Hypertriglyceridemia and adverse outcomes during pregnancy
Hipertrigliceridemia y consecuencias adversas en el embarazo
DOI:
https://doi.org/10.15446/revfacmed.v66n2.60791Palabras clave:
Pregnancy, Hypertriglyceridemia, Maternal-Fetal Exchange, Fetal Development, Pregnancy Complications (en)Embarazo, Hipertrigliceridemia, Intercambio materno-fetal, Desarrollo fetal, Complicaciones del embarazo (es)
Descargas
Introduction: During pregnancy, levels of maternal serum triglycerides increase as a physiological adaptation mechanism to meet the needs of the developing fetus. However, although an excessive increase has been associated with preeclampsia, macrosomia and preterm delivery, the levels from which measurements should be taken in each trimester to prevent complications have not been established conclusively.
Objective: To review the physiopathology, effects on mother and child, expected values in each trimester and therapeutic interventions in maternal hypertriglyceridemia.
Materials and methods: A review was made based on a search of articles in the ScienceDirect, Pubmed, Scopus, LILACS, Cochrane and SciELO databases, with the terms: Pregnancy; Hypertriglyceridemia; Maternal-Fetal Exchange; Fetal Development; Pregnancy Complications in English and its equivalents in Spanish.
Results: 59 articles met the search criteria and responded to the objectives.
Conclusions: The limited amount and the great variability of the data indicate the need to carry out further research to establish the normal triglycerides ranges during the three trimesters of pregnancy and to determine risks and effective interventions before pregnancy in order to reduce maternal and child morbidity and mortality.
Introducción. Durante el embarazo, los niveles séricos de triglicéridos maternos aumentan como un mecanismo de adaptación fisiológica para suplir las necesidades del feto en desarrollo. Pese a que el incremento excesivo se ha asociado a preeclampsia, macrosomía y parto pretérmino, no se han establecido de manera contundente los niveles a partir de los cuales se deben tomar medidas en cada trimestre para prevenir complicaciones.
Objetivo. Hacer una revisión sobre fisiopatología, efectos en madre e hijo, valores esperados en cada trimestre e intervenciones terapéuticas en hipertrigliceridemia gestacional.
Materiales y métodos. Se realizó una revisión con la búsqueda de artículos en las bases de datos ScienceDirect, PubMed, Scopus, LILACS, Cochrane y SciELO con los términos: Pregnancy; Hypertriglyceridemia; Maternal-Fetal Exchange; Fetal Development; Pregnancy Complications y sus equivalentes en español.
Resultados. Se encontraron 59 artículos que cumplieron los criterios de búsqueda y daban respuesta a los objetivos.
Conclusiones. El número limitado y la gran variabilidad de los datos indican la necesidad de realizar más investigaciones que establezcan los rangos de normalidad de los triglicéridos durante los tres trimestres del embarazo y así determinar riesgos e intervenciones eficaces antes de la gestación y reducir la morbimortalidad materno-infantil.
review article
DOI: https://doi.org/10.15446/revfacmed.v66n2.60791
Hypertriglyceridemia and adverse outcomes during pregnancy
Hipertrigliceridemia y consecuencias adversas en el embarazo
Received: 30/10/2016. Accepted: 15/12/2016.
Jonathan Cortés-Vásquez1 • Islendy Noreña1 • Ismena Mockus1
1 Universidad Nacional de Colombia - Sede Bogotá - Faculty of Medicine - Departament of Physiological Sciences - Lipids and Diabetes Unit - Bogotá D.C. - Colombia.
Corresponding author: Jonathan Cortés-Vásquez. Lipids and Diabetes Unit, Departament of Physiological Sciences, Faculty of Medicine, Universidad Nacional de Colombia. Carrera 30 No. 45-03, building 471, floor 4. Telephone number: +57 1 3165000, ext.: 15054; Mobile number: +57 3045467063. Bogotá D.C. Colombia. Email: joacortesva@unal.edu.co.
| Abstract |
Introduction: During pregnancy, levels of maternal serum triglycerides increase as a physiological adaptation mechanism to meet the needs of the developing fetus. However, although an excessive increase has been associated with preeclampsia, macrosomia and preterm delivery, the levels from which measurements should be taken in each trimester to prevent complications have not been established conclusively.
Objective: To review the physiopathology, effects on mother and child, expected values in each trimester and therapeutic interventions in maternal hypertriglyceridemia.
Materials and methods: A review was made based on a search of articles in the ScienceDirect, Pubmed, Scopus, LILACS, Cochrane and SciELO databases, with the terms: Pregnancy; Hypertriglyceridemia; Maternal-Fetal Exchange; Fetal Development; Pregnancy Complications in English and its equivalents in Spanish.
Results: 59 articles met the search criteria and responded to the objectives.
Conclusions: The limited amount and the great variability of the data indicate the need to carry out further research to establish the normal triglycerides ranges during the three trimesters of pregnancy and to determine risks and effective interventions before pregnancy in order to reduce maternal and child morbidity and mortality.
Keywords: Pregnancy; Hypertriglyceridemia; Maternal-Fetal Exchange; Fetal Development; Pregnancy Complications (MeSH).
Cortés-Vásquez J, Noreña I, Mockus I. Hypertriglyceridemia and adverse outcomes during pregnancy. Rev. Fac. Med. 2018;66(2):247-53. English. doi: https://doi.org/10.15446/revfacmed.v66n2.60791.
| Resumen |
Introducción. Durante el embarazo, los niveles séricos de triglicéridos maternos aumentan como un mecanismo de adaptación fisiológica para suplir las necesidades del feto en desarrollo. Pese a que el incremento excesivo se ha asociado a preeclampsia, macrosomía y parto pretérmino, no se han establecido de manera contundente los niveles a partir de los cuales se deben tomar medidas en cada trimestre para prevenir complicaciones.
Objetivo. Hacer una revisión sobre fisiopatología, efectos en madre e hijo, valores esperados en cada trimestre e intervenciones terapéuticas en hipertrigliceridemia gestacional.
Materiales y métodos. Se realizó una revisión con la búsqueda de artículos en las bases de datos ScienceDirect, PubMed, Scopus, LILACS, Cochrane y SciELO con los términos: Pregnancy; Hypertriglyceridemia; Maternal-Fetal Exchange; Fetal Development; Pregnancy Complications y sus equivalentes en español.
Resultados. Se encontraron 59 artículos que cumplieron los criterios de búsqueda y daban respuesta a los objetivos.
Conclusiones. El número limitado y la gran variabilidad de los datos indican la necesidad de realizar más investigaciones que establezcan los rangos de normalidad de los triglicéridos durante los tres trimestres del embarazo y así determinar riesgos e intervenciones eficaces antes de la gestación y reducir la morbimortalidad materno-infantil.
Palabras clave: Embarazo; Hipertrigliceridemia; Intercambio materno-fetal; Desarrollo fetal; Complicaciones del embarazo (DeCS).
Cortés-Vásquez J, Noreña I, Mockus I. [Hipertrigliceridemia y consecuencias adversas en el embarazo]. Rev. Fac. Med. 2018;66(2):247-53. English. doi:
https://doi.org/10.15446/revfacmed.v66n2.60791.
Introduction
During pregnancy, mother’s physiology adapts to provide nutrients to the growing fetus. However, the imbalance in the amount of triglycerides (TG) —either before or during pregnancy— has been related to maternal-perinatal pathologies such as preeclampsia (PE) and gestational diabetes mellitus (GDM). In neonates, reported pathologies include preterm delivery (PD), dystocia, macrosomia, hypoglycemia or intrauterine growth restriction. (1-6)
In Colombia, 7 482 cases of severe maternal morbidity (SMM) were reported during the first semester of 2016, which corresponds to 27.9 mothers for every 1 000 live births (LB), and 182 cases of maternal mortality, with 49.2 cases for every 100 000 LB. (7) The main causes of SMM and maternal mortality were hypertensive disorders (62.4% and 15.5%, respectively) and hemorrhagic complications (15.5% and 18%, respectively). (7)
Exacerbated hypertriglyceridemia and insulin resistance condition an oxidative environment that leads to endothelial injury, which, in turn, has a predisposing effect on the development of PE as a hypertensive disorder of higher incidence. (8-10) Intrauterine weight gain may be associated with the elevation of TG passage through the placenta and TG production by the fetus, which would lead to a large for gestational age (LGA) fetus and, thus, possible hemorrhagic complications during delivery by overdistension and uterine rupture. (6,11,12)
Besides short-term complications associated with hypertriglyceridemia, other long-term complications involve metabolic disorders and cardiovascular diseases (CVD) that may affect the well-being of the child during adulthood and the health of the mother in her middle and late adulthood. (13-16) The objective of this work is to perform a comprehensive literature review on the pathophysiological causes, effects on mother and child, expected TG values in each trimester of pregnancy and possible therapies to manage maternal hypertriglyceridemia in a timely manner.
