Complicated congenital malaria due to Plasmodium vivax
Paludismo congénito complicado debido a Plasmodium vivax
Palabras clave:
Congenital malaria, Plasmodium vivax, relapse, complicated malaria, treatment. (en)Malaria congénita, Plasmodium vivax, recaída malaria complicada, tratamiento. (es)
Descargas
COMPLICATED CONGENITAL MALARIA DUE TO PLASMODIUM VIVAX
Angélica Contreras-Díaz 1,
Germán Camacho-Moreno 2,
Edgar Rojas-Soto 3.
1. Resident in pediatrics.
2. Full instructor of pediatrics,
department of pediatrics,
Universidad Nacional de Colombia.
Correspondence to:
Angélica Contreras-Díaz.
Bogotá DC, Colombia.
Email : amdiazco@unal.edu.co
Congenital malaria is a disease that appears in the neonatal period and that, if not treated in a timely manner, may have fatal consequences for the newborn. According to statistics published in The State of the World Children 2009 Report, 3.7 million children under the age of 28 days die annually around the world at present. 8% of cases correspond to children under 5 years of age with malaria (1). Similarly, studies in endemic areas have reported incidences of congenital malaria of between 0.83 and 5.93% (2). Here, we present a case of congenital malaria in a one-month-old nursing infant whose mother received treatment for malaria from Plasmodium vivax (P. vivax) during gestation but suffered a relapse with a consequent compromise of the infant in utero. There is a need to recognize the high prevalence of this disease in our context and to know how to monitor and treat the disease in special cases like those of gestating mothers and newborn infants with congenital infections.
Keywords: Congenital malaria, Plasmodium vivax, relapse, complicated malaria, treatment.
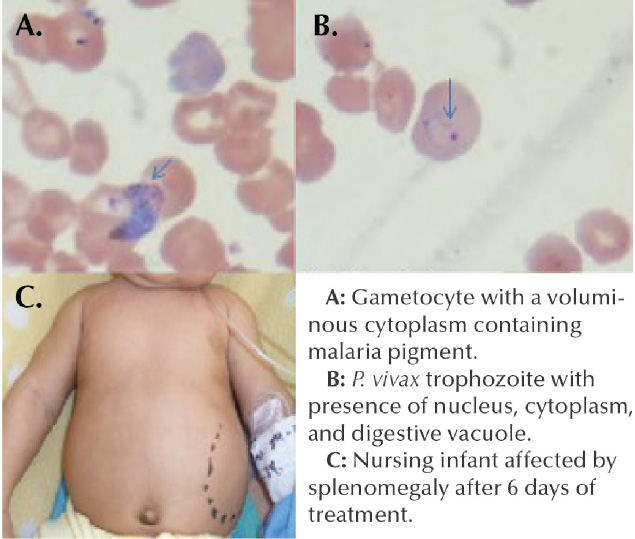
Fig 1. A: Gametocyte with a voluminous cytoplasm containing malaria pigment.
B: P. vivax trophozoite with presence of nucleus, cytoplasm, and digestive vacuole.
C: Nursing infant affected by splenomengaly after 6 days of treatment.
Colombia is recognized globally as the country with the second highest level of biodiversity after Brazil. This condition gives the country the characteristic of endemicity for many tropical diseases that affect a large portion of the population across the national territory and that can be fatal if they are not properly managed in a timely fashion. Malaria has a high prevalence in our context. One quarter of Colombians inhabit zones where there is a risk of contracting the disease, with a endemicity of 75% in areas under 1500 meters below sea level (3), Nevertheless, the congenital presentation of the parasitosis due to Plasmodium vivax is very infrequent. Here, we present a case of congenital malaria complicated by P. vivax to warn clinicians about this entity.
