Acylhydrazone-based dynamic combinatorial libraries: study of the thermodynamic/kinetic evolution, configurational and coordination dynamics
Librerías combinatorias dinámicas basadas en acil-hidrazona: estudio del desarrollo termodinámico/cinético, dinámicas de configuración y de coordinación
DOI:
https://doi.org/10.15446/rev.colomb.quim.v45n3.61408Palabras clave:
dynamic combinatorial chemistry, acylhydrazones, coordination and configurational dynamics (en)librerías combinatorias dinámicas, acil-hidrazonas, dinámicas de coordinación y configuración (es)
Descargas
The kinetic and thermodynamic selectivity of acylhydrazone formation in dynamic combinatorial libraries (DCL) is described. Competition reactions were generated from hydrazides: isoniazid, 4-nitro-benzohydrazide, 4-dimethylamino-benzohydrazide, and nicotinic hydrazide; as well as the aldehyde derivatives: benzaldehyde and 2-pyridine-carboxaldehyde. The obtained species and the distribution of the DCLs were monitored by 1H-NMR spectroscopy finding that those acylhydrazones containing the 4-dimethylamino-benzohydrazide are both the kinetic and thermodynamic product of their respective libraries. Configurational and coordination dynamics for some of these libraries were also investigated. The obtained results allowed the study of the redistribution of components and the amplification of one or more products using light and metal ions as physical and chemical templates, respectively.
Se describe la selectividad cinética y termodinámica de la formación de acil-hidrazona en librerías combinatorias dinámicas (DCL). Se generaron reacciones competitivas a partir de hidrazidas: isoniazida, 4-nitro-benzohidrazida, 4-dimetilamino-benzohidrazida y hidrazida nicotínica; así como a partir de los derivados de aldehído: benzaldehído y 2-piridin-carboxaldehido. Las especies obtenidas y la distribución de los DCLs fueron monitoreados mediante espectroscopia 1H-NMR, encontrándose que las acil-hidrazonas que contenían la 4-dimetilamino-benzohidrazida son tanto el producto cinético, como el termodinámico de sus respectivas librerías. También se investigaron las dinámicas de configuración y de coordinación para algunas de estas librerías. Los resultados obtenidos permitieron estudiar la redistribución de los componentes y la amplificación de uno o más productos usando luz e iones metálicos como plantillas físicas y químicas, respectivamente.
Doi: https://doi.org/10.15446/rev.colomb.quim.v45n3.61408
Acylhydrazone-based dynamic combinatorial libraries: study of the thermodynamic/kinetic evolution, configurational and coordination dynamics
Librerías combinatorias dinámicas basadas en acil-hidrazona: estudio del desarrollo termodinámico/cinético, dinámicas de configuración y de coordinación
Livrarias combinatórias dinâmicas baseadas na acil-hidrazona: estudo da evolução cinética/termodinâmica, dinâmicas de configuração e da coordenação
Mónica A. Gordillo1, Fabio Zuluaga1, Manuel N. Chaur1,*
1 Grupo de Investigación Síntesis y Mecanismos de Reacción en Química Orgánica (SIMERQO) Departamento de Química, Universidad del Valle, A.A., 25360 Cali, Colombia.
* Autor para correspondencia: manuel.chaur@correounivalle.edu.co
Article citation:
Gordillo, M. A.; Zuluaga, F.; Chaur, M. N. Acylhydrazone-based dynamic combinatorial libraries: study of the thermodynamic/kinetic evolution, configurational and coordination dynamics. Rev. Colomb. Quim. 2016, 45 (3), 39-50. DOI: https://doi.org/10.15446/rev.colomb.quim.v45n3.61408.
Recibido: 6 de Junio de 2016. Aceptado: 27 de Septiembre de 2016.
Abstract
The kinetic and thermodynamic selectivity of acylhydrazone formation in dynamic combinatorial libraries (DCL) is described. Competition reactions were generated from hydrazides: isoniazid, 4-nitro-benzohydrazide, 4-dimethylamino-benzohydrazide, and nicotinic hydrazide as well as the aldehyde derivatives: benzaldehyde and 2-pyridine-carboxaldehyde. The obtained species and the distribution of the DCLs were monitored by 'H-NMR spectroscopy finding that those acylhydrazones containing the 4-dimethylamino-benzohydrazide moiety are both the kinetic and thermodynamic product of their respective libraries. Configurational and coordination dynamics for some of these libraries were also investigated. The obtained results allowed the study of the redistribution of components and the amplification of one or more products using light and metal ions as physical and chemical templates, respectively.