Increase in circulating triglycerides during pregnancy
Pregnancy is a state of metabolic stress associated with high TG levels (17), which increase during this period; the highest concentrations are observed during the third trimester. (1) This increase is related to the decrease in the synthesis of fatty acids and the activity of the lipoprotein lipase (LPL) that catalyzes the hydrolysis of TG-rich lipoproteins in the adipose tissue. (1) The activity of this enzyme decreases about 85% during a normal pregnancy. (2,17) TG levels decrease in the postpartum period and this decrease is faster in women who lactate. (18)
The abovementioned events are related to the insulin resistance that occurs during pregnancy, which may be caused by the increase of non-esterified fatty acids, changes in adipokines secretion and inflammatory factors. (1,2,17) Increased lipolysis has been associated with increased placental lactogen, progesterone, prolactin, cortisol and estrogen. (2,19) Adiponectin and apelin, which favor insulin sensitivity, decrease in the third trimester, while other adipokines and cytokines that reduce insulin sensitivity increase at the end of pregnancy, including resistin, retinol binding protein 4 (RBP4), leptin, visfatin, chemerin, adipocyte fatty acid binding protein (AFABP), tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) (2). In addition, the expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) in adipose tissue decreases in the third trimester, contributing to insulin resistance. (2)
Placental passage of maternal triglycerides
1-3% of maternal fatty acids circulate in non-esterified form and enter the syncytiotrophoblast through diffusion or receptor-mediated endocytosis. (3) Low-density lipoproteins associated with TG (LDL-TG), more abundant in circulation, are hydrolyzed by intracellular lipases and cholesterol ester hydrolases. On the other hand, high density lipoproteins (HDL) and very low density lipoproteins (VLDL) bind to surface receptors and are hydrolysed extracellularly by endothelial lipase. (2,3,16) Transporters such as fatty acid transport protein 1 and 4 (FATP-1 and FATP-4), fatty acids translocase (FAT/CD36) and plasma membrane fatty acid-binding protein (FABPpm) make fatty acid uptake a more efficient process. (3,16)
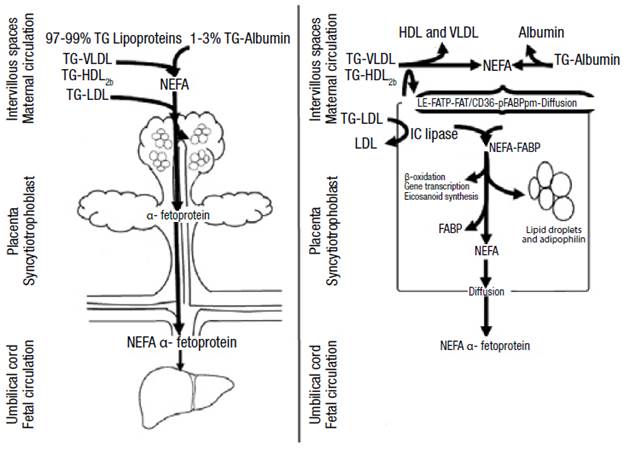
At the intracellular level, the fatty acids bind to the fatty acid binding protein (FABP), which has intrinsic acetyl-CoA ligase activity. (16) Then, the syncytiotrophoblast releases fatty acids into the fetal circulation to bind to the α-fetoprotein (AFP) that takes them to the fetal liver where they are metabolized (Figure 1). (2,12,16) It should be noted that lipid droplets have been observed in the placenta and that their formation is stimulated by adipophilin. (16)
White adipose tissue is observed in the fetus since week 14 or 15, while fetal lipogenesis begins at week 12 or 20; PPAR-γ activation plays an important role in the subsequent increase of adipose tissue size. (20) Fetus weight increases fourfold from 1.6 g/kg/day to 3.4 g/kg/day since the 26th week of pregnancy, but the increase in fetal body fat depends by 20% on placental lipid transfer; the remaining tissue results from fetal lipogenesis. It is worth noting that only one third of circulating maternal glucose is used by placenta through lipolytic routes and the demand is not modified by increasing glucose levels because the utilization is saturated between 90 mg/dL and 143 mg/dL. (2)
Hypertriglyceridemia and pathologies
A study carried out in Amsterdam (n=4 008) revealed that TG increase in the first trimester of pregnancy is directly associated with pregnancy-induced hypertension, PD and LGA. (3) Likewise, a research carried out in India (n=180) found that high TG levels (≥195 mg/dL) in the second trimester are associated with a higher incidence of PD, GDM, PE and LGA. (4) Another complication is maternal pancreatitis, which must be mentioned due to its severity (Table 1). (14,18)
Table 1. Outcomes of gestational hypertriglyceridemia according to different studies.
|
Author |
TG levels (mg/dL) |
Outcome |
Conclusion |
|
El Khouly, et al. (5). Egypt |
182.46+16.62 |
PE |
The elevation of lipids during the first trimester of pregnancy may predict the development of PE and its severity: mild (178.31+11.42 mg/dL) or severe (190.40+11.95 mg/dL) |
|
Jin et al. (21). China
|
278.25 |
LGA |
In the third trimester, each time TG rises by 87.5 mg/dL, it is associated with a greater risk of GDM (OR: 1.37, IC95%: 1.18-1.58), PE (OR: 1.50, IC95%: 1.16-1.93), LGA (OR: 1.13, IC95%: 1.02-1.26) and macrosomia (OR: 1.19, CI95%: 1.02-1.39) |
|
308.88 |
PE |
||
|
338.63 |
GDM |
||
|
Siddiqui (22) Saudi Arabia |
271.25+70.59 |
PE |
Hypertriglyceridemia ≥273.53 mg/dL in the third trimester may be associated with endothelial damage that leads to PE |
PE: preeclampsia; TG: triglycerides; GDM: gestational diabetes mellitus; LGA: large for gestational age fetus.
Source: Own elaboration based on the data obtained in the study.

Figure 1. Passage of triglycerides through the placenta.
TG: triglycerides; VLDL: very low density lipoprotein; LDL: low density lipoprotein; HDL: high density lipoprotein; FATP: fatty acid transport protein; FAT/CD 36: fatty acid translocase; FABPpm: plasma membrane fatty acid-binding protein; IC: intracellular; FABP: fatty acid binding protein; NEFA: non-esterified fatty acids.
Source: Own elaboration based on Barrett et al. (16)
In the mother
Preeclampsia
Between 2% and 8% of pregnancies are complicated by PE, which is the third cause of maternal-fetal death after hemorrhage and sepsis. (14,23-25) In the mother, this condition is associated with endothelial dysfunction, hypertension, kidney disease and diabetes mellitus (DM). It also increases the risk of developing CVD and death due to kidney and liver impairment. (14) Hypertriglyceridemia, before or during pregnancy, alters the vascular development of the placenta and results from inadequate implantation or placental perfusion. (3,5,8) PE and dyslipidemia are correlated because LPL dysfunction, metabolic syndrome and increased plasma lipids occur in both. (4) These disorders stimulate peroxidation of placental lipids and trophoblast components that promote oxidative stress and form deleterious complexes in endothelial cells that cause vascular dysfunction. (4,5)
A prospective cohort study conducted in Egypt (n=251) showed that TG levels between weeks 4 and 12 of pregnancy can be predictors of PE development. (5,8) This study found that the increase in total cholesterol (TC), TG and LDL greater than 231 mg/dL, 149.5 mg/dL and 161 mg/dL, respectively, and the decrease in HDL below 42.5 mg/dL are cutoff points with positive predictive value for the development of PE, while TC and TG increase was related to severity. (5) Another study conducted in Turkey (n=52) found that TC, TG and LDL increase greater than 4%, 5% and 9.8%, respectively, and a decrease in HDL by more than 9% are associated with worse prognosis of gestational hypertensive disease. (26)
The study by Manna et al. (27) in Bangladesh (n=90) showed that increased TG levels are directly associated with an increase in blood pressure. In the study group, systolic and diastolic pressures were 152.4±19.8 mmHg and 103.1±12.2 mmHg, respectively, while in pregnant controls, they were 112.0±8.9 mmHg and 75.5±6.6 mmHg, respectively. (27) These numbers were associated with TG levels in the study group (242.9±36.8 mg/dL) and in the control group (184.6±12.5 mg/dL). (27) TG >181 mg/dL before week 20 increased the risk of PE by 3 to 7 times. (28)
A study conducted in Mexico with 47 normotensive women and 27 with PE or gestational hypertension in the seventh month of pregnancy showed that hypertriglyceridemia is related to hypertensive disorders in pregnancy. Researchers found that nitric oxide (NO) synthesis decreased proportionally to the increase of TG levels. (8) NO decrease may be secondary to oxidative stress increase considering the high concentration of TG or glucose that inhibits NO synthesis. (29) In this study, women with hypertensive disorders of pregnancy were treated with hydralazine, which induces NO synthesis. (8) It has been observed that the increase of fatty acids in the placenta of women with DM1 is related to reduced fetal-placental circulation and that having a family history of DM2 is closely related to an increased risk of developing hypertensive disorders during pregnancy. (2)
Gestational diabetes mellitus
The prevalence of GDM has gone from 4% to 20% in 27 years and its current incidence is 1-14%. (30,31) This pathology not only increases the risk of PE and macrosomia during pregnancy, but also predisposes the mother to the development of DM2 and CVD. (12) Mothers with GDM have hypertriglyceridemia, hypercholesterolemia, insulin resistance, low levels of adiponectin and increased intracellular concentrations of AFABP (2); therefore, they are characterized by having insulin resistance. (4,12,29)
Alterations have been found in the fetus and placenta related to imbalances in the expression and function of PPARs isotypes that increase lipid flow in women with hyperlipidemia and GDM. (2,9,10)
Concentrations of PPAR-γ and PPAR-α are low in term-placental tissue of women who developed GDM, whereas no changes are detected in PPAR-δ concentrations. (9,10) No changes were found in PPAR-γ concentrations in women with DM1; however, decreased levels of 15-deoxy-δ-12, 14-prostaglandin J2 (15dPGJ2) were observed. (9) Also, alterations in the expression of PPAR-α during the first trimester of pregnancy are associated with spontaneous abortions. (10) 15dPGJ2 increases lipid concentrations and significantly reduces NO expression in the placenta of healthy women, while it regulates the increase in the concentrations of phospholipids and cholesterol esters in the placenta of diabetic women. (9) In addition, its receptor (PPAR-γ) is decreased in these patients, which could increase NO and lipid peroxidation, markers of pro-inflammatory and pro-oxidant states. (9)
A case-control study (n=254) conducted in the USA by Han et al. (32) found that the measurement of the pregestational lipid profile is a predictor of GDM. This study revealed that women who developed GDM, in comparison with the controls, presented smaller LDL diameters, low concentrations of HDL and high levels of small VLDL regardless of other risk factors (body mass index —BMI—, weight gain during pregnancy, age or ethnicity) in measurements taken even up to 7 years before pregnancy. (32,33) On the other hand, the risk of developing GDM is 3.5 times higher if TG >137 mg/dL in the first trimester. In addition, every time TG increase by 20 mg/dL, the risk of developing DMG increases by 10%. (34)
Pancreatitis
Acute pancreatitis caused by gestational hypertriglyceridemia is a rare complication of pregnancy but, when it occurs, it represents high maternal and fetal morbidity and mortality (1,15,16,18) and may be complicated by pancreatic necrosis, shock, hypokalemia, PE or eclampsia. (15) The risk of developing pancreatitis increases progressively with TG >500 mg/dL. (15) Thus, during the first trimester, these increased levels are associated with a 19% risk of pancreatitis, in the second with 26%, in the third with 53% and in the postpartum with 2% (1,15), depending on the activity of pancreatic lipase and liver involvement. (15,18)