CASE REPORT
A 54 day old patient at the time of the consultation, son of a gestating mother native to and coming from La Chorrera-Amazonas department. The mother had moved to Bogotá, the capital of Colombia, located at around 2600 meters above sea level in a non-malarial zone, since the vector of the parasite is not viable at this altitude. The gestating mother visited a doctor in the third trimester of gestation, and on the second day of her stay in the city, she started to experience general discomfort, fever, headache, and nausea, symptoms that led her to seek medical attention at another institution. Malaria due to P. vivax was diagnosed at 32 weeks of gestation and she was treated with oral chloroquine. A post-treatment check-up was performed at 35 weeks of gestation in which a negative thick drop test was found. Therefore, prenatal checkups continued as normally.
The infant was born in that institution at 38 weeks of gestation by cesarean section due to an unsatisfactory fetal state during labor that made an emergency C-section necessary.
The newborn showed a spontaneous neonatal adaptation, an Apgar score of 8-9-9, and a weight of 3315 grams. The mother suffered from postpartum endometritis, which required curettage. She showed satisfactory progression and was discharged with primaquine treatment for 14 days in the postpartum period. There were no further check-ups.
The infant was admitted to hospital with a presentation of fever measured at 39°C and irritability associated with diarrheal semi-solid stools, and the vomiting of food on 4 occasions. In the physical examination, the following was found: Heart rate at 168 beats per minute, respiratory rate at 42xmin, blood pressure at 82/46xmin, temperature at 39°C. Weight at 3.8Kg. The infant appeared pale with first degree jaundice and third degree dehydration, the liver palpable at 2 cm below the edge of the ribs. Fluid resuscitation
was initiated with crystalloids at 20 mL/Kg. 2 boluses were required. The laboratory reported the following in the CBC: Leukocytes: 7930, lymphocytes: 48.9%, neutrophils: 24.4%, eosinophils: 13%, hemoglobin: 3.6g/dL, hematocrit: 11.6%, platelets: 65 000, C-reactive proteint 271.4, and the thick drop test was positive for P. vivax. The patient required a red blood cell transfusion and antimalarial treatment for complicated malaria was initiated with clindamycin and quinine dichlorohydrate over 7 days. The patient was monitored in the intermediate care unit and showed a satisfactory progression, with resolution of the fever after 24 hours of antimicrobial management, control of tachycardia, reduction of jaundice, and persistence of splenomegaly. Prior to discharge, the thick drop test was performed with a negative result.
DISCUSSION
The diagnosis of malaria in the mother during gestation was correct and timely since there was a high degree of suspicion due to her origin in an area endemic for the disease and her general symptoms that, although they are unspecific and can point to any febrile process, must be taken with the clinical context so that proper diagnosis and treatment can be made in order to reduce complications in the maternal- fetal unit. The treatment of gestating mothers with malaria caused by P. vivax should be limited to chloroquine. This is due to the fact that primaquine, which eradicates P. vivax tissue schizonts and hypnozoites, is counter-indicated during gestation and breast-feeding due to the risk of generating hemolytic anemia in the infant(4). As such, these parasitic forms can remain dormant, and, therefore, patients that are gestating or breast-feeding merit monthly monitoring with the thick drop test over the period in which P. vivax relapses are most frequent (2-6 months post-infection) (5). This is to evaluate relapses that require new rounds of treatment until the hypnozoites can be eradicated when breast-feeding has ended. Likewise, the newborn should be monitored with the thick drop test during the first month of life. If the parasitemia is detected, chloroquine should be administered again.
However, the child may develop complicated congenital malaria due to P. vivax. The incidence of congenital malaria in endemic zones is low, between 0.01 and 1.4% of live births. This is due to the passage of anti-plasmodium antibodies through the placental barrier and to the lower susceptibility of infection by this agent shown by fetal erythrocytes that have fetal hemoglobin (4).
Malaria is considered to be congenital when it develops within 7 days following birth in endemic zones, and within up to 2 months in non-endemic zones, as in this case (4). The most frequent agent is P. falciparum. Nevertheless, cases of infection by P. vivax have been described (6). The clinical manifestations are fever (100%), splenomegaly (93%), hepatomegaly (84%), anemia (85%), irritability (85%), vomiting (79%), jaundice (79%), and diarrhea (65%)(2,4), all of which were present in our patient. In this case, the alteration of the state of consciousness, the hemodynamic instability, and the severe anemia also define the diagnosis of complicated malaria, which is also more frequent in infections of P. falciparum. Mortality due to congenital malaria is 1%, but this increases to up to 20% if the patient has complicated malaria. The treatment for non-complicated congenital malaria due to P. vivax is chloroquine. The treatment from complicated malaria in infants under 6 months of age is quinine with a loading dose of 20 mg/Kg IV in a DAD syringe at 5% or 10% to be infused over 4 hours, followed by a dose of 10mg/ Kg every 8 hours to be infused over 2 hours. The medication should be administered orally once the patient can tolerate it over 7 days. In infants over 1 month, clindamycin at 20mg/Kg/day is added in 3 to 4 doses for 5 to 7 days (3,7) .
CONCLUSION
It is important to have a high degree of suspicion for tropical diseases given the context in which we live that has a high prevalence of malaria. In addition, physicians should be aware of the special treatment to be administered to infected gestating mothers and the monitoring to be conducted, both of her and the newborn, since there is a possibility of congenital infection, even when the mother has been adequately treated. Monitoring of the mother should be done monthly over the entire gestation period and will end once primaquine can be administered to eradicate dormant parasitic forms that reactivate the disease, and that can lead to consequences for both the mother and the newborn.
REFERENCES
1. Carmona J. Incidencia de las malarias gestacional, congénita y placentaria en Urabá (Antioquia, Colombia), 2005-2007. Revista colombiana de obstetricia y ginecología 2009; 60(1):19-33
2. Moya-Alvarez et al. Pregnancy-associated malaria and malaria in infants: an old problem with present consequences. Malaria Journal 2014, 13:271
3. Padilla JC, Montoya R. Guía de Atención Clínica de Malaria en Colombia. Infectio 2011; 15(4): 302-323.
4. Palau JM. Paludismo congénito. In: González N, Saltigeral P, Macías M. Infectología neonatal 2 ed. McGraw Hill 2006; 307 – 312.
5. Equipo de trabajo Convenio 637/09 de Cooperación OPS/OMS-MPS. Guía de Atención Clínica de Malaria 2010. Ministerio de protección social, Organización panamericana de la salud.
6. Singh J et al. Placental and Neonatal Outcome in Maternal Malaria. Indian pediatrics, 2014; 51: 285-289
7. Baird JK. Effectiveness of antimalarial drugs. N Engl J Med 2005; 352: 1565-77.
Referencias
Carmona J. Incidencia de las malarias gestacional, congénita y placentaria en Urabá (Antioquia, Colombia), 2005-2007. Revista colombiana de obstetricia y ginecología 2009; 60(1):19-33
Moya-Alvarez et al. Pregnancy-associated malaria and malaria in infants: an old problem with present consequences. Malaria Journal 2014, 13:271
Padilla JC, Montoya R. Guía de Atención Clínica de Malaria en Colombia. Infectio 2011; 15(4): 302-323.
Palau JM. Paludismo congénito. In: González N, Saltigeral P, Macías M. Infectología neonatal 2 ed. McGraw Hill 2006; 307 – 312.
Equipo de trabajo Convenio 637/09 de Cooperación OPS/OMS-MPS. Guía de Atención Clínica de Malaria 2010. Ministerio de protección social, Organización panamericana de la salud.
Singh J et al. Placental and Neonatal Outcome in Maternal Malaria. Indian pediatrics, 2014; 51: 285-289
Baird JK. Effectiveness of antimalarial drugs. N Engl J Med 2005; 352: 1565-77.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2015 Case reports

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Los autores al someter sus manuscritos conservarán sus derechos de autor. La revista tiene el derecho del uso, reproducción, transmisión, distribución y publicación en cualquier forma o medio. Los autores no podrán permitir o autorizar el uso de la contribución sin el consentimiento escrito de la revista.
El Formulario de Divulgación Uniforme para posibles Conflictos de Interés y los oficios de cesión de derechos y de responsabilidad deben ser entregados junto con el original.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cual estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons 4.0 que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).


























