Comparación entre métodos de extracción para la obtención de volátiles a partir de pulpa de lulo (Solanum quitoense)
Comparison between extraction methods to obtain volatiles from lulo (Solanum quitoense) pulp
DOI:
https://doi.org/10.15446/rev.colomb.quim.v45n3.61359Palabras clave:
análisis químico, Solanum quitoense, perfil de metabolitos, HS-SPME, SDE, compuestos orgánicos volátiles (es)Chemical analysis, Solanum quitoense, metabolite profile, HS-SPME, SDE, volatile organic compounds (en)
Descargas
Se compararon los métodos de extracción y destilación simultánea (SDE) y microextracción en fase sólida con espacio de cabeza (HS-SPME), acoplados a cromatografía de gases con detector de espectrometría de masas (GC-MS), para la recuperación de volátiles a partir de pulpa de lulo (Solanum quitoense). Se realizó un diseño completamente al azar aplicado al tipo de solvente para SDE/GC-MS, mientras que para HS-SPME/GC-MS se ejecutó un diseño a dos vías, teniendo como factores el tipo de fibra y la temperatura de adsorción. En el primer caso se obtuvieron principalmente hidrocarburos, aldehídos y ésteres; en el segundo, se recuperaron ésteres y aldehídos. El análisis de varianza mostró una interacción significativa entre el tipo de fibra, la temperatura de adsorción y los grupos funcionales.
Simultaneous Distillation-Solvent Extraction (SDE) and Headspace Solid Phase Micro-extraction (HS-SPME), coupled to Gas Chromatography-Mass Spectrometry (GC-MS), for the recovery of volatiles from lulo pulp (Solanum quitoense) were compared. A completely randomized SDE/GC-MS design was applied to establish differences between the areas obtained with different solvents, whereas a two-way HS-SPME/GC-MS indicated the most appropriate extraction conditions of volatiles, having the type of fiber and the adsorption temperature as factors. SDE/GC-MS mainly promoted the extraction of hydrocarbons, aldehydes, and esters; whereas esters and aldehydes had higher areas using HS-SPME/GC-MS. Furthermore, the variance analysis showed a significant interaction among the type of fiber, the adsorption temperature, and the functional groups
Doi: https://doi.org/10.15446/rev.colomb.quim.v45n3.61359
Comparison between extraction methods to obtain volátiles from lulo (Solatium quitoense) pulp
Comparación entre métodos de extracción para la obtención de volátiles a partir de pulpa de lulo (Solanum qu toense)
Comparação entre métodos de extração para a obtenção de voláteis a partir da polpa de lulo (Solanum quitoense)
Eduardo J. Corpas1*, Gonzalo Taborda2, Omar A. Tapasco2, Aristófeles Ortiz3
1 Universidad Católica de Manizales, Instituto de Investigación en Microbiología y Biotecnología Agroindustrial, Carrera 23 # 60-63, Manizales, Caldas, Colombia.
2 Universidad de Caldas, Facultad de Ciencias Exactas y naturales, Calle 65 # 26-10, Manizales, Caldas, Colombia.
3 Centro Nacional de Investigaciones de Café CENICAFÉ, Área de Calidad y Producción, Km 4 vía Manizales Chinchiná, Caldas, Colombia.
*Corresponding author: ecorpasjucm.edu.co
Article citation:
Corpas, E. J.; Taborda, G.; Tapasco, O. A.; Ortiz, A. Comparison between extraction methods to obtain volatiles from lulo (Solanum quitoense) pulp. Rev. Colomb. Quim. 2016, 45 (3), 12-21. DOI: https://doi.org/10.15446/rev.colomb.quim.v45n3.61359.
Recibido: 26 de Agosto de 2016. Aceptado: 1 de Octubre de 2016.
Abstract
Simultaneous Distillation-Solvent Extraction (SDE) and Headspace Solid Phase Micro-extraction (HS-SPME), coupled to Gas Chromatography-Mass Spectrometry (GC-MS), for recovery of volatiles from lulo pulp (Solanum quitoense) were compared. A completely randomized SDE/GC-MS design was applied to establish differences between the areas obtained with different solvents, whereas a two-way HS-SPME/GC-MS indicated the most appropriate extraction conditions of volatiles, having the type of fiber and the adsorption temperature as factors. SDE/GC-MS mainly promoted the extraction of hydrocarbons, aldehydes, and esters; whereas esters and aldehydes had higher areas using HS-SPME/GC-MS. Furthermore, the variance analysis showed a significant interaction among the type of fiber, the adsorption temperature, and the functional groups.
Keywords: Chemical analysis, Solanum quitoense, metabolite profile, HS-SPME, SDE, volatile organic compounds.
Resumen
Se compararon los métodos de extracción y destilación simultánea (SDE) y microextracción en fase sólida con espacio de cabeza (HS-SPME), acoplados a cromatografía de gases con detector de espectrometría de masas (GC-MS), para la recuperación de volátiles a partir de pulpa de lulo (Solanum quitoense). Se realizó un diseño completamente al azar aplicado al tipo de solvente para SDE/GC-MS, mientras que para HS-SPME/GC-MS se ejecutó un diseño a dos vías, teniendo como factores el tipo de fibra y la temperatura de adsorción. En el primer caso se obtuvieron principalmente hidrocarburos, aldehídos y ésteres; en el segundo, se recuperaron ésteres y aldehídos. El análisis de varianza mostró una interacción significativa entre el tipo de fibra, la temperatura de adsorción y los grupos funcionales.
Palabras clave: Análisis químico, Solanum quitoense, perfil de metabolitos, HS-SPME, SDE, compuestos orgánicos volátiles.
Resumo
Foram comparados os métodos de extração e destilação simultânea (SDE) e microextração em fase sólida com espaço de cabeça (HS-SPME), acopladas à cromatografia gasosa-espectrometria de massa (GC-MS), para à recuperação de voláteis a partir da polpa de lulo (Solanum quitoense). Foi realizado um delineamento completamente casualizado aplicado ao tipo de solvente para à SDE/GC-MS, enquanto à HS-SPME/GC-MS foi executado um desenho de duas vias, tendo como fatores o tipo de fibra e a temperatura de adsorção. No primeiro caso foram obtidos sobretudo hidrocarbonetos, aldeídos e ésteres; no segundo foram obtidos ésteres e aldeídos. A análise de variância mostrou uma interação significativa entre o tipo de fibra, a temperatura de adsorção e os grupos funcionais.
Palavras-Chave: Análise química, Solanum quitoense, perfil de metabolitos, HS-SPME, SDE, compostos orgânicos voláteis.
Introduction
Volatile organic compounds (VOC) are responsible for the distinctive flavor in each fruit, even though some of these components are not able to interact with the human olfactory receptors for triggering the subsequent sensory impact (1). Obtaining a complete volatile profile from a ripe fruit constitutes a relevant evidence regarding its sensorial quality features (2). A predominance of esters, alcohols, and aldehydes has been denoted in several types of fruit, mostly climacteric (3-7). On the contrary, in other climacteric fruits the hydrocarbons were the outstanding group (8- 10).
The diverse chemical nature of volatile compounds arises due to the different metabolic pathways that exist in fruits (11, 12). The metabolites obtained depend on the extraction method employed. The Simultaneous Distillation-Solvent Extraction (SDE) method, based on the recovery of compounds by polar affinity to a simultaneously distilled organic solvent, promotes the extraction of diverse chemical classes (13). Nevertheless, SDE is a sensitive method for obtaining compounds at trace concentrations (14). It requires great amount of sample, has a prolonged extraction time (2), and promotes the loss of highly volatile metabolites (15). On the other hand, Solid Phase Micro-extraction (SPME), supported on the partition equilibrium of the metabolites between both fiber and matrix analyzed (16) is fast, easy, sensitive, solventless, and avoids loss of volatiles with low boiling point (17, 18).
Previous studies have demonstrated the complementarity between SDE and HS-SPME to obtain more complete volatile profiles in several fruits (15, 17, 19, 20). The increase in the compounds using SDE and HS-SPME methods occurs due to the affinity of each method for compounds with a specific polarity and molecular weight. The extracts from SDE contain high molecular weight compounds and are poor in highly volatile metabolites (21), but using HS-SPME the obtaining of heavy volatile compounds is lower (2). In addition, each fruit has a volatile profile with different characteristics, which justifies in some cases the extraction with nonpolar solvents such as diethyl ether (1, 22, 23), or solvents of intermediate polarity such as dichloromethane (17, 18, 22, 24, 25). In addition to SDE, the extraction with HS-SPME has been carried out in several fruits using fibers with a specific polarity (2, 15, 18, 20), after the selection of this as the higher performance fiber in the extraction of volatile metabolites.
Lulo (Solanum quitoense Lam.) is a Solanaceae species native to South America, whose pulp has potential for both processing and marketing at industrial scale (26). A comparative referent between the volatile profiles of frozen lulo pulp cultivated in Colombia and Costa Rica, obtained by extraction with pentane and ether (2:1), showed differences attributed to the different environmental conditions in each country (27). Moreover, supercritical CO2 enabled to recover the volatile profiling from the lulo pulp and to identify 52 compounds, mainly alcohols and esters (among which, decane, methyl benzoate, acetic acid, hexadecane, and methyl hexanoate had the highest concentrations (28)).
In addition, 65 compounds from S. vestissimum, another lulo species, were identified with SDE/GC-MS, using diethyl ether and pentane (1:1). Among the volatiles obtained, those of highest concentration were methyl propionate, methyl butanoate, butyl acetate, 3-methylbutyl acetate, methyl hexanoate, methyl (E)-2-methyl-2-butenoate, (Z)-3-hexenylacetate, methyl benzoate, (Z)-3-hexenol, linalool, α-terpineol, and geraniol (29).
This study aimed to obtain volatile profiles from lulo pulp, using two extraction methods: SDE with solvents of different polarity and HS-SPME by using several fibers. The extracts were analyzed by GC-MS. In both experiments, the comparison of total volatile areas and those of the functional groups allowed to establish which treatment was the most efficient for the extraction of volatiles from lulo pulp.
Materials and methods
Fruit selection
Lulo fruit, harvested in stage five (30), came from seedlings which were generated through in vitro propagation by the company Agro in-vitro S.A.S. (Manizales, Colombia) and harvested at the Villa Malicia farm, placed at 1 km from Manizales. In addition, the fruit grew from a developed crop in controlled conditions with Green Seal fungicides and had the following features as a selection criteria: diameter of 5-6 cm, orange skin, and brix degrees of 10.3 ± 0.2 (30). Moreover, fruit with spoilage signs, triggered by insects or molds, was discarded.
Reagents and materials
Sodium chloride was acquired from Carlo Erba Reagents® (Barcelone, Spain). The solvents hexane, dichloromethane, and ethyl acetate were provided by Sigma-Aldrich® (Saint Louis, USA). The SPME holder and the fibers used in the adsorption of volatile metabolites were obtained from Supelco® (Bellenfonte, PA, USA). Four fibers for were employed: polydimethysiloxane (PDMS, 100 μm), carboxen/polydimethylsiloxane (CAR/PDMS, 75 μm), polydimethylsiloxane/divinylbenzene (PDMS/DVB, 65 μm), divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS, 50/30 μm), which were conditioned prior to their use as indicated by the manufacturer. The alkane standard solution C7-C40 was provided by Sigma-Aldrich Chemical S.A.
SDE procedure
The fruit was washed with distilled water for 20 s and cut for separating the peel and obtaining the pulp. 200 g of pulp were weighed in a sample flask with 500 mL capacity. The extraction was conducted in a modified Likens-Nickerson apparatus. In the first one side, the flask containing the sample was adapted, and in the second one, another flask with 50 mL of the respective solvent was installed. The flasks underwent the boiling temperature of each solvent and SDE extraction was carried out for 1 h. Thereafter, an extracted volume of approximately 20 mL was collected and completed to a fixed volume of 50 mL with each solvent. Subsequently, 1 mL ofthis sample was added on a vial with capacity of 2 mL. Finally, 1 μL of extract was inserted to desorb in the injection port ofthe gas chromatograph.
HS-SPME procedure
Each fruit was washed with distilled water for 20 s and 10 g of the pulp were added into a vial with 20 mL of capacity. Subsequently, the vial was closed with a rubber cap and placed on a water bath. Thereafter, the respective SPME fiber was manually inserted into the headspace (HS) of the pulp and exposed at temperature of 40 or 60 °C for 30 min, according to the experimental design proposed. After removing, the fiber was inserted into the injection port of the gas chromatograph to desorb the extracted compounds at 230 °C in splitless mode for 2 min.
Analysis of volatile compounds
In order to analyze the volatile compounds from lulo pulp, a gas chromatograph Shimadzu GCMS-QP2010 Plus coupled to a mass spectrometry detector was used. Regarding the samples extracted by HS-SPME, a liner of 0.75 mm I.D. (Supelco, Bellefonte, PA) was used to conduct the metabolites to the column, whereas for the extracts obtained by SDE, a 3.4 mm I.D. liner (Shimadzu) was used. As a carrier gas, helium at a constant flow rate of 4 mL/min was used. A Shimadzu 5% polysiloxane (30 m x 0.25 mm ID x 1.4 um DF) semi-polar analytical column with a temperature range of-40 °C to 260 °C was used. Flow control worked at a linear velocity of 36 cm/s, the pressure was 55.2 kPa and the column flow was 0.98 mL/ min. The temperature ramp program was as follows: one min at 50 °C, increasing at 2.5 °C/min up to 150 °C, in which remained for seven min; subsequently, it was increased at 15 °C/min up to 220 °C, remaining in this state for three min; and finally, the temperature was increased at 15 °C/min up to 230 °C and maintained for two min.
On the other side, the mass spectrometer was operated with ionization energy (IE) 70 eV, ion source temperature 235 °C, time of solvent cut-off 3 min, threshold of 1000, and mass range between 33-350 Da. The detector operated was operated at 1.0 kV and the mass spectrum had a scan speed of 666 Hz. The analyses of volatiles from extractions by HS-SPME were carried out for 50 min, whereas each assay of the SDE treatments lasted 60 min. The identification of each peak was based on the comparison between the mass spectrum of each compound and generated compounds from the NIST library version 8, having as an identification criteria a concordance equal or superior to 93%. In addition, a verification of the Kovats retention index was made from the analysis of a mixture of alkanes (C7-C24) under the same conditions used with the samples.
Statistical analysis
In relation to SDE experiments, a completely randomized design was performed having the type of solvent with three treatments (hexane, dichloromethane, and ethyl acetate) as a factor, and the total area of volatiles and functional groups areas as a response variable. Six replicates per treatment were carried out. After evaluating the statistical assumptions, an analysis of variance (ANOVA) was performed to establish differences between treatments and the Tukey test to define for which of the treatments there were differences.
Regarding the SPME fiber treatments, a two-way design was performed: the first factor was the type of fiber with four levels (PDMS, CAR/PDMS, PDMS/DVB, and DVB/CAR/PDMS), and the second factor was the adsorption temperature with two levels (40 °C and 60 °C). The response variable was the total area of volatile compounds. Five repetitions were carried out for each treatment. Moreover, the areas of the functional groups in each treatment were analyzed.
Volatiles data were submitted to an ANOVA to establish differences between both the total areas and the functional groups areas. The relative standard deviation (RSD) of the functional groups areas was lower than 12% in all experiments. Finally, a t-test for the areas of the functional groups of the most efficient treatments from each experiment was made. Using the SPSS software version 22, the obtained data from the treatments were analyzed.
Results and discussion
Volatile compounds from lulo pulp by SDE/GC-MS using different solvents
Total area
Through SDE/GC-MS, 47 volatile compounds with molecular weights ranging from 60 to 282 Da were obtained, mainly hydrocarbons (42.55%), followed by aldehydes (17.02%), esters (17.02%), alcohols (10.63%), ketones (6.38%), and acids (4.25%). Furthermore, 34 of these compounds were identified as well. In addition, there was a higher percentage of the area obtained from compounds such as decanal, furfural, benzeneacetaldehyde, methylbutanoate, (Z)-3-hexen-1-ol acetate, and hexadecane (Table 1).
The assumptions of normality were confirmed through the Shapiro-Wilk from the SDE data with the solvents hexane (P = 0.369), dichloromethane (P = 0.496), and ethyl acetate (P = 0.914), as well as through the homogeneity of the variances of these datasets from Levene test statistic (P = 0.566).
Firstly, a lower total area of volatiles was presented from the hexane extraction, whereas ethyl acetate enabled to recover a mean area higher than that obtained with the other solvents. Secondly, the ANOVA showed statistically significant differences among the treatments considering the type of solvent (P = 0.00), whereas the Tukey test showed that extraction using hexane (mean area: 2.2 x 108) was less effective than those obtained with dichloromethane (mean area: 3.7 x 108) and ethyl acetate (mean area: 1.12 x 109). However, there were no statistical differences between the mean areas using the last two mentioned solvents.
Area of the functional groups
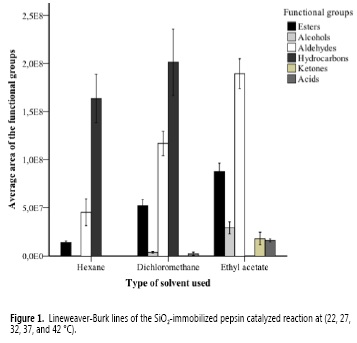
When comparing the areas, a predominance of hydrocarbons in the treatments using hexane and dichloromethane was observed, but through ethyl acetate the aldehydes predominated and the hydrocarbons were not recovered due to its nonpolar nature (Figure 1).
Furthermore, a higher area of esters, alcohols, and aldehydes was observed when increasing the polarity of the solvent. However, when hexane was used, neither alcohols nor ketones were extracted. The compounds of higher area extracted with ethyl acetate were furfural and benzeneacetaldehyde. Nonanal had the highest extraction with dichloromethane, followed by decanal, which was the compound with the highest mean area using hexane.
The ANOVA indicated statistical differences among the areas of the functional groups obtained with different extraction solvents (P = 0.00), whereas the Tukey multiple comparison test showed that esters (mean area: 8.7 x 107), alcohols (mean area: 3.7 x 107), aldehydes (mean area: 1.8 x 108), ketones (mean area: 1.8 x 107), and acids (mean area: 7.2 x 108) extracted with ethyl acetate belong to a different subset with means statistically higher than those obtained with dichloromethane and hexane. Besides, the hydrocarbons recovered with dichloromethane belong to a different subset of higher area (mean area: 2.0 x 108) in relation to the areas obtained using other solvents.
Volatile compounds from lulo pulp by HS-SPME/GC-MS
Total area
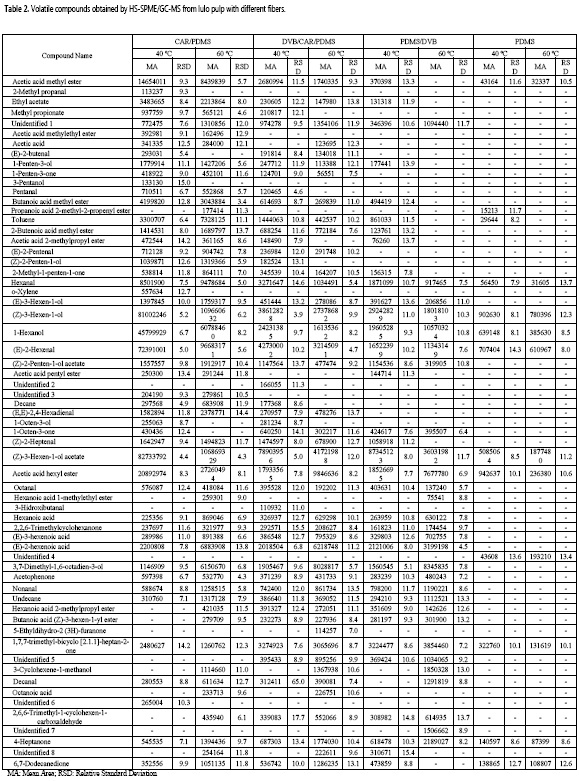
A number of 63 volatiles were obtained and 55 were identified, among them, 28.8% were esters and 23.1% were aldehydes. The identified metabolites had molecular weights ranging from 60 to198 Da (C3 to C12). Moreover, by using the fiber CAR/PDMS, a larger number of compounds (42 at both temperatures) was obtained, whereas with the fiber of PDMS less than 15 compounds were recovered. The compounds with the highest abundance were (Z)-3-hexen-1-ol acetate, (Z)-3-hexen-1-ol, and (E)-2-hexenal (Table 2).
In order to establish differences among the areas of volatiles, the data normality of the total areas from different fibers was verified through Shapiro-Wilk test (P = 0.243), the homogeneity of variances via the Levene statistic (P = 0.082), and the absence of correlation among the residuals of the data by the Durbin-Watson test (P = 0.141). When performing the ANOVA from the total areas, an interaction between the type of fiber and the adsorption temperature (P = 0.00) was found. Using the fiber coated of CAR/PDMS, a higher total area of volatiles at 40 °C and 60 °C was obtained, as compared to those produced by PDMS/DVB and CAR/PDMS/DVB fibers; nevertheless, the last two mentioned fibers promoted higher total areas at 40 °C than at 60 °C, in contrast to the fiber coated with CAR/PDMS, which was more efficient at 60 °C.
Area of the functional groups
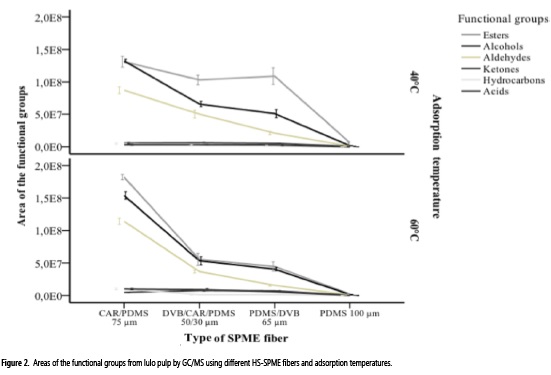
The ANOVA applied to the areas of the functional groups showed a significant interaction among the factors: type of fiber, adsorption temperature, and functional groups (P = 0.00). At 40 °C, the fiber made of CAR/PDMS had greater affinity than the other fibers for the extraction of alcohols (mean area: 1.3 x 108), esters (mean area: 1.3 x 108), and aldehydes (mean area: 8.7 x 107). The fiber coated of CAR/ PDMS/DVB yielded the second highest level of extraction, having a higher area of alcohols (mean area: 6.6 x 107) and aldehydes (mean area: 5.0 x 107), and a lower area of esters (mean area: 1.0 x 108) as compared to the fiber coated with PDMS/DVB (Figure 2).
Moreover, the groups of ketones, hydrocarbons, and acids behaved similarly in terms of extraction using different fibers at 40 °C. The extraction at 60 °C also showed a better performance with the fiber made of CAR/PDMS for the alcohols extraction (mean area: 1.8 x 108), esters (mean area: 1.5 x 108), and aldehydes (mean area: 1.1 x 108). Finally, in both temperatures the fiber coated with PDMS showed the lowest extraction to the different functional groups.
Comparison between SDE/GC-MS and HS-SPME/GC-MS
A t-test to establish differences between the means of the functional groups obtained with the most efficient treatments of HS-SPME (CAR/PDMS) and SDE (ethyl acetate) was performed (Table 3).
Differences between the mean area (P = 0.00) of the functional groups acids, aldehydes, and ketones were found by SDE/GC-MS. For the areas of the esters, alcohols, and hydrocarbons, statistical differences were obtained, suggesting higher extraction by HS-SPME/GC-MS.
The current study constitutes not only the first comparative antecedent among SPME fibers to obtain volatile compounds from lulo, but it is also the first work in which SDE/GC-MS and HS-SPME/GC-MS are contrasted in this fruit. Regarding the HS-SPME method, the denoted differences are attributable to the polarity and molecular weight of the volatiles in each fruit. The fibers CAR/PDMS, PDMS/DVB, and DVB/CAR/PDMS have affinity for low molecular weight volatile (C3-C12), polar and nonpolar, whereas the fiber coated of PDMS mainly promotes the recovery of nonpolar volatile compounds. In regard to the SDE method, the polarity of the solvent used influences the extraction of volatile compounds.
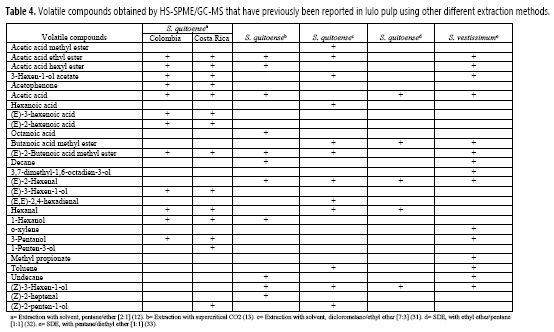
Comparison between volatile compounds obtained by HS-SPME with other extraction methods
Considering that HS-SPME is a modern method, Table 4 shows the volatile compounds extracted by HS-SPME in the current study, which have previously been reported using other extraction methods from the lulo species S. quitoense (27, 28, 31, 32) and S. vestissimum (33). Fourteen out of the 28 volatile compounds previously reported belong to the esters and alcohols, for instance: acetic acid ethyl ester, 3-hexen-l-ol acetate, butanoic acid methyl ester, (E)-2-butenoic acid methyl ester, acetic acid, hexanal, (E)-2-hexenal, and (Z)-3-hexen-l-ol. On the contrary, some volatile compounds obtained by HS-SPME were not previously identified when analyzing the species of the fruit through the traditional methods such as: (Z)-2-penten-l-ol acetate, pentanal, acetic acid pentyl ester, butanoic acid (Z)-3-hexen-l-yl ester, l-penten-3-one, 4-heptanone, and 6,7-dodecanodione. These compounds had lower areas than most of the other volatiles obtained from the same analysis, thus its difficulty of recovering using traditional methods, where there are higher losses compared to HS-SPME, could be related to the low sensitivity of these methods. As a matter of fact, the thermal degradation of these compounds during the conventional extraction should not be discarded.
Sensorial relevance of some of the compounds obtained
In regard to the HS-SPME method, there are referents on the extraction and analysis of odor active volatiles from dried lulo solids using the CAR/PDMS/DVB fiber. Among the compounds identified in the current study, hexanal, (E)-2-hexenal, and (Z)-3-hexen-l-ol were described as green odor volatiles. In addition, the compounds methyl butanoate, methyl hexanoate, and methyl benzoate had a fruity odor; whereas acetic acid, and benzoic acid were associated with descriptors of vinegar and rancid, respectively (31). The compounds (Z)-3-hexen-l-ol, hexyl acetate, and (Z)-3-hexenyl acetate have also been considered as relevant volatiles for curuba (Passiflora mollissima (Kunth) L. H. Bailey) (34), whereas hexanal showed a grassy flavor in pink Colombian guavas (Psidium guajava L.)(35).
Conclusions
This study allowed to select the most efficient HS-SPME fiber for the extraction of volatile compounds for the first time in lulo pulp, as well as to compare the extracted volatiles with those recovered by a traditional method such as SDE. Among the tested solvents, ethyl acetate was the most appropriate solvent for the extraction using SDE/GC-MS; statistically higher areas for esters, alcohols, aldehydes, ketones, and acids were obtained. Also SPME fibers coated with CAR/PDMS promoted a higher efficiency in the extraction; with esters as were the main group of compounds. The differences between the mean areas of acids, aldehydes, and ketones by using SDE/GC-MS and the higher abundances of esters, alcohols, and hydrocarbons through HS-SPME/GC-MS indicated complementarity between these extraction methods. Finally, by using HS-SPME/GC-MS, the compounds (Z)-2-penten-l-ol acetate, pentanal, acetic acid pentyl ester, butanoic acid (Z)-3-hexen-l-yl ester, l-penten-3-one, 4-heptanone, and 6,7-dodecanodione were obtained, which were not identified in previous studies by traditional extraction methods.
Acknowledgements
The researchers express their gratitude to the Administrative Department of Science, Technology and Innovation (Colciencias, Colombia) for their contribution in financing their study process (call for grant number 528-20H).
References
1. Pino, J.; Febles, Y. Odour-active compounds in banana fruit cv. Giant Cavendish. Food Chem. 2013, 141, 795-801. DOI: https://doi.org/10.1016/j.foodchem.2013.03.064.
2. Chen, M.; Chen, X.; Wang, X.; Ci, Z.; Liu, X.; He, T. et al. Comparison of heads pace solid-phase microextraction with simultaneous steam distillation extraction for the analysis of the volatile constituents in chinese apricot. Agric. Sci. China. 2006, 5, 879-884. DOI: https://doi.org/10.1016/s1671-2927(06)60139-9.
3. Arizala, M.; Monsalvo, A.; Betancourth, C.; Salazar, C.; Lagos, T. Evaluación de solanaceas silvestres como patrones de lulo (Solanum quitoense Lam) y su reacción a Fusarium sp. Rev. Cienc. Agric. 2011, 28, 147-160.
4. Brunerie, P.; Maugeais, P. Comparison of volatile components in two naranjilla fruit (Solanum quitoense Lam ) pulp from different origin. Dev Food Sci. 1992, 29, 163-174. DOI: https://doi.org/10.1016/b978-0-444-88834-1.50019-3.
5. Parada, F.; Guerrero, E.; Suárez, M.; Duque, C. Obtención de esencias de lulo (Solanum quitoense L.) utilizando CO2 supercritico. Rev. Colomb. Quim. 1993, 22, 53-62.
6. Suárez, M.; Duque, C.; Wintoch, H.; Schreier, P. Glycosidically bound aroma compounds from the pulp and the of lulo fruit (Solanum vestissimum D.). J. Agr. Food Chem. 1991, 39, 1643-1645. DOI: https://doi.org/10.1021/jf00009a022.
7. Mejía, C.; Gaviria, D.; Duque, A.; Rengifo, L.; Aguilar, E.; Alegría, A. Physicochemical characterization of the lulo (Solanum quitoense Lam.) castilla variety in six ripening stages. Vitae 2012, 19, 157-165.
8. Grant, S.; Golding, J.; McGlasson, W.; Williams, M. The relationship between ethylene and aroma volatiles production in ripening climacteric fruit. Dev. Food Sci. 1998, 40, 375-384. DOI: https://doi.org/10.1016/s0167-4501(98)80061-7.