In the fetus
Macrosomia
Lipids availability and accumulation in the fetus of a mother with dyslipidemia increases the risk of developing macrosomia and PD. (6) A LB presents macrosomia if its weight is >4kg and LGA if its size is above the 90th percentile. (12,14) Giving birth to a LGA fetus increases the likelihood of prolonged labor, cesarean or postpartum hemorrhage. In addition, giving birth to fetuses with macrosomia makes women 4.2 times more susceptible to developing DM2 throughout their lives. (35) Hypoglycemia, hyperbilirubinemia, respiratory distress, cardiac hypertrophy, shoulder dystocia, clavicle fracture, and brachial plexus injuries may occur in the newborn. (36,37)
A Cuban case-control study (n=236) showed that the increase in TG levels during the third trimester was a predictor of the development of fetal macrosomia (OR:4.80, CI95%: 2.34-9.84). (6) Another study conducted in Chile in patients with well-controlled GDM and hypertriglyceridemia found that macrosomia was more frequent in women with pre-pregnancy overweight or obesity. (12,38,39) A BMI >26.1 kg/m2 was a predictor of macrosomia regardless of hypertriglyceridemia during the first trimester of pregnancy (40), perhaps due to the fact that free fatty acids act as growth factors and, that in high concentrations, they compete for the binding site of sex hormones to albumin, which increases the levels of free hormones that could act on the placenta and the fetus, thus modifying its growth and development. (3) It is noteworthy that the levels of circulating leptin in pregnant women with GDM, PE, intrauterine growth restriction or macrosomia have not been shown to have a predictive value on the weight of the newborn. (41)
Contrary to the increase in TG, recent studies suggest that low levels of omega 3 fatty acids during prenatal life influence adiposity in children, not only in intrauterine life, but also in the postnatal period. (42,43) Low concentrations of omega 3 fatty acids are associated with lower weights. In turn, a study conducted in India identified that the placenta of term infants with low weight had lower levels of docosahexaenoic acid (DHA) compared to term neonates weighing > 2 500g. (43)
Preterm delivery
PD is the leading cause of neonatal morbidity and mortality and occurs in 12% of births. (14,44) Its risk increases to 60% if there is a history of DM1, DM2 or pregestational hyperlipidemia and to 33.3%, if there is hypertriglyceridemia in the third trimester. (44-46)
Variants in LPL
Polymorphisms in the LPL gene (S447X, N291S and D9N) are associated with exaggerated increase in TG levels during pregnancy. (17,18) In addition, two APOAV polymorphisms, -1131T>C and S19W, are related to increased VLDL secretion and increased circulating TG levels of 11% and 16.2%, respectively. (18) An interesting correlation has been found between APOAV (-1131T>C), maternal size and crown-rump length of the fetus. (18)
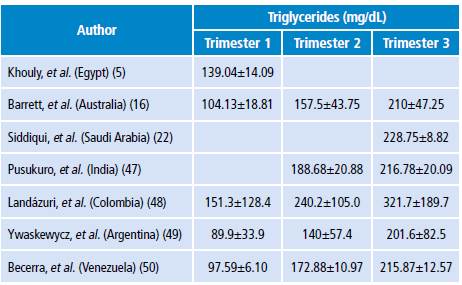
Measurement of triglycerides during pregnancy
In practice, measuring the concentrations of circulating TG once every trimester is advisable, since increased TG levels are normal during pregnancy (Table 2). Clinically, xanthomas on the external surface of arms, legs and buttocks, retinal lipemia, hepatosplenomegaly and lipemic serum are very suggestive of severe hypertriglyceridemia. (15)
Table 2. Normal triglycerides levels during pregnancy.
|
Author |
Triglycerides (mg/dL) |
||
|
Trimester 1 |
Trimester 2 |
Trimester 3 |
|
|
Khouly, et al. (Egypt) (5) |
139.04±14.09 |
||
|
Barrett, et al. (Australia) (16) |
104.13±18.81 |
157.5±43.75 |
210±47.25 |
|
Siddiqui, et al. (Saudi Arabia) (22) |
228.75±8.82 |
||
|
Pusukuro, et al. (India) (47) |
188.68±20.88 |
216.78±20.09 |
|
|
Landázuri, et al. (Colombia) (48) |
151.3±128.4 |
240.2±105.0 |
321.7±189.7 |
|
Ywaskewycz, et al. (Argentina) (49) |
89.9±33.9 |
140±57.4 |
201.6±82.5 |
|
Becerra, et al. (Venezuela) (50) |
97.59±6.10 |
172.88±10.97 |
215.87±12.57 |
Source: Own elaboration based on the data obtained in the study.
A study conducted by Landázuri et al. (48) in Colombia (n=422) found that TG levels were 86% higher during the second trimester of pregnancy and 137.8% in the third compared to the first. The authors followed 56 of these pregnant women throughout their pregnancy and observed a TG increase of 58.8% from the first to the second trimester and of 112.6% from the first to the third trimester (p<0.001). (48) In this study, low HDL levels were observed compared to the European or North American population during pregnancy. (46,47) Low HDL serum levels could be an additional risk factor for cardiovascular or gestational diseases in Colombian mothers and their children. (51)
Studies to evaluate the lipid profile of normal pregnant women have allowed proposing physiological levels for different populations. Thus, the study conducted by Ywaskewycz et al. (49) (n=291) in pregnant women without complications showed a TG increase of 56% from the first to second trimester and of 124% from the first to the third trimester; in the third trimester, the increase was twofold as compared to non-pregnant controls, and no differences were observed between TG in the first trimester and TG in non-pregnant women. (49) In addition, the TG/cHDL ratio increases during pregnancy, thus indicating the presence of LDL proteins of smaller size, rich in TG, denser and with higher atherogenic risk. (49)
On the other hand, the study by Becerra et al. (50) (n=91) with healthy pregnant women found that TG levels increased significantly (p<0.001) from the first to the second and third trimesters, and that the TG/cHDL ratio was correlated to pre-pregnancy BMI, fetal abdominal circumference, estimated fetal weight and uterine height. (p<0.01)
Treatment of hypertriglyceridemia during pregnancy
The treatment of hypertriglyceridemia depends on how high lipids are. If moderate (200-999 mg/dL), a strict low-fat diet with nutritional support with medium-chain TG and ω-3 fatty acids is initiated while increasing physical activity. Dietary supplementation with DHA and eicosapentaenoic acid reduces the production of proinflammatory cytokines (TNF-α, IL-1, IL-6 and IL-8) and inhibits the synthesis of VLDL without altering that of HDL. (15,16,52)
If hypertriglyceridemia is severe (>1000 mg/dL), using medications such as fibrates (PPAR-α agonists), statins, niacin, heparin or insulin is considered. Other possible alternatives are carbaprostacycline and iloprost, drugs that activate PPAR-δ and glitazones, which are synthetic ligands for PPAR-γ. (15,53) However, to the extent possible, using medications should be avoided during the first trimester of pregnancy, since many are contraindicated due to the potential harm to the fetus. It should be noted that using statins and fibrates has been described in case reports.
In case dyslipidemia is refractory to drugs or nutrition, plasmapheresis is initiated. (54) This procedure is also indicated when serum lipase levels are >3 times the normal limit of this enzyme or when hypocalcemia, lactic acidosis and worsening of inflammation or organic dysfunction occur; this can also be combined with heparin infusion. (55,56) When TG levels are below 500 mg/dL, this procedure should be stopped (55,56); however, if contraindicated or not available, infusion of regular insulin with 5% dextrose is used and glucose levels must be maintained between 150 mg/dL and 200 mg/dL during therapy. (15,53) When TG levels are normalized, the next step is to perform a rigorous control of the lipid profile, long-term dietary restriction and administration of fenofibrate. (15,16) In severe hypertriglyceridemia, PD is induced considering the high risks of maternal and fetal mortality (20% and 50%, respectively) and secondary pancreatitis. (1,15,16)
A study conducted by Kern-Pessoa et al. (57) in Sao Paulo (n=73) found that TC and TG levels increased during pregnancy and decreased from the third to the sixth postpartum week in women with GDM. (57) In this study, LDL and TG levels increased during pregnancy in patients who received insulin, and decreased in those treated with a strict low-fat diet, rich in ω-3 fatty acids as therapy. (57)
Conclusions
During pregnancy, TG concentrations increase as a physiological adaptation mechanism, but if they reach very high levels, they become a risk factor for the mother and the child in the short and long term. The metabolic stress observed during this stage is associated with a decrease in the synthesis of fatty acids and the activity of lipoprotein lipase that elevates non-esterified fatty acids and increases insulin resistance. Greater lipolysis is associated with the increase of hormones (progesterone, prolactin and estrogens), cytokines (TNF-α and IL-6) and adipokines (leptin, visfatin and resistin), and with the decrease in adiponectin and apelin, which together reduce insulin sensitivity. For this reason, the availability of TG bound to lipoproteins such as VLDL increases for transplacental passage of fatty acids to the fetus through diffusion, hydrolysis and membrane transporters. These fatty acids reach the fetal liver attached to the α-fetoprotein.
Hypertriglyceridemia increases the risk of pregnancy complications, especially in pregnant women with a history of obesity or overweight, uncontrolled diabetes and familial dyslipidemia. In pregnant women, GDM and acute pancreatitis are the main complications of hypertriglyceridemia; on the other hand, PD, macrosomia and LGA fetuses are the main complications for the product of pregnancy.
For treatment, some case reports have described the use of statins and fibrates in pregnant women with severe hypertriglyceridemia; however, the use of these medications could generate more risks than benefits for the fetus, so its formulation is not recommended during pregnancy.
The limited number of studies and the great variability of the data indicate the need to conduct more research in Colombia to establish the normal ranges of TG during the three trimesters of pregnancy. This could facilitate diagnosis and monitoring of hypertriglyceridemia throughout pregnancy. On the other hand, it is important that health professionals understand the importance of measuring TG levels before pregnancy to determine risks and effective interventions and reduce maternal and child morbidity and mortality.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgements
None stated by the authors.
References
1.Wild R, Weedin EA, Gill EA. Women’s Health Considerations for Lipid Management. Endocrinol Metab Clin North Am. 2016;45(1):65-85. http://doi.org/f8fxx5.
2.Herrera E, Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm Mol Biol Clin Investig. 2016;26(2):109-27. http://doi.org/cgf8.
3.Vrijkotte TG, Krukziener N, Hutten BA, Vollebregt KC, Van Eijsden M, Twickler MB. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: The ABCD study. J Clin Endocrinol Metab. 2012;97(11):3917-25. http://doi.org/f4b9ns.
4.Niromanesh S, Shirazi M, Dastgerdy E, Sharbaf FR, Shirazi M, Khazaeipour Z. Association of hypertriglyceridaemia with pre-eclampsia, preterm birth, gestational diabetes and uterine artery pulsatility index. Natl Med J India. 2012;25(5):265-7.
5.El Khouly NI, Sanad ZF, Saleh SA, Shabana AA, Elhalaby AF, Badr EE. Value of first-trimester serum lipid profile in early prediction of preeclampsia and its severity: A prospective cohort study. Hypertens Pregnancy. 2016;35(1):73-81. http://doi.org/cgf9.
6.Cruz J, Grandía R, Padilla L, Rodríguez S, Hernández-García P, Lang-Prieto J, et al. Macrosomia Predictors in Infants Born to Cuban Mothers with Gestational Diabetes. MEDICC Rev. 2015;17(3):27-32.
7.Instituto Nacional de Salud. Enfermedades no trasmitibles. Bogotá, D.C.: Boletín Epidemiológico No 21; 2016.
8.Mayret-Mesquiti M, Pérez-Méndez O, Rodríguez ME, Fortoul TI, Gorocica P, Bernal-Alcántara D, et al. Hypertriglyceridemia is linked to reduced nitric oxide synthesis in women with hypertensive disorders of pregnancy. Hypertens pregnancy. 2007;26(4):423-31. http://doi.org/dtkhxg.
9.Capobianco E, Martínez N, Fornes D, Higa R, Di Marco I, Basualdo MN, et al. PPAR activation as a regulator of lipid metabolism, nitric oxide production and lipid peroxidation in the placenta from type 2 diabetic patients. Mol Cell Endocrinol. 2013;377(1-2):7-15. http://doi.org/f48stf.
10.Jawerbaum A, Capobianco E. Review: Effects of PPAR activation in the placenta and the fetus: Implications in maternal diabetes. Placenta. 2011;32(Suppl 2):S212-7. http://doi.org/bgxzfh.
11.Olmos PR, Rigotti A, Busso D, Berkowitz L, Santos JL, Borzone GR, et al. Maternal hypertriglyceridemia: A link between maternal overweight-obesity and macrosomia in gestational diabetes. Obesity (Silver Spring). 2014;22(10):2156-63. http://doi.org/cggb.
12.Olmos P, Martelo G, Reimer V, Rigotti A, Busso D, Belmar C, et al. La hipótesis de Pedersen no es suficiente: Otros nutrientes además de la glucosa explicarían la macrosomía fetal en pacientes diabéticas gestacionales con sobrepeso y buen control glicémico. Rev méd Chile. 2013;141(11):1441-8. http://doi.org/cggh.
13.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592-7. http://doi.org/df2w76.
14.Ferriols E, Rueda C, Gamero R, Vidal M, Payá A, Carreras R, et al. Comportamiento de los lípidos durante la gestación y su relación con acontecimientos obstétricos desfavorables. Clin Invest Arterioscl. 2016;28(5):232-44. http://doi.org/f3gxhv.
15.Gupta N, Ahmed S, Shaffer L, Cavens P, Blankstein J. Severe hypertriglyceridemia induced pancreatitis in pregnancy. Case Rep Obstet Gynecol. 2014;2014:485-93. http://doi.org/f3gxhv.
16.Barrett HL, Dekker-Nitert M, McIntyre HD, Callaway LK. Normalizing metabolism in diabetic pregnancy: is it time to target lipids? Diabetes Care. 2014;37(5):1484-93. http://doi.org/f5zbr4.
17.McGladdery SH, Frohlich JJ. Lipoprotein lipase and apoE polymorphisms: relationship to hypertriglyceridemia during pregnancy. J Lipid Res. 2001;42(11):1905-12.
18.Ward KJ, Shields B, Knight B, Salzmann MB, Hattersley AT, Frayling TM. Genetic variants in Apolipoprotein AV alter triglyceride concentrations in pregnancy. Lipids Health Dis. 2003;2:9. http://doi.org/dccv7x.
19.Montelongo A, Lasunción MA, Pallardo LF, Herrera E. Longitudinal-Study of Plasma lipoproteins and Hormones During Pregnancy in Normal and Diabetic Women. Diabetes. 1992;41(12):1651-9. http://doi.org/cggn.
20.Sjöström L, William-Olsson T. Prospective studies on adipose tissue development in man. Int J Obes. 1981;5(6):597-604.
21.Jin WY, Lin SL, Hou RL, Chen XY, Han T, Jin Y, et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth. 2016;16(1):60. http://doi.org/cggp.
22.Siddiqui I. Maternal Serum Lipids in Women with Pre-eclampsia. Ann Med Health Sci Res. 2014;4(4):638-41. http://doi.org/gcb4px.
23.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631-44. http://doi.org/d3cbtp.
24.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066-74. http://doi.org/dst3wb.
25.Duley L. The Global Impact of Pre-eclampsia and Eclampsia. Semin Perinatol. 2009;33(3):130-7. http://doi.org/bqvddg.
26.Kumru S, Aydin S, Gursu MF, Ozcan Z. Changes of serum paraoxonase (an HDL-cholesterol-associated lipophilic antioxidant) and arylesterase activities in severe preeclamptic women. Eur J Obstet Gynecol Reprod Biol. 2004;114(2):177-81. http://doi.org/b8bnmg.
27.Manna FN, Khanam NN, Chowdhury KA, Das SN, Kabir MA, Zubyra SJ, et al. Study on Association of Maternal Serum Triglyceride with Pre-eclampsia. Mymensingh Med J. 2015;24(3):578-84.
28.Qiu C, Phung TT, Vadachkoria S, Muy-Rivera M, Sanchez SE, Williams MA. Oxidized low-density lipoprotein (Oxidized LDL) and the risk of preeclampsia. Physiol Res. 2006;55(5):491-500.
29.Kadam L, Kohan-Ghadr HR, Drewlo S. The balancing act - PPAR-γ’s roles at the maternal-fetal interface. Syst Biol Reprod Med. 2015;61(2):65-71. http://doi.org/cghz.
30.Bowers K, Tobias DK, Yeung E, Hu FB, Zhang C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am J Clin Nutr. 2012;95(2):446-53. http://doi.org/fx2bnx.
31.Khan R, Ali K, Khan Z, Ahmad T. Lipid profile and glycosylated hemoglobin status of gestational diabetic patients and healthy pregnant women. Indian J Med Sci. 2012;66(7-8):149-54. http://doi.org/cgh2.
32.Han ES, Krauss RM, Xu F, Sridhar SB, Ferrara A, Quesenberry CP, et al. Prepregnancy adverse lipid profile and subsequent risk of gestational diabetes. J Clin Endocrinol Metab. 2016;101(7):2721-7. http://doi.org/f8wn58.
33.Gunderson EP, Quesenberry CP Jr, Jacobs DR Jr, Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: The CARDIA study. Am J Epidemiol. 2010;172(10):1131-43. http://doi.org/bdcc96.
34.Enquobahrie DA, Williams MA, Butler CL, Frederick IO, Miller RS, Luthy DA. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17(7):574-81. http://doi.org/bwbc23.
35.Merzouk H, Khan NA. Implication of lipids in macrosomia of diabetic pregnancy: can n-3 polyunsaturated fatty acids exert beneficial effects? Clin Sci (Lond). 2003;105(5):519-29. http://doi.org/bkxcc8.
36.Zhang X, Decker A, Platt RW, Kramer MS. How big is too big? The perinatal consequences of fetal macrosomia. Am J Obstet Gynecol. 2008;198(5):517.e1-6. http://doi.org/c9z643.
37.Chauhan SP, Grobman WA, Gherman RA, Chauhan VB, Chang G, Magann EF, et al. Suspicion and treatment of the macrosomic fetus: A review. Am J Obstet Gynecol. 2005;193(2):332-46. http://doi.org/bqm56b.
38.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007;109(2 Pt 1):419-33. http://doi.org/fw6tnr.
39.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964-8. http://doi.org/d38phg.
40.Ricart W, López J, Mozas J, Pericot A, Sancho MA, González N, et al. Body mass index has a greater impact on pregnancy outcomes than gestational hyperglycaemia. Diabetologia. 2005;48(9):1736-42. http://doi.org/bsq766.
41.Verhaeghe J, Pintiaux A, van Herck E, Hennen G, Foidart J-M, Igout A. Placental GH, IGF-I, IGF-Binding Protein-1, and Leptin during a Glucose Challenge Test in Pregnant Women: Relation with Maternal Body Weight, Glucose Tolerance, and Birth Weight. J Clin Endocrinol Metab. 2002;87(6):2875-82. http://doi.org/cgh4.
42.de Vries PS, Gielen M, Rizopoulos D, Rump P, Godschalk R, Hornstra G, et al. Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: The MEFAB cohort. Prostaglandins Leukot Essent Fatty Acids. 2014;91(3):81-5. http://doi.org/f6fh2b.
43.Meher AP, Wadhwani N, Randhir K, Mehendale S, Wagh G, Joshi SR. Placental DHA and mRNA levels of PPARγ and LXRα and their relationship to birth weight. J Clin Lipidol. 2016;10(4):767-74. http://doi.org/f83xmg.
44.Steffen KM, Cooper ME, Shi M, Caprau D, Simhan HN, Dagle JM, et al. Maternal and fetal variation in genes of cholesterol metabolism is associated with preterm delivery. J Perinatol. 2007;27(11):672-80. http://doi.org/bxgk2r.
45.Magnussen EB, Vatten LJ, Myklestad K, Salvesen KÅ, Romundstad PR. Cardiovascular risk factors prior to conception and the length of pregnancy: population-based cohort study. Am J Obstet Gynecol. 2011;204(6):526.e1-8. http://doi.org/b5qbkw.
46.Mattison DR, Damus K, Fiore E, Petrini J, Alter C. Preterm delivery: a public health perspective. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):7-16. http://doi.org/dxt4g7.
47.Pusukuru R, Shenoi AS, Kyada PK, Ghodke B, Mehta V, Bhuta K, et al. Evaluation of Lipid Profile in Second and Third Trimester of Pregnancy. J Clin Diagnostic Res. 2016;10(3):12-6. http://doi.org/cgh6.
48.Landázuri P, Restrepo B, Trejos J, Gallego ML, Loango-Chamorro N, Ocampo R. Perfil lipídico por trimestres de gestación en una población de mujeres colombianas. Rev Colomb Obstet Ginecol. 2006;57(4):256-63.
49.Ywaskewycz-Benítez LR, Bonneau GA, Castillo-Rascón MS, López DL, Pedrozo WR. Perfil lipídico por trimestre de gestación en una población de mujeres adultas. Rev Chil Obstet Ginecol. 2010;75(4):227-33. http://doi.org/dpqh2r.
50.Becerra-Leal AV, Salas-Paredes A, Buela L, Sosa MA, Arata-Bellabarba G, Valeri L, et al. Índice trigliceridos/cHDL en el embarazo: Interrelación con índices de resistencia a la insulina y antropometría fetal. Rev Venez Endocrinol y Metab. 2013;11(3):141-6.
51.Hersberger M, von Eckardstein A. Low high-density lipoprotein cholesterol: physiological background, clinical importance and drug treatment. Drugs. 2003;63(18):1907-45. http://doi.org/dvm9pp.
52.Valenzuela BR, Barrera RC, Gonzalez-Astorga M, Sanhueza CJ, Valenzuela BA. Alpha linolenic acid (ALA) from Rosa canina, sacha inchi and chia oils may increase ALA accretion and its conversion into n-3 LCPUFA in diverse tissues of the rat. Food Funct. 2014;5(7):1564–72. http://doi.org/ckck.
53.Gürsoy A, Kulaksizoglu M, Sahin M, Ertugrul DT, Ozer F, Tutuncu NB, et al. Severe hypertriglyceridemia-induced pancreatitis during pregnancy. J Natl Med Assoc. 2006;98(4):655-7.
54.Safi F, Toumeh A, Abuissa-Qadan MA, Karaz R, AlAkdar B, Assaly R. Management of familial hypertriglyceridemia-induced pancreatitis during pregnancy with therapeutic plasma exchange: a case report and review of literature. Am J Ther. 2014;21(5):e134-6. http://doi.org/cgh7.
55.Altun D, Eren G, Cukurova Z, Hergünsel O, Yasar L. An alternative treatment in hypertriglyceridemia-induced acute pancreatitis in pregnancy: Plasmapheresis. J Anaesthesiol Clin Pharmacol. 2012;28(2):252-4. http://doi.org/f3xftm.
56.Vandenbroucke L, Seconda S, Lassel L, Le Bouar G, Poulain P. Pancréatite aiguë secondaire à une hypertriglycéridémie majeure au cours de la grossesse. À propos d’un cas. J Gynecol Obstet Biol Reprod (Paris). 2009;38(5):436-9. http://doi.org/cbq964.
57.Kern-Pessôa VN, Rodacki M, Negrato CA, Zajdenverg L. Changes in lipid profile after treatment of women with gestational diabetes mellitus. J Clin Lipidol. 2016;10(2):350-5. http://doi.org/f8htrw.

Jean Marc Bourgery
“Traité complet de l’anatomie de l’homme”
Paris 1832-1854
Recibido: 30 de octubre de 2016; Aceptado: 15 de diciembre de 2016
Abstract
Introduction:
During pregnancy, levels of maternal serum triglycerides increase as a physiological adaptation mechanism to meet the needs of the developing fetus. However, although an excessive increase has been associated with preeclampsia, macrosomia and preterm delivery, the levels from which measurements should be taken in each trimester to prevent complications have not been established conclusively.
Objective:
To review the physiopathology, effects on mother and child, expected values in each trimester and therapeutic interventions in maternal hypertriglyceridemia.
Materials and methods:
A review was made based on a search of articles in the ScienceDirect, Pubmed, Scopus, LILACS, Cochrane and SciELO databases, with the terms: Pregnancy; Hypertriglyceridemia; Maternal-Fetal Exchange; Fetal Development; Pregnancy Complications in English and its equivalents in Spanish.
Results:
59 articles met the search criteria and responded to the objectives.
Conclusions:
The limited amount and the great variability of the data indicate the need to carry out further research to establish the normal triglycerides ranges during the three trimesters of pregnancy and to determine risks and effective interventions before pregnancy in order to reduce maternal and child morbidity and mortality.
Keywords:
Pregnancy, Hypertriglyceridemia, Maternal-Fetal Exchange, Fetal Development, Pregnancy Complications (MeSH).Resumen
Introducción.
Durante el embarazo, los niveles séricos de triglicéridos maternos aumentan como un mecanismo de adaptación fisiológica para suplir las necesidades del feto en desarrollo. Pese a que el incremento excesivo se ha asociado a preeclampsia, macrosomía y parto pretérmino, no se han establecido de manera contundente los niveles a partir de los cuales se deben tomar medidas en cada trimestre para prevenir complicaciones.
Objetivo.
Hacer una revisión sobre fisiopatología, efectos en madre e hijo, valores esperados en cada trimestre e intervenciones terapéuticas en hipertrigliceridemia gestacional.
Materiales y métodos.
Se realizó una revisión con la búsqueda de artículos en las bases de datos ScienceDirect, PubMed, Scopus, LILACS, Cochrane y SciELO con los términos: Pregnancy; Hypertriglyceridemia; Maternal-Fetal Exchange; Fetal Development; Pregnancy Complications y sus equivalentes en español.
Resultados.
Se encontraron 59 artículos que cumplieron los criterios de búsqueda y daban respuesta a los objetivos.
Conclusiones.
El número limitado y la gran variabilidad de los datos indican la necesidad de realizar más investigaciones que establezcan los rangos de normalidad de los triglicéridos durante los tres trimestres del embarazo y así determinar riesgos e intervenciones eficaces antes de la gestación y reducir la morbimortalidad materno-infantil.
Palabras clave:
Embarazo, Hipertrigliceridemia, Intercambio materno-fetal, Desarrollo fetal, Complicaciones del embarazo (DeCS).Introduction
During pregnancy, mother's physiology adapts to provide nutrients to the growing fetus. However, the imbalance in the amount of triglycerides (TG) -either before or during pregnancy- has been related to maternal-perinatal pathologies such as preeclampsia (PE) and gestational diabetes mellitus (GDM). In neonates, reported pathologies include preterm delivery (PD), dystocia, macrosomia, hypoglycemia or intrauterine growth restriction. 1-6
In Colombia, 7 482 cases of severe maternal morbidity (SMM) were reported during the first semester of 2016, which corresponds to 27.9 mothers for every 1 000 live births (LB), and 182 cases of maternal mortality, with 49.2 cases for every 100 000 LB. 7 The main causes of SMM and maternal mortality were hypertensive disorders (62.4% and 15.5%, respectively) and hemorrhagic complications (15.5% and 18%, respectively). 7
Exacerbated hypertriglyceridemia and insulin resistance condition an oxidative environment that leads to endothelial injury, which, in turn, has a predisposing effect on the development of PE as a hypertensive disorder of higher incidence. 8-10 Intrauterine weight gain may be associated with the elevation of TG passage through the placenta and TG production by the fetus, which would lead to a large for gestational age (LGA) fetus and, thus, possible hemorrhagic complications during delivery by overdistension and uterine rupture. 6,11,12
Besides short-term complications associated with hypertriglyceridemia, other long-term complications involve metabolic disorders and cardiovascular diseases (CVD) that may affect the well-being of the child during adulthood and the health of the mother in her middle and late adulthood. 13-16 The objective of this work is to perform a comprehensive literature review on the pathophysiological causes, effects on mother and child, expected TG values in each trimester of pregnancy and possible therapies to manage maternal hypertriglyceridemia in a timely manner.
Increase in circulating triglycerides during pregnancy
Pregnancy is a state of metabolic stress associated with high TG levels 17, which increase during this period; the highest concentrations are observed during the third trimester. 1 This increase is related to the decrease in the synthesis of fatty acids and the activity of the lipoprotein lipase (LPL) that catalyzes the hydrolysis of TG-rich lipoproteins in the adipose tissue. 1 The activity of this enzyme decreases about 85% during a normal pregnancy. 2,17 TG levels decrease in the postpartum period and this decrease is faster in women who lactate. 18
The abovementioned events are related to the insulin resistance that occurs during pregnancy, which may be caused by the increase of non-esterified fatty acids, changes in adipokines secretion and inflammatory factors. 1,2,17 Increased lipolysis has been associated with increased placental lactogen, progesterone, prolactin, cortisol and estrogen. 2,19 Adiponectin and apelin, which favor insulin sensitivity, decrease in the third trimester, while other adipokines and cytokines that reduce insulin sensitivity increase at the end of pregnancy, including resistin, retinol binding protein 4 (RBP4), leptin, visfatin, chemerin, adipocyte fatty acid binding protein (AFABP), tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) 2. In addition, the expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) in adipose tissue decreases in the third trimester, contributing to insulin resistance. 2
Placental passage of maternal triglycerides
1-3% of maternal fatty acids circulate in non-esterified form and enter the syncytiotrophoblast through diffusion or receptor-mediated endocytosis. 3 Low-density lipoproteins associated with TG (LDL-TG), more abundant in circulation, are hydrolyzed by intracellular lipases and cholesterol ester hydrolases. On the other hand, high density lipoproteins (HDL) and very low density lipoproteins (VLDL) bind to surface receptors and are hydrolysed extracellularly by endothelial lipase. 2,3,16 Transporters such as fatty acid transport protein 1 and 4 (FATP-1 and FATP-4), fatty acids translocase (FAT/ CD36) and plasma membrane fatty acid-binding protein (FABPpm) make fatty acid uptake a more efficient process. 3,16
At the intracellular level, the fatty acids bind to the fatty acid binding protein (FABP), which has intrinsic acetyl-CoA ligase activity. 16 Then, the syncytiotrophoblast releases fatty acids into the fetal circulation to bind to the α-fetoprotein (AFP) that takes them to the fetal liver where they are metabolized (Figure 1). 2,12,16 It should be noted that lipid droplets have been observed in the placenta and that their formation is stimulated by adipophilin. 16
Figure 1: Passage of triglycerides through the placenta. TG: triglycerides; VLDL: very low density lipoprotein; LDL: low density lipoprotein; HDL: high density lipoprotein; FATP: fatty acid transport protein; FAT/CD 36: fatty acid translocase; FABPpm: plasma membrane fatty acid-binding protein; IC: intracellular; FABP: fatty acid binding protein; NEFA: non-esterified fatty acids.
White adipose tissue is observed in the fetus since week 14 or 15, while fetal lipogenesis begins at week 12 or 20; PPAR-γ activation plays an important role in the subsequent increase of adipose tissue size. 20 Fetus weight increases fourfold from 1.6 g/kg/day to 3.4 g/ kg/day since the 26th week of pregnancy, but the increase in fetal body fat depends by 20% on placental lipid transfer; the remaining tissue results from fetal lipogenesis. It is worth noting that only one third of circulating maternal glucose is used by placenta through lipolytic routes and the demand is not modified by increasing glucose levels because the utilization is saturated between 90 mg/dL and 143 mg/dL. 2
Hypertriglyceridemia and pathologies
A study carried out in Amsterdam (n=4 008) revealed that TG increase in the first trimester of pregnancy is directly associated with pregnancy-induced hypertension, PD and LGA. 3 Likewise, a research carried out in India (n=180) found that high TG levels (>195 mg/dL) in the second trimester are associated with a higher incidence of PD, GDM, PE and LGA. 4 Another complication is maternal pancreatitis, which must be mentioned due to its severity (Table 1). 14,18
PE: preeclampsia; TG: triglycerides; GDM: gestational diabetes mellitus; LGA: large for gestational age fetus. Source: Own elaboration based on the data obtained in the study.Table 1: Outcomes of gestational hypertriglyceridemia according to different studies.

In the mother
Preeclampsia
Between 2% and 8% of pregnancies are complicated by PE, which is the third cause of maternal-fetal death after hemorrhage and sepsis. 14,23-25 In the mother, this condition is associated with endothelial dysfunction, hypertension, kidney disease and diabetes mellitus (DM). It also increases the risk of developing CVD and death due to kidney and liver impairment. 14 Hypertriglyceridemia, before or during pregnancy, alters the vascular development of the placenta and results from inadequate implantation or placental perfusion. 3,5,8 PE and dyslipidemia are correlated because LPL dysfunction, metabolic syndrome and increased plasma lipids occur in both. 4 These disorders stimulate peroxidation of placental lipids and trophoblast components that promote oxidative stress and form deleterious complexes in endothelial cells that cause vascular dysfunction. 4,5
A prospective cohort study conducted in Egypt (n=251) showed that TG levels between weeks 4 and 12 of pregnancy can be predictors of PE development. 5,8 This study found that the increase in total cholesterol (TC), TG and LDL greater than 231 mg/dL, 149.5 mg/ dL and 161 mg/dL, respectively, and the decrease in HDL below 42.5 mg/dL are cutoff points with positive predictive value for the development of PE, while TC and TG increase was related to severity. 5 Another study conducted in Turkey (n=52) found that TC, TG and LDL increase greater than 4%, 5% and 9.8%, respectively, and a decrease in HDL by more than 9% are associated with worse prognosis of gestational hypertensive disease. 26
The study by Manna et al. 27 in Bangladesh (n=90) showed that increased TG levels are directly associated with an increase in blood pressure. In the study group, systolic and diastolic pressures were 152.4±19.8 mmHg and 103.1±12.2 mmHg, respectively, while in pregnant controls, they were 112.0±8.9 mmHg and 75.5±6.6 mmHg, respectively. 27 These numbers were associated with TG levels in the study group (242.9±36.8 mg/dL) and in the control group (184.6±12.5 mg/dL). 27TG>181 mg/dL before week 20 increased the risk of PE by 3 to 7 times. 28
A study conducted in Mexico with 47 normotensive women and 27 with PE or gestational hypertension in the seventh month of pregnancy showed that hypertriglyceridemia is related to hypertensive disorders in pregnancy. Researchers found that nitric oxide (NO) synthesis decreased proportionally to the increase of TG levels. 8 NO decrease may be secondary to oxidative stress increase considering the high concentration of TG or glucose that inhibits NO synthesis. 29 In this study, women with hypertensive disorders of pregnancy were treated with hydralazine, which induces NO synthesis. 8 It has been observed that the increase of fatty acids in the placenta of women with DM1 is related to reduced fetal-placental circulation and that having a family history of DM2 is closely related to an increased risk of developing hypertensive disorders during pregnancy. 2
Gestational diabetes mellitus
The prevalence of GDM has gone from 4% to 20% in 27 years and its current incidence is 1-14%. 30,31 This pathology not only increases the risk of PE and macrosomia during pregnancy, but also predisposes the mother to the development of DM2 and CVD. 12 Mothers with GDM have hypertriglyceridemia, hypercholesterolemia, insulin resistance, low levels of adiponectin and increased intracellular concentrations of AFABP 2; therefore, they are characterized by having insulin resistance. 4,12,29
Alterations have been found in the fetus and placenta related to imbalances in the expression and function of PPARs isotypes that increase lipid flow in women with hyperlipidemia and GDM. 2,9,10
Concentrations of PPAR-γ and PPAR-α are low in term-placental tissue of women who developed GDM, whereas no changes are detected in PPAR-δ concentrations. 9,10 No changes were found in PPAR-γ concentrations in women with DM1; however, decreased levels of 15-deoxy δ-12, 14-prostaglandin J2 (15dPGJ2) were observed. 9 Also, alterations in the expression of PPAR-α during the first trimester of pregnancy are associated with spontaneous abortions. 10 15dPGJ2 increases lipid concentrations and significantly reduces NO expression in the placenta of healthy women, while it regulates the increase in the concentrations of phospholipids and cholesterol esters in the placenta of diabetic women. 9 In addition, its receptor (PPAR-γ) is decreased in these patients, which could increase NO and lipid peroxidation, markers of pro-inflammatory and pro-oxidant states. 9
A case-control study (n=254) conducted in the USA by Han et al. 32 found that the measurement of the pregestational lipid profile is a predictor of GDM. This study revealed that women who developed GDM, in comparison with the controls, presented smaller LDL diameters, low concentrations of HDL and high levels of small VLDL regardless of other risk factors (body mass index -BMI-, weight gain during pregnancy, age or ethnicity) in measurements taken even up to 7 years before pregnancy. 32,33 On the other hand, the risk of developing GDM is 3.5 times higher if TG >137 mg/dL in the first trimester. In addition, every time TG increase by 20 mg/ dL, the risk of developing DMG increases by 10%. 34
Pancreatitis
Acute pancreatitis caused by gestational hypertriglyceridemia is a rare complication of pregnancy but, when it occurs, it represents high maternal and fetal morbidity and mortality 1,15,16,18 and may be complicated by pancreatic necrosis, shock, hypokalemia, PE or eclampsia. 15 The risk of developing pancreatitis increases progressively with TG >500 mg/dL. 15 Thus, during the first trimester, these increased levels are associated with a 19% risk of pancreatitis, in the second with 26%, in the third with 53% and in the postpartum with 2% 1,15, depending on the activity of pancreatic lipase and liver involvement. 15,18
In the fetus
Macrosomia
Lipids availability and accumulation in the fetus of a mother with dyslipidemia increases the risk of developing macrosomia and PD. 6 A LB presents macrosomia if its weight is >4kg and LGA if its size is above the 90th percentile. 12,14 Giving birth to a LGA fetus increases the likelihood of prolonged labor, cesarean or postpartum hemorrhage. In addition, giving birth to fetuses with macrosomia makes women 4.2 times more susceptible to developing DM2 throughout their lives. 35 Hypoglycemia, hyperbilirubinemia, respiratory distress, cardiac hypertrophy, shoulder dystocia, clavicle fracture, and brachial plexus injuries may occur in the newborn. 36,37
A Cuban case-control study (n=236) showed that the increase in TG levels during the third trimester was a predictor of the development of fetal macrosomia (OR:4.80, CI95%: 2.34-9.84). 6 Another study conducted in Chile in patients with well-controlled GDM and hypertriglyceridemia found that macrosomia was more frequent in women with pre-pregnancy overweight or obesity. 12,38,39 A BMI >26.1 kg/m2 was a predictor of macrosomia regardless of hypertriglyceridemia during the first trimester of pregnancy 40, perhaps due to the fact that free fatty acids act as growth factors and, that in high concentrations, they compete for the binding site of sex hormones to albumin, which increases the levels of free hormones that could act on the placenta and the fetus, thus modifying its growth and development. 3 It is noteworthy that the levels of circulating leptin in pregnant women with GDM, PE, intrauterine growth restriction or macrosomia have not been shown to have a predictive value on the weight of the newborn. 41
Contrary to the increase in TG, recent studies suggest that low levels of omega 3 fatty acids during prenatal life influence adiposity in children, not only in intrauterine life, but also in the postnatal period. 42,43 Low concentrations of omega 3 fatty acids are associated with lower weights. In turn, a study conducted in India identified that the placenta of term infants with low weight had lower levels of docosahexaenoic acid (DHA) compared to term neonates weighing > 2 500g. 43
Preterm delivery
PD is the leading cause of neonatal morbidity and mortality and occurs in 12% of births. 14,44 Its risk increases to 60% if there is a history of DM1, DM2 or pregestational hyperlipidemia and to 33.3%, if there is hypertriglyceridemia in the third trimester. 44-46
Variants in LPL
Polymorphisms in the LPL gene (S447X, N291S and D9N) are associated with exaggerated increase in TG levels during pregnancy. 17,18 In addition, two APOAV polymorphisms, -1131T>C and S19W, are related to increased VLDL secretion and increased circulating TG levels of 11% and 16.2%, respectively. 18 An interesting correlation has been found between APOAV (-1131T>C), maternal size and crown-rump length of the fetus. 18
Measurement of triglycerides during pregnancy
In practice, measuring the concentrations of circulating TG once every trimester is advisable, since increased TG levels are normal during pregnancy (Table 2). Clinically, xanthomas on the external surface of arms, legs and buttocks, retinal lipemia, hepatosplenomegaly and lipemic serum are very suggestive of severe hypertriglyceridemia. 15
Source: Own elaboration based on the data obtained in the study.Table 2: Normal triglycerides levels during pregnancy.
A study conducted by Landázuri et al. 48 in Colombia (n=422) found that TG levels were 86% higher during the second trimester of pregnancy and 137.8% in the third compared to the first. The authors followed 56 of these pregnant women throughout their pregnancy and observed a TG increase of 58.8% from the first to the second trimester and of 112.6% from the first to the third trimester (p<0.001). 48 In this study, low HDL levels were observed compared to the European or North American population during pregnancy. 46,47 Low HDL serum levels could be an additional risk factor for cardiovascular or gestational diseases in Colombian mothers and their children. 51
Studies to evaluate the lipid profile of normal pregnant women have allowed proposing physiological levels for different populations. Thus, the study conducted by Ywaskewycz et al. 49 (n=291) in pregnant women without complications showed a TG increase of 56% from the first to second trimester and of 124% from the first to the third trimester; in the third trimester, the increase was twofold as compared to non-pregnant controls, and no differences were observed between TG in the first trimester and TG in non-pregnant women. 49 In addition, the TG/cHDL ratio increases during pregnancy, thus indicating the presence of LDL proteins of smaller size, rich in TG, denser and with higher atherogenic risk. 49
On the other hand, the study by Becerra et al. (50) (n=91) with healthy pregnant women found that TG levels increased significantly (p<0.001) from the first to the second and third trimesters, and that the TG/cHDL ratio was correlated to pre-pregnancy BMI, fetal abdominal circumference, estimated fetal weight and uterine height. (p<0.01)
Treatment of hypertriglyceridemia during pregnancy
The treatment of hypertriglyceridemia depends on how high lipids are. If moderate (200-999 mg/dL), a strict low-fat diet with nutritional support with medium-chain TG and ω-3 fatty acids is initiated while increasing physical activity. Dietary supplementation with DHA and eicosapentaenoic acid reduces the production of proinflammatory cytokines (TNF-α, IL-1, IL-6 and IL-8) and inhibits the synthesis of VLDL without altering that of HDL. 15,16,52
If hypertriglyceridemia is severe (>1000 mg/dL), using medications such as fibrates (PPAR-α agonists), statins, niacin, heparin or insulin is considered. Other possible alternatives are carbaprostacycline and iloprost, drugs that activate PPAR-δ and glitazones, which are synthetic ligands for PPAR-γ. 15,53 However, to the extent possible, using medications should be avoided during the first trimester of pregnancy, since many are contraindicated due to the potential harm to the fetus. It should be noted that using statins and fibrates has been described in case reports.
In case dyslipidemia is refractory to drugs or nutrition, plasmapheresis is initiated. 54 This procedure is also indicated when serum lipase levels are >3 times the normal limit of this enzyme or when hypocalcemia, lactic acidosis and worsening of inflammation or organic dysfunction occur; this can also be combined with heparin infusion. 55,56 When TG levels are below 500 mg/dL, this procedure should be stopped 55,56; however, if contraindicated or not available, infusion of regular insulin with 5% dextrose is used and glucose levels must be maintained between 150 mg/dL and 200 mg/dL during therapy. 15,53 When TG levels are normalized, the next step is to perform a rigorous control of the lipid profile, long-term dietary restriction and administration of fenofibrate. 15,16 In severe hypertriglyceridemia, PD is induced considering the high risks of maternal and fetal mortality (20% and 50%, respectively) and secondary pancreatitis. 1,15,16
A study conducted by Kern-Pessoa et al. 57 in Sao Paulo (n=73) found that TC and TG levels increased during pregnancy and decreased from the third to the sixth postpartum week in women with GDM. 57 In this study, LDL and TG levels increased during pregnancy in patients who received insulin, and decreased in those treated with a strict low-fat diet, rich in ω-3 fatty acids as therapy. 57
Conclusions
During pregnancy, TG concentrations increase as a physiological adaptation mechanism, but if they reach very high levels, they become a risk factor for the mother and the child in the short and long term. The metabolic stress observed during this stage is associated with a decrease in the synthesis of fatty acids and the activity of lipoprotein lipase that elevates non-esterified fatty acids and increases insulin resistance. Greater lipolysis is associated with the increase of hormones (progesterone, prolactin and estrogens), cytokines (TNF-α and IL-6) and adipokines (leptin, visfatin and resistin), and with the decrease in adiponectin and apelin, which together reduce insulin sensitivity. For this reason, the availability of TG bound to lipoproteins such as VLDL increases for transplacental passage of fatty acids to the fetus through diffusion, hydrolysis and membrane transporters. These fatty acids reach the fetal liver attached to the α-fetoprotein.
Hypertriglyceridemia increases the risk of pregnancy complications, especially in pregnant women with a history of obesity or overweight, uncontrolled diabetes and familial dyslipidemia. In pregnant women, GDM and acute pancreatitis are the main complications of hypertriglyceridemia; on the other hand, PD, macrosomia and LGA fetuses are the main complications for the product of pregnancy.
For treatment, some case reports have described the use of statins and fibrates in pregnant women with severe hypertriglyceridemia; however, the use of these medications could generate more risks than benefits for the fetus, so its formulation is not recommended during pregnancy.
The limited number of studies and the great variability of the data indicate the need to conduct more research in Colombia to establish the normal ranges of TG during the three trimesters of pregnancy. This could facilitate diagnosis and monitoring of hypertriglyceridemia throughout pregnancy. On the other hand, it is important that health professionals understand the importance of measuring TG levels before pregnancy to determine risks and effective interventions and reduce maternal and child morbidity and mortality.
Acknowledgements
None stated by the authors.
References
Referencias
Wild R, Weedin EA, Gill EA. Women’s Health Considerations for Lipid Management. Endocrinol Metab Clin North Am. 2016;45(1):65-85. http://doi.org/f8fxx5.
Herrera E, Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm Mol Biol Clin Investig. 2016;26(2):109-27. http://doi.org/cgf8.
Vrijkotte TG, Krukziener N, Hutten BA, Vollebregt KC, Van Eijsden M, Twickler MB. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: The ABCD study. J Clin Endocrinol Metab. 2012;97(11):3917-25. http://doi.org/f4b9ns.
Niromanesh S, Shirazi M, Dastgerdy E, Sharbaf FR, Shirazi M, Khazaeipour Z. Association of hypertriglyceridaemia with pre-eclampsia, preterm birth, gestational diabetes and uterine artery pulsatility index. Natl Med J India. 2012;25(5):265-7.
El Khouly NI, Sanad ZF, Saleh SA, Shabana AA, Elhalaby AF, Badr EE. Value of first-trimester serum lipid profile in early prediction of preeclampsia and its severity: A prospective cohort study. Hypertens Pregnancy. 2016;35(1):73-81. http://doi.org/cgf9.
Cruz J, Grandía R, Padilla L, Rodríguez S, Hernández-García P, Lang-Prieto J, et al. Macrosomia Predictors in Infants Born to Cuban Mothers with Gestational Diabetes. MEDICC Rev. 2015;17(3):27-32.
Instituto Nacional de Salud. Enfermedades no trasmitibles. Bogotá, D.C.: Boletín Epidemiológico No 21; 2016.
Mayret-Mesquiti M, Pérez-Méndez O, Rodríguez ME, Fortoul TI, Gorocica P, Bernal-Alcántara D, et al. Hypertriglyceridemia is linked to reduced nitric oxide synthesis in women with hypertensive disorders of pregnancy. Hypertens pregnancy. 2007;26(4):423-31. http://doi.org/dtkhxg.
Capobianco E, Martínez N, Fornes D, Higa R, Di Marco I, Basualdo MN, et al. PPAR activation as a regulator of lipid metabolism, nitric oxide production and lipid peroxidation in the placenta from type 2 diabetic patients. Mol Cell Endocrinol. 2013;377(1-2):7-15. http://doi.org/f48stf.
Jawerbaum A, Capobianco E. Review: Effects of PPAR activation in the placenta and the fetus: Implications in maternal diabetes. Placenta. 2011;32(Suppl 2):S212-7. http://doi.org/bgxzfh.
Olmos PR, Rigotti A, Busso D, Berkowitz L, Santos JL, Borzone GR, et al. Maternal hypertriglyceridemia: A link between maternal overweight-obesity and macrosomia in gestational diabetes. Obesity (Silver Spring). 2014;22(10):2156-63. http://doi.org/cggb.
Olmos P, Martelo G, Reimer V, Rigotti A, Busso D, Belmar C, et al. La hipótesis de Pedersen no es suficiente: Otros nutrientes además de la glucosa explicarían la macrosomía fetal en pacientes diabéticas gestacionales con sobrepeso y buen control glicémico. Rev méd Chile. 2013;141(11):1441-8. http://doi.org/cggh.
Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592-7. http://doi.org/df2w76.
Ferriols E, Rueda C, Gamero R, Vidal M, Payá A, Carreras R, et al. Comportamiento de los lípidos durante la gestación y su relación con acontecimientos obstétricos desfavorables. Clin Invest Arterioscl. 2016;28(5):232-44. http://doi.org/f3gxhv.
Gupta N, Ahmed S, Shaffer L, Cavens P, Blankstein J. Severe hypertriglyceridemia induced pancreatitis in pregnancy. Case Rep Obstet Gynecol. 2014;2014:485-93. http://doi.org/f3gxhv.
Barrett HL, Dekker-Nitert M, McIntyre HD, Callaway LK. Normalizing metabolism in diabetic pregnancy: is it time to target lipids? Diabetes Care. 2014;37(5):1484-93. http://doi.org/f5zbr4.
McGladdery SH, Frohlich JJ. Lipoprotein lipase and apoE polymorphisms: relationship to hypertriglyceridemia during pregnancy. J Lipid Res. 2001;42(11):1905-12.
Ward KJ, Shields B, Knight B, Salzmann MB, Hattersley AT, Frayling TM. Genetic variants in Apolipoprotein AV alter triglyceride concentrations in pregnancy. Lipids Health Dis. 2003;2:9. http://doi.org/dccv7x.
Montelongo A, Lasunción MA, Pallardo LF, Herrera E. Longitudinal-Study of Plasma lipoproteins and Hormones During Pregnancy in Normal and Diabetic Women. Diabetes. 1992;41(12):1651-9. http://doi.org/cggn.
Sjöström L, William-Olsson T. Prospective studies on adipose tissue development in man. Int J Obes. 1981;5(6):597-604.
Jin WY, Lin SL, Hou RL, Chen XY, Han T, Jin Y, et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth. 2016;16(1):60. http://doi.org/cggp.
Siddiqui I. Maternal Serum Lipids in Women with Pre-eclampsia. Ann Med Health Sci Res. 2014;4(4):638-41. http://doi.org/gcb4px.
Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631-44. http://doi.org/d3cbtp.
Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066-74. http://doi.org/dst3wb.
Duley L. The Global Impact of Pre-eclampsia and Eclampsia. Semin Perinatol. 2009;33(3):130-7. http://doi.org/bqvddg.
Kumru S, Aydin S, Gursu MF, Ozcan Z. Changes of serum paraoxonase (an HDL-cholesterol-associated lipophilic antioxidant) and arylesterase activities in severe preeclamptic women. Eur J Obstet Gynecol Reprod Biol. 2004;114(2):177-81. http://doi.org/b8bnmg.
Manna FN, Khanam NN, Chowdhury KA, Das SN, Kabir MA, Zubyra SJ, et al. Study on Association of Maternal Serum Triglyceride with Pre-eclampsia. Mymensingh Med J. 2015;24(3):578-84.
Qiu C, Phung TT, Vadachkoria S, Muy-Rivera M, Sanchez SE, Williams MA. Oxidized low-density lipoprotein (Oxidized LDL) and the risk of preeclampsia. Physiol Res. 2006;55(5):491-500.
Kadam L, Kohan-Ghadr HR, Drewlo S. The balancing act - PPAR-γ’s roles at the maternal-fetal interface. Syst Biol Reprod Med. 2015;61(2):65-71. http://doi.org/cghz.
Bowers K, Tobias DK, Yeung E, Hu FB, Zhang C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am J Clin Nutr. 2012;95(2):446-53. http://doi.org/fx2bnx.
Khan R, Ali K, Khan Z, Ahmad T. Lipid profile and glycosylated hemoglobin status of gestational diabetic patients and healthy pregnant women. Indian J Med Sci. 2012;66(7-8):149-54. http://doi.org/cgh2.
Han ES, Krauss RM, Xu F, Sridhar SB, Ferrara A, Quesenberry CP, et al. Prepregnancy adverse lipid profile and subsequent risk of gestational diabetes. J Clin Endocrinol Metab. 2016;101(7):2721-7. http://doi.org/f8wn58.
Gunderson EP, Quesenberry CP Jr, Jacobs DR Jr, Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: The CARDIA study. Am J Epidemiol. 2010;172(10):1131-43. http://doi.org/bdcc96.
Enquobahrie DA, Williams MA, Butler CL, Frederick IO, Miller RS, Luthy DA. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17(7):574-81. http://doi.org/bwbc23.
Merzouk H, Khan NA. Implication of lipids in macrosomia of diabetic pregnancy: can n-3 polyunsaturated fatty acids exert beneficial effects? Clin Sci (Lond). 2003;105(5):519-29. http://doi.org/bkxcc8.
Zhang X, Decker A, Platt RW, Kramer MS. How big is too big? The perinatal consequences of fetal macrosomia. Am J Obstet Gynecol. 2008;198(5):517.e1-6. http://doi.org/c9z643.
Chauhan SP, Grobman WA, Gherman RA, Chauhan VB, Chang G, Magann EF, et al. Suspicion and treatment of the macrosomic fetus: A review. Am J Obstet Gynecol. 2005;193(2):332-46. http://doi.org/bqm56b.
Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007;109(2 Pt 1):419-33. http://doi.org/fw6tnr.
Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964-8. http://doi.org/d38phg.
Ricart W, López J, Mozas J, Pericot A, Sancho MA, González N, et al. Body mass index has a greater impact on pregnancy outcomes than gestational hyperglycaemia. Diabetologia. 2005;48(9):1736-42. http://doi.org/bsq766.
Verhaeghe J, Pintiaux A, van Herck E, Hennen G, Foidart J-M, Igout A. Placental GH, IGF-I, IGF-Binding Protein-1, and Leptin during a Glucose Challenge Test in Pregnant Women: Relation with Maternal Body Weight, Glucose Tolerance, and Birth Weight. J Clin Endocrinol Metab. 2002;87(6):2875-82. http://doi.org/cgh4.
de Vries PS, Gielen M, Rizopoulos D, Rump P, Godschalk R, Hornstra G, et al. Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: The MEFAB cohort. Prostaglandins Leukot Essent Fatty Acids. 2014;91(3):81-5. http://doi.org/f6fh2b.
Meher AP, Wadhwani N, Randhir K, Mehendale S, Wagh G, Joshi SR. Placental DHA and mRNA levels of PPARγ and LXRα and their relationship to birth weight. J Clin Lipidol. 2016;10(4):767-74. http://doi.org/f83xmg.
Steffen KM, Cooper ME, Shi M, Caprau D, Simhan HN, Dagle JM, et al. Maternal and fetal variation in genes of cholesterol metabolism is associated with preterm delivery. J Perinatol. 2007;27(11):672-80. http://doi.org/bxgk2r.
Magnussen EB, Vatten LJ, Myklestad K, Salvesen KÅ, Romundstad PR. Cardiovascular risk factors prior to conception and the length of pregnancy: population-based cohort study. Am J Obstet Gynecol. 2011;204(6):526.e1-8. http://doi.org/b5qbkw.
Mattison DR, Damus K, Fiore E, Petrini J, Alter C. Preterm delivery: a public health perspective. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):7-16. http://doi.org/dxt4g7.
Pusukuru R, Shenoi AS, Kyada PK, Ghodke B, Mehta V, Bhuta K, et al. Evaluation of Lipid Profile in Second and Third Trimester of Pregnancy. J Clin Diagnostic Res. 2016;10(3):12-6. http://doi.org/cgh6.
Landázuri P, Restrepo B, Trejos J, Gallego ML, Loango-Chamorro N, Ocampo R. Perfil lipídico por trimestres de gestación en una población de mujeres colombianas. Rev Colomb Obstet Ginecol. 2006;57(4):256-63.
Ywaskewycz-Benítez LR, Bonneau GA, Castillo-Rascón MS, López DL, Pedrozo WR. Perfil lipídico por trimestre de gestación en una población de mujeres adultas. Rev Chil Obstet Ginecol. 2010;75(4):227-33. http://doi.org/dpqh2r.
Becerra-Leal AV, Salas-Paredes A, Buela L, Sosa MA, Arata-Bellabarba G, Valeri L, et al. Índice trigliceridos/cHDL en el embarazo: Interrelación con índices de resistencia a la insulina y antropometría fetal. Rev Venez Endocrinol y Metab. 2013;11(3):141-6.
Hersberger M, von Eckardstein A. Low high-density lipoprotein cholesterol: physiological background, clinical importance and drug treatment. Drugs. 2003;63(18):1907-45. http://doi.org/dvm9pp.
Valenzuela BR, Barrera RC, Gonzalez-Astorga M, Sanhueza CJ, Valenzuela BA. Alpha linolenic acid (ALA) from Rosa canina, sacha inchi and chia oils may increase ALA accretion and its conversion into n-3 LCPUFA in diverse tissues of the rat. Food Funct. 2014;5(7):1564–72. http://doi.org/ckck.
Gürsoy A, Kulaksizoglu M, Sahin M, Ertugrul DT, Ozer F, Tutuncu NB, et al. Severe hypertriglyceridemia-induced pancreatitis during pregnancy. J Natl Med Assoc. 2006;98(4):655-7.
Safi F, Toumeh A, Abuissa-Qadan MA, Karaz R, AlAkdar B, Assaly R. Management of familial hypertriglyceridemia-induced pancreatitis during pregnancy with therapeutic plasma exchange: a case report and review of literature. Am J Ther. 2014;21(5):e134-6. http://doi.org/cgh7.
Altun D, Eren G, Cukurova Z, Hergünsel O, Yasar L. An alternative treatment in hypertriglyceridemia-induced acute pancreatitis in pregnancy: Plasmapheresis. J Anaesthesiol Clin Pharmacol. 2012;28(2):252-4. http://doi.org/f3xftm.
Vandenbroucke L, Seconda S, Lassel L, Le Bouar G, Poulain P. Pancréatite aiguë secondaire à une hypertriglycéridémie majeure au cours de la grossesse. À propos d’un cas. J Gynecol Obstet Biol Reprod (Paris). 2009;38(5):436-9. http://doi.org/cbq964.
Kern-Pessôa VN, Rodacki M, Negrato CA, Zajdenverg L. Changes in lipid profile after treatment of women with gestational diabetes mellitus. J Clin Lipidol. 2016;10(2):350-5. http://doi.org/f8htrw.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Sapna Ramdin, Thajasvarie Naicker, Virushka Pillay, Sanil D. Singh, Sooraj Baijnath, Blessing N Mkhwanazi, Nalini Govender. (2022). Physiological characterization of an arginine vasopressin rat model of preeclampsia. Systems Biology in Reproductive Medicine, 68(1), p.55. https://doi.org/10.1080/19396368.2021.1981486.
2. Sudarshan Dash, Malvika Tiwari, Putul Dash, Kaustav Kar, Nirmal K Mohakud. (2022). Complications of Hypertriglyceridemia in Pregnancy and Its Impact on Neonates: a Hospital-Based Study From Odisha. Cureus, https://doi.org/10.7759/cureus.28399.
3. Chun Yang, Wu Jing, Sheng Ge, Wenguang Sun. (2021). Vitamin D status and vitamin D deficiency risk factors among pregnancy of Shanghai in China. BMC Pregnancy and Childbirth, 21(1) https://doi.org/10.1186/s12884-021-03889-0.
4. Olatunji Anthony Akerele, Sarah Jane Manning, Sarah Emily Dixon, Amelia Estelle Lacey, Sukhinder Kaur Cheema. (2021). Maternal omega-3 fatty acids maintained positive maternal lipids and cytokines profile, and improved pregnancy outcomes of C57BL/6 mice. The Journal of Nutritional Biochemistry, 98, p.108813. https://doi.org/10.1016/j.jnutbio.2021.108813.
5. Gheorghe Cruciat, Georgiana Nemeti, Iulian Goidescu, Stefan Anitan, Andreea Florian. (2020). Hypertriglyceridemia triggered acute pancreatitis in pregnancy – diagnostic approach, management and follow-up care. Lipids in Health and Disease, 19(1) https://doi.org/10.1186/s12944-019-1180-7.
6. Julia Cristina Coronado Arroyo, Marcio José Concepción Zavaleta, Eilhart Jorge García Villasante, Mikaela Kcomt Lam, Luis Alberto Concepción Urteaga, Francisca Elena Zavaleta Gutiérrez. (2021). Familial Chylomicronemia Syndrome-Induced Acute Necrotizing Pancreatitis during Pregnancy. Revista Brasileira de Ginecologia e Obstetrícia / RBGO Gynecology and Obstetrics, 43(03), p.220. https://doi.org/10.1055/s-0040-1722173.
7. Indu G. Poornima, Mahathi Indaram, Joyce D. Ross, Anandita Agarwala, Robert A. Wild. (2022). Hyperlipidemia and risk for preclampsia. Journal of Clinical Lipidology, 16(3), p.253. https://doi.org/10.1016/j.jacl.2022.02.005.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2018 Revista de la Facultad de Medicina

Esta obra está bajo una licencia Creative Commons Reconocimiento 3.0 Unported.
-