Keywords: Dynamic combinatorial chemistry, acylhydrazones, coordination and configurational dynamics.
Resumen
Se describe la selectividad cinética y termodinámica de la formación de acil-hidrazona en bibliotecas combinatorias dinámicas (DCL). Se generaron reacciones competitivas a partir de hidrazidas: isoniazida, 4-nitro-benzohidrazida, 4-dimetilamino-benzohidrazida y hidrazida nicotínica; así como a partir de los derivados de aldehído: benzaldehído y 2-piridin-carboxaldehido. Las especies obtenidas y la distribución de los DCLs fueron monitoreados mediante espectroscopia 'H-NMR, encontrándose que las acil-hidrazonas que contenían la 4-dimetilamino-benzohidrazida son tanto el producto cinético, como el termodinámico de sus respectivas bibliotecas. También se investigaron las dinámicas de configuración y de coordinación para algunas de estas bibliotecas. Los resultados obtenidos permitieron estudiar la redistribución de los componentes y la amplificación de uno o más productos usando luz e iones metálicos como plantillas físicas y químicas, respectivamente.
Palabras clave: Bibliotecas combinatorias dinámicas, acil-hidrazonas, dinámicas de coordinación y configuración.
Resumo
É descrita a seletividade cinética e termodinâmica da formação de acil-hidrazonas em livrarias combinatórias dinâmicas (DLC). Foram geradas reações competitivas a partir das hidrazidas: isoniazida, 4-nitro-benzohidrazida, 4-dimetilamino-benzohidrazida e hidrazida nicotínica; além dos derivados de aldeído: benzaldeído e 2-piridin-carboxaldeído. As espécies obtidas e a distribuição dos DLCs foram monitorados mediante espectroscopia 'H-NMR, foi encontrado que as acil-hidrazonas que continham à 4-dimetilamino-benzohidrazida são tanto o produto cinético como o termodinâmico de suas respectivas livrarias. Também investigaram-se as dinâmicas de configuração e coordenação para algumas destas livrarias. Os resultados obtidos permitem estudar a redistribuicao dos componentes e a amplificação de um ou mais produtos usando luz e íons metálicos como modelos físicos e químicos, respectivamente.
Palavras-Chave: Livrarias combinatórias dinâmicas, acil-hidrazonas, dinâmicas de coordenação e configuração.
Introduction
Dynamic combinatorial chemistry (DCC) is a powerful tool to study and create complex chemical systems in a relative simple manner. DCC was defined by Sanders as molecular or supramolecular combinatorial chemistry under thermodynamic control (1). When a system is formed by molecular fragments that can react with each other, combining them, a mixture of many compounds that interconverts constantly, will be obtained, i.e. building blocks are connected together by reversible bonds which are continuously forming and breaking in the reaction medium (Figure 1). This product mixture is known as Dynamic Combinatorial Library (DCL). The system is reversible and it is in equilibrium, thus, any external effect could shift this equilibrium. A clean-cut example of these systems and relating Emil Fisher's concept is placing a template in the system, which fits precisely with one member of the library, and subsequently amplify or shift the equilibrium towards the formation of a product (2).
DCC and DCL's have been widely used for the synthesis and identification of small molecular receptors (3-7). These tools have also helped to generate effective ligands for biomacromolecules and biosensors (8-10), synthesis of catalysts (11-13), crosslinked materials (14-16), capsules and cages (17-19), self-replication (20), nanomachines (21 ), among others.
Based on previous work done by Lehn's group (22-24), we have chosen a set of four hydrazides and two aldehydes as building blocks in order to generate several dynamic combinatorial libraries. These building blocks were selected since the acylhydrazones, which can be formed, have a number of characteristics that make them attractive for DCL's formation: i) unlike the hydrazones, the acylhydrazones have a much weaker double bond making them favorable to perform exchange reactions; ii) these compounds have an imino double bond, which has been widely investigated in our research group and it is known that is sensitive to light (25-26); iii) some of these compounds have coordination sites in their chemical structure that serve as tridentate ligand to coordinate to cation metals. Having in mind these characteristics, we have analyzed how the distributions of the formed libraries vary by the presence of the metals and UV light as external stimuli. For this purpose, nuclear magnetic resonance technique was used as a tool for monitoring the evolution of the dynamic library.
Materials and methods
All starting materials, reagents and solvents, were purchased from Sigma-Aldrich and Alfa Aesar. The hydrazides were used without any further purification. The benzaldehyde was distilled under reduced pressure. 1H and 13C-NMR spectra were taken in a 400 MHz Bruker UltraShield spectrometer. UV-Vis spectra were recorded in a Shimadzu UV-1700 PharmaSpec spectrophotometer.
Standard procedure for the preparation of acylhydrazones
One eq of aldehyde A-B was added to an ethanol solution (5.0 mL) of the corresponding hidrazide 1-4 (1 eq) with 5.0 µL of glacial acetic acid. The mixture was heated under reflux of ethanol for 3 to 6 h. The resulting precipitate was collected by vacuum filtration and recrystallized from cold ethanol to afford the pure acylhydrazones in their E-configuration.
Standard procedure for the preparation of DCLs
The DCLs were prepared by mixing in a sealed NMR tube (under inert atmosphere of N2, done in a Aldrich AtmosBag) 1 eq of the corresponding aldehydes (225 μL) and acylhydrazines (225 μL) in DMSO-d6 or CD3OD at 25 °C. The starting time of reaction (t = 0) was considered as the time in which the solution of aldehydes was poured into the NMR tube and entered in contact with the hydrazines solution. 1H-NMR spectroscopy was used as a tool to monitor the evolution of the library. For those experiments involving UV radiation, a 250 W mercury lamp was used as an UV source. The NMR tube was irradiated while pouring the reagents; in a second experiment the NMR tube with the compounds was allowed to equillibrate during 24 h. Afterwards, the tube was irradiated during 1 h. For the addition of metal ions, solutions of the corresponding M2+ ion were standarized by atomic absorption spectroscopy calculating the concentration in a calibration curve.
Results and discussion
When mixing aldehydes and hydrazines redacción: a large number of 1H-NMR signals are obtained making difficult to identify the products. Therefore, each possible acylhydrazone, as part of the library, was synthesized from each corresponding hydrazide and aldehyde derivatives, according to a methodology reported previously (22) (Figure 2). The reactions were monitored by thin layer chromatography (TLC), and the spectroscopic data were consistent with the proposed structures (E configuration) of compounds A-1 to B-4 (Figure 3). Details of the synthesis were described in the Materials and methods section.
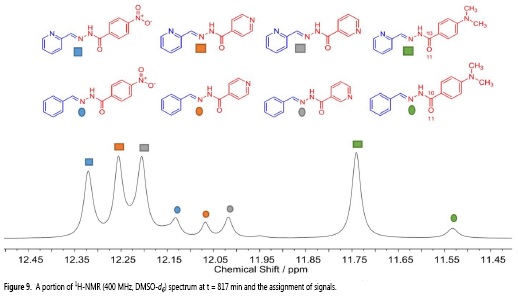
The synthesis was performed with the aim to identify characteristic signals in the 1H-NMR spectra of each acylhydrazone. Signals found in the region between 11.5 and 12.5 ppm, which correspond to the N-H protons (as determined by 2D NMR techniques), were chosen to determine the distribution of the products on the libraries (see Characterization data for acylhydrazones) and further confirmed by DOSY experiments to corroborate the asignment of the N-H proton signals.
The fact that the signals of the N-H protons of each acylhydrazone appear at different chemical shifts, results from the type of substituent, which is present in every one of them, since they contain either electron-withdrawing groups, electron-releasing groups or an electronegative nitrogen at different positions on the ring.
Characterization data for acylhydrazones
2-pyridinecarboxaldehyde isonicotinoyl hydrazone (A-1): Using the method described above, the compound was synthesized and obtained in a 86% yield. M.p.: 166-167°C. Elemental analysis calcd. (%) for C12H10N4O: C, 63.71; H, 4.46; N, 24.76; found: C, 62.37; H, 4.23; N, 23.82. FT-IR (ATR) v/cm-1 3292 (N-H), 1665 (C=O), 1539 (C=N). 1H-NMR (400 MHz, DMSO-d6) δ 12.28 (s, 1H), 8.84-8.78 (m, 2H), 8.64 (d, J 4.29 Hz, 1 H), 8.48 (s, 1 H), 8.03-7.97 (m, 1 H), 7.91 (td, J 7.71, 1.56 Hz, 1 H), 7.86-7.82 (m, 2 H), 7.45 (ddd, J 7.27, 4.93, 1.07 Hz, 1 H). 13C-NMR (100.60 MHz, DMSO-d6) δ 161.93, 152.96, 150.44, 149.65, 149.23, 140.26, 137.04, 124.74, 121.61, 120.13.
2-pyridinecarboxaldehyde p-nitrobenzoyl hydrazone (A-2): Using the method described above, the compound was synthesized and obtained in a 96% yield. M.p.:227-228°C. Elemental analysis calcd. (%) for C13H10N4O3: C, 57.78; H, 3.73; N, 20.73; found : C, 57.86; H, 3.72; N, 20.62. FT-IR (ATR) v/cm-1 3221 (N-H), 1659 (C=O), 1595(C=N). 1H-NMR (400 MHz, DMSO-d6) δ 12.31 (s, 1 H), 8.64 (d, J 4.29 Hz, 1 H), 8.49 (s, 1 H), 8.39 (d, J 8.59 Hz, 2 H), 8.17 (d, J 8.59 Hz, 2 H), 8.01 (d, J 7.80 Hz, 1 H) 7.94-7.87 (m, 1 H) 7.47-7.42 (m, 1 H). 13C-NMR (100.60 MHz, DMSO-d6) δ 161.76, 152.96, 149.59, 149.38, 149.12, 138.80, 136.95, 129.26, 124.65, 123.69, 120.08.
2-pyridinecarboxaldehyde p-dimethylamino-benzoyl hydrazone (A-3): Using the method described above, the compound was synthesized and obtained in a 87% yield. M.p.: 224-225°C. Elemental analysis calcd. (%) for C15H16N4O: C, 67.15; H, 6.05; N, 20.88; found: C, 67.19; H, 6.07; N, 20.78. FT-IR (ATR) v/cm-1 3244 (N-H), 1611(C=O), 1516(C=N). ). 1H-NMR (400 MHz, DMSO-d6) δ 11.77 (s, 1 H), 8.60 (d, J 4.49 Hz, 1 H), 8.45 (s, 1 H), 7.95 (d, J 7.80 Hz, 1 H), 7.88 (dd, J 7.61, 1.56 Hz, 1 H), 7.83 (d, J 8.98 Hz, 2 H), 7.39 (dd, J 7.41, 4.88 Hz, 1 H), 6.76 (d, J 8.98 Hz, 2 H), 3.00 (s, 6 H). 13C-NMR (100.60 MHz, DMSO-d6) δ 163.09, 153.66, 152.61, 149.48, 146.32, 136.83, 129.24, 124.12, 119.67, 119.08, 110.84, 39.51.
2-pyridinecarboxaldehyde nicotinoyl hydrazone (A-4): Using the method described above, the compound was synthesized and obtained in a 91% yield. M.p.: 148-150°C. Elemental analysis calcd. (%) for C12H10N4O: C, 63.71; H, 4.46; N, 24.76; found: C, 58.63; H, 4.74; N, 22.81. FT-IR (ATR) v/cm-1 3474 (N-H), 1668(C=O), 1593 (C=N). 1H-NMR (400 MHz, DMSO-d6) δ 12.24 (s, 1 H), 9.08 (d, J = 1.56 Hz, 1 H), 8.82-8.75 (m, 1 H), 8.63 (d, J = 4.68 Hz, 1 H), 8.46 (s, 1 H), 8.28 (d, J = 7.80 Hz, 1 H), 8.00 (d, J = 7.80 Hz, 1 H), 7.94-7.86 (m, 1 H), 7.59 (dd, J = 7.80, 4.88 Hz, 1 H), 7.48-7.40 (m, 1 H). 13C-NMR (100.60 MHz, DMSO-d6) δ 162.12, 153.10, 152.61, 149.70, 148.75, 148.73, 137.13, 135.73, 129.05, 124.76, 123.83, 120.20.
Benzaldehyde isonicotinoyl hydrazone (B-1): Using the method described above, the compound was synthesized and obtained in a 71% yield. M.p.: 198-199°C. Elemental analysis calcd. (%) for C13H11N3O: C, 69.32; H, 4.92; N, 18.66; found: C, 68.73; H, 4.89; N, 18.38. FT-IR (KBr) