega AD, Willers I, Sanchez-Cenizo L, Aldea M, Sánchez-Aragó M. The tumor suppressor function of mitochondria: translation into the clinics. Biochim Biophys Acta. 2009;1792(12):1145- 58. http://doi.org/bgzqtz.
Devita VT, Hellman S, Roseenberg SA. Cáncer: Principios y Práctica de Oncología . Vol 2. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
Martínez-Hezquerro JD, Herrera LA. Angiogénesis: VEGF/VEGFRs como blancos terapéuticos en el tratamiento contra el cáncer. Cancerología. 2006;1(1):83-96.
Saavedra Torres Jhan Sebastian, Zuñiga Cerón Luisa Fernanda, Freyre Bernal Sofía Isabel, Muñoz Ordoñez Guillermo Wilson, Salguero Carolina. El rol de vegf en la angiogénesis fisiológica y tumoral. Revista Medicina 2017; 39 (3) : 190-20920.
Duffy AM, Bouchier-Hayes DJ, Harmey JH. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF. Firts. Austin: Bioscience; 200-2013.
Reichardt LF, Tomaselli KJ. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531-70. http://doi.org/frrkc4.
Mandal A. ¿Cuál es Metástasis? London: News Medica Life Sciences & Medicine; 2014.
Saavedra-Torres JS, Zúñiga-Cerón LF, Vásquez-López JA, Navia- Amézquita CA, Mosquera-Sánchez LP, Freyre-Bernal SI. La matriz extracelular : un ecosistema influyente en la forma y comportamiento de las células. Morfolia. 2015;7(1):12-35.
Kuo TH, Kubota T, Watanabe M, Furukawa T, Teramoto T, Ishibiki K, et al. Liver colonization competence governs colon cancer matastasis. Proc Natl Acad Sci U S A. 1995;92(26):12085-9.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298-307. http://doi.org/b95kmv.
Marín-Hernández A. El factor inducido por la hipoxia-1 (HIF-1) y la glucólisis en las células tumorales. REB. 2009;28(2):42-51.
Simonnet H, Alazard N, Pfeiffer K, Gallou C, Bérud C, Demont J, et al. Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis. 2002;23(5):759-68.
Bergström J, Fürst P, Norée LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974;36(6):693-7. http://doi.org/chtx.
Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2018 Revista de la Facultad de Medicina

Esta obra está bajo una licencia Creative Commons Reconocimiento 3.0 Unported.
-





