9. Márquez, C. Caracterización fisiológica, físico-química, reológica, nutraceútica, estructural y sensorial de la guanábana (Annona muricata L. cv. ELITA). Tesis de Doctorado, Universidad Nacional de Colombia, Medellín, Colombia, 2009.
10. Ceva-Antunes, P.; Ribeiro, H.; Carvalho, C.; Antunes, O. Analysis of volatile composition of siriguela (Spondias purpurea L.) by solid phase microextraction (SPME). Food Sci. Technol. 2006, 39, 437-443. DOI: https://doi.org/10.1016/j.lwt.2005.02.007.
11. Matich, A.; Young, H.; Allen, J.; Wang, M.; Fielder, S.; McNeilage, M. et al. Actinidia arguta: volatile compounds in fruit and flowers. Phytochemistry. 2003, 63, 285-301. DOI: https://doi.org/10.1016/s0031-9422(03)00142-0.
12. Almora, K.; Pino, J.; Hernández, M.; Duarte, C.; González, J.; Roncal, E. Evaluation ofvolatiles from ripening papaya (Carica papaya L., var. Maradol roja). Food Chem. 2004, 86, 127-130. DOI: https://doi.org/10.1016/j.foodchem.2003.09.039.
13. Pino, J.; Cuevas-Glory, L.; Marbot, R.; Fuentes, V. Volatile compounds of grosella (Phyllanthus acidus [L.] Skeels) fruit. Rev. CENIC, Cienc. Quim 2008, 39, 3-5.
14. Aurore, G.; Ginies, C.; Ganou-parfait, B.; Renard, C.; Fahrasmane, L. Comparative study of free and glycoconjugated volatile compounds of three banana cultivars from French West Indies: Cavendish, Frayssinette and Plantain. Food Chem. 2011, 129, 28-34. DOI: https://doi.org/10.1016/j.foodchem.2011.01.104.
15. Restrepo, P.; Duque, C. Componentes volátiles de la gulupa Passiflora maliformis. Rev. Colomb. Quim. 1988, 17, 57-63.
16. Min, S.; Zhang, Q. Effects of commercial-scale pulsed electric field processing on flavor and color of tomato juice. J. Food Sci. 2003, 68, 1600-1606. DOI: https://doi.org/10.1111/j.1365-2621.2003.tb12298.x.
17. Qin, G.; Tao, S.; Cao, Y.; Wu, J.; Zhang, H.; Huang, W. et al. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC-MS. Food Chem. 2012, 134, 2367-2382. DOI: https://doi.org/10.1016/j.foodchem.2012.04.053.
18. Yang, D.; Balandrán-Quintana, R.; Ruiz, C.; Toledo, R.; Kays, S. Effect of hyperbaric, controlled atmosphere, and UV treatments on peach volatiles. Postharvest Biol. Technol. 2007, 51, 334-341. DOI: https://doi.org/10.1016/j.postharvbio.2008.09.005.
19. Cai, J.; Liu, B.; & Su, Q. Comparison of simultaneous distillation extraction and solid-phase microextraction for the determination of volatile flavor components. J. Chromatogr. A. 2001, 930, 1-7. DOI: https://doi.org/10.1016/s0021-9673(01)01187-6.
20. Pino, J. Odour-active compounds in papaya fruit cv. Red Maradol. Food Chem. 2014, 146, 120-126. DOI: https://doi.org/10.1016/j.foodchem.2013.09.031.
21. Soler, S.; De Oliveira, W.; Re-Poppi, N.; Simionatto, E.; Carasek, E. Volatile compounds of leaves and fruits of Mangifera indica var. coquinho (Anacardiaceae) obtained using solid phase microextraction and hydrodistillation. Food Chem. 2011, 127, 689-693. DOI: https://doi.org/10.1016/j.foodchem.2010.12.123.
22. Nunes, C.; Coimbra, M.; Saraiva, J.; Rocha, S. Study of the volatile components of a candied plum and estimation of their contribution to the aroma. Food Chem 2008, 111, 897-905 DOI: https://doi.org/10.1016/j.foodchem.2008.05.003.
23. Cheong, M.; Liu, S.; Zhou, W.; Curran, P.; Yu, B. Chemical composition and sensory profile of pomelo (Citrus grandis (L ) Osbeck) juice. Food Chem 2012, 135, 2505-2513 DOI: https://doi.org/10.1016/j.foodchem.2012.07.012.
24. Santos, T.; Nogueira, P. Volatile components of mangaba fruit ( Hancornia speciosa Gomes) at three stages of maturity. Food Chem. 2006, 95, 606-610. DOI: https://doi.org/10.1016/j.foodchem.2005.01.038.
25. Pontes, M.; Marques, J.; Câmara, J. Headspace solid-phase microextraction-gas chromatography-quadrupole mass spectrometric methodology for the establishment of the volatile composition of Passiflora fruit species. Microchem J 2009, 93, 1-11 DOI: https://doi.org/10.1016/j.microc.2009.03.010.
26. Diniz, F.; Pereira, T.; Maio, M.; Monteiro, A. Volatile and nonvolatile chemical composition of the white guava fruit (Psidium guajava) at different stages of maturity. Food Chem. 2007, 100, 15-21. DOI: https://doi.org/10.1016/j.foodchem.2005.07.061.
27. Pandit, S.; Chidley, H.; Kulkarni, R.; Pujari, K.; Giri, A.; Gupta, V. Cultivar relationships in mango based on fruit volatile profiles Food Chem. 2009, 114, 363-372. DOI: https://doi.org/10.1016/j.foodchem.2008.09.107.
28. El Arem, A.; Flamini, G.; Saafi, E.; Issaoui, M.; Zayene, N.; Ferchichi, A et al Chemical and aroma volatile compositions of date palm (Phoeni dactylifera L.) fruits at three maturation stages. Food Chem. 2011, 127, 1744-1754. DOI: https://doi.org/10.1016/j.foodchem.2011.02.051.
29. Dharmawan, J.; Barlow, P.; Curran, P Characterisation of volatile compounds in selected citrus fruits from Asia. Dev. Food Sci. 2006, 43, 319-322. DOI: https://doi.org/10.1016/s0167-4501(06)80076-2.
30. Meret, M.; Brat, P.; Mertz, C.; Lebrun, M.; Gü nata, Z. Contribution to aroma potential of Andean blackberry (Rubus glaucus Benth.). Food Res. Int. 2011, 44, 54-60. DOI: https://doi.org/10.1016/j.foodres.2010.11.016.
31. Forero, D.; Orrego, C.; Grant, D.; Osorio, C. Chemical and sensory comparison of fresh and dried lulo (Solanum quitoense Lam.) fruit aroma. Food Chem. 2015, 169, 85-91. DOI: https://doi.org/10.1016/j.foodchem.2014.07.111.
32. Suárez, M.; Duque, C. Volatile constituents of lulo (Solanum vestissimun D.) fruit. J. Agr. Food Chem. 1991, 39, 1498-1500. DOI: https://doi.org/10.1021/jf00008a026.
33. Mora, R.; Pinzón, M. Principales constituyentes volátiles del aroma de lulo "La Selva" durante la maduración. V Seminario Nacional e Internacional de Frutales. Manizales, 2004, 261-267.
34. Conde-Martínez, N.; Sinuco, D.; Osorio, C. Chemical studies on curuba (Passiflora mollissima (Kunth) L. H. Bailey) fruit flavour. Food Chem. 2014, 157, 356-363. DOI: https://doi.org/10.1016/j.foodchem.2014.02.056.
35. Steinhaus, M.; Sinuco, D.; Polster, J.; Osorio, C.; Schieberle, P. Characterization of the key aroma compounds in pink guava (Psidium guajava L.) by means of aroma re-engineering experiments and omission tests. J. Agric. Food Chem. 2009, 57, 2882-2888. DOI: https://doi.org/10.1021/jf803728n.
Referencias
Pino, J.; & Febles, Y. Odour-active compounds in banana fruit cv. Giant Cavendish. Food Chem. 2013, 141, 795–801. DOI: http://dx.doi.org/10.1016/j.foodchem.2013.03.064
Chen, M.; Chen, X.; Wang, X.; Ci, Z.; Liu, X.; He, T. et al. Comparison of heads pace solid- phase microextraction with simultaneous steam distillation extraction for the analysis of the volatile constituents in chinese apricot. Agric. Sci. China. 2006, 5, 879-884. DOI: http://dx.doi.org/10.1016/s1671-2927(06)60139-9
Arizala, M.; Monsalvo, A.; Betancourth, C.; Salazar, C.; Lagos, T. Evaluación de solanaceas silvestres como patrones de lulo (Solanum quitoense Lam) y su reacción a Fusarium sp. Revista de ciencias agrícolas. 2011, 28, 147-160.
Brunerie, P.; & Maugeais, P. Comparison of volatile components in two naranjilla fruit (Solanum quitoense Lam.) pulp from different origin. Dev Food Sci. 1992, 29, 163-174. DOI: https://doi.org/10.1016/b978-0-444-88834-1.50019-3
Parada, F.; Guerrero, E.; Suárez, M.; Duque, C. Obtención de esencias de lulo (Solanum quitoense L) utilizando CO2 supercritico. Rev Colomb Quim. 1993, 22, 53-62.
Suárez, M.; Duque, C.; Wintoch, H.; Schreier, P. Glycosidically bound aroma compounds from the pulp and the of lulo fruit (Solanum vestissimum D.). J Agr Food Chem. 1991, 39, 1643-1645. DOI: https://doi.org/10.1021/jf00009a022
Mejía, C.; Gaviria, D.; Duque, A.; Rengifo, L.; Aguilar, E.; Alegría, A. Physicochemical characterization of the lulo (Solanum quitoense Lam.) castilla variety in six ripening stages. Vitae. 2012, 19, 157-165.
Grant, S.; Golding, J.; McGlasson, W.; Williams, M. The relationship between ethylene and aroma volatiles production in ripening climacteric fruit. Dev Food Sci. 1998, 40, 375-384. DOI: https://doi.org/10.1016/s0167-4501(98)80061-7
Márquez, C. Caracterización fisiológica, físico-química, reológica, nutraceútica, estructural y sensorial de la guanábana (Annona muricata L. cv. ELITA). [Tesis de Doctorado]. [Medellín, Colombia]. Universidad Nacional: 2009. 274 p.
Ceva-Antunes, P.; Ribeiro, H.; Carvalho, C.; Antunes, O. Analysis of volatile composition of siriguela (Spondias purpurea L.) by solid phase microextraction (SPME). Food Sci Technol. 2006, 39, 437-443. DOI: https://doi.org/10.1016/j.lwt.2005.02.007
Matich, A.; Young, H.; Allen, J.; Wang, M.; Fielder, S.; McNeilage, M. et al. Actinidia arguta: volatile compounds in fruit and flowers. Phytochemistry. 2003, 63, 285-301. DOI: https://doi.org/10.1016/s0031-9422(03)00142-0
Almora, K.; Pino, J.; Hernández, M.; Duarte, C.; González, J.; Roncal, E. Evaluation of volatiles from ripening papaya (Carica papaya L., var. Maradol roja). Food Chem. 2004, 86, 127–130. DOI: https://doi.org/10.1016/j.foodchem.2003.09.039
Pino, J.; Cuevas-Glory, L.; Marbot, R.; Fuentes, V. Volatile compounds of grosella (Phyllanthus acidus [L.] Skeels) fruit. Revista CENIC Ciencias Químicas. 2008, 39, 3-5.
Aurore, G.; Ginies, C.; Ganou-parfait, B.; Renard, C.; Fahrasmane, L. Comparative study of free and glycoconjugated volatile compounds of three banana cultivars from French West Indies: Cavendish, Frayssinette and Plantain. Food Chem. 2011, 129, 28–34. DOI: https://doi.org/10.1016/j.foodchem.2011.01.104
Restrepo, P.; Duque, C. Componentes volátiles de la gulupa Passiflora maliformis. Rev Colomb Quim. 1988, 17, 57-63.
Min, S.; & Zhang, Q. Effects of commercial-scale pulsed electric field processing on flavor and color of tomato juice. J. Food Sci. 2003, 68, 1600-1606. DOI: http://dx.doi.org/10.1111/j.1365-2621.2003.tb12298.x
Qin, G.; Tao, S.; Cao, Y.; Wu, J.; Zhang, H.; Huang, W. et al. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC–MS. Food Chem. 2012, 134, 2367-2382. DOI: http://dx.doi.org/10.1016/j.foodchem.2012.04.053
Yang, D.; Balandrán-Quintana, R.; Ruiz, C.; Toledo, R.; Kays, S. Effect of hyperbaric, controlled atmosphere, and UV treatments on peach volatiles. Postharvest Biol Tec. 2007, 51, 334-341. DOI: http://dx.doi.org/10.1016/j.postharvbio.2008.09.005
Cai, J.; Liu, B.; & Su, Q. Comparison of simultaneous distillation extraction and solid-phase microextraction for the determination of volatile flavor components. J Chromatogr A. 2001, 930, 1-7. DOI: http://dx.doi.org/10.1016/s0021-9673(01)01187-6
Pino, J. Odour-active compounds in papaya fruit cv. Red Maradol. Food Chem. 2014, 146, 120–126. DOI: http://dx.doi.org/10.1016/j.foodchem.2013.09.031
Soler, S.; De Oliveira, W.; Ré-Poppi, N.; Simionatto, E.; Carasek, E. Volatile compounds of leaves and fruits of Mangifera indica var. coquinho (Anacardiaceae) obtained using solid phase microextraction and hydrodistillation. Food Chem. 2011, 127, 689–693. DOI: http://dx.doi.org/10.1016/j.foodchem.2010.12.123
Nunes, C.; Coimbra, M.; Saraiva, J.; & Rocha, S. Study of the volatile components of a candied plum and estimation of their contribution to the aroma. Food Chem. 2008, 111, 897–905. DOI: http://dx.doi.org/10.1016/j.foodchem.2008.05.003
Cheong, M.; Liu, S.; Zhou, W.; Curran, P.; Yu, B. Chemical composition and sensory profile of pomelo (Citrus grandis (L.) Osbeck) juice. Food Chem. 2012, 135, 2505–2513. DOI: http://dx.doi.org/10.1016/j.foodchem.2012.07.012
Santos, T.; & Nogueira, P. Volatile components of mangaba fruit (Hancornia speciosa Gomes) at three stages of maturity. Food Chem. 2006, 95, 606-610. DOI: https://doi.org/10.1016/j.foodchem.2005.01.038
Pontes, M.; Marques, J.; Câmara, J. Headspace solid-phase microextraction-gas chromatography-quadrupole mass spectrometric methodology for the establishment of the volatile composition of Passiflora fruit species. Microchem J. 2009, 93, 1-11. DOI: https://doi.org/10.1016/j.microc.2009.03.010
Diniz, F.; Pereira, T.; Maio, M.; Monteiro, A. Volatile and non-volatile chemical composition of the white guava fruit (Psidium guajava) at different stages of maturity. Food Chem. 2007, 100, 15-21. DOI: https://doi.org/10.1016/j.foodchem.2005.07.061
Pandit, S.; Chidley, H.; Kulkarni, R.; Pujari, K.; Giri, A.; Gupta, V. Cultivar relationships in mango based on fruit volatile profiles. Food Chem. 2009, 114, 363-372. DOI: https://doi.org/10.1016/j.foodchem.2008.09.107
El Arem, A.; Flamini, G.; Saafi, E.; Issaoui, M.; Zayene, N.; Ferchichi, A. et al. Chemical and aroma volatile compositions of date palm (Phoenix dactylifera L.) fruits at three maturation stages. Food Chem. 2011, 127, 1744-1754. DOI: https://doi.org/10.1016/j.foodchem.2011.02.051
Dharmawan, J.; Barlow, P.; Curran, P. Characterisation of volatile compounds in selected citrus fruits from Asia. Dev Food Sci. 2006, 43, 319-322. DOI: https://doi.org/10.1016/s0167-4501(06)80076-2
Meret, M.; Brat, P.; Mertz, C.; Lebrun, M.; Günata, Z. Contribution to aroma potential of Andean blackberry (Rubus glaucus Benth.). Food Res Int. 2011, 44, 54–60. DOI: https://doi.org/10.1016/j.foodres.2010.11.016
Forero, D.; Orrego, C.; Grant, D.; Osorio, C. Chemical and sensory comparison of fresh and dried lulo (Solanum quitoense Lam.) fruit aroma. Food Chem. 2015, 169, 85-91. DOI: https://doi.org/10.1016/j.foodchem.2014.07.111
Suárez, M.; Duque, C. Volatile constituents of lulo (Solanum vestissimun D) fruit. J Agr Food Chem. 1991, 39, 1498-1500. DOI: https://doi.org/10.1021/jf00008a026
Mora, R.; Pinzón, M. Principales constituyentes volátiles del aroma de lulo “La Selva” durante la maduración. V Seminario Nacional e Internacional de Frutales. Manizales, 2004, 261-267.
Conde-Martínez, N.; Sinuco, D.; Osorio, C. Chemical studies on curuba (Passiflora mollissima (Kunth) L. H. Bailey) fruit flavour. Food Chem. 2014, 157, 356-363. DOI: https://doi.org/10.1016/j.foodchem.2014.02.056
Steinhaus, M.; Sinuco, D.; Polster, J.; Osorio, C.; Schieberle, P. Characterization of the key aroma compounds in pink guava (Psidium guajava L.) by means of aroma re-engineering experiments and omission tests. J. Agric. Food Chem. 2009, 57, 2882-2888. DOI: https://doi.org/10.1021/jf803728n
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Lorenzo N. Bizzio, Denise Tieman, Patricio R. Munoz. (2022). Branched-Chain Volatiles in Fruit: A Molecular Perspective. Frontiers in Plant Science, 12 https://doi.org/10.3389/fpls.2021.814138.
2. Muhammad Usman, Shuo Cheng, Sasipa Boonyubol, Jeffrey S. Cross. (2024). Nitrogen Minimization in Hydrothermal Liquefaction Biocrude from Sewage Sludge with Green Extraction Solvents. ACS Omega, 9(12), p.14530. https://doi.org/10.1021/acsomega.4c00455.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons (CC. Atribución 4.0) que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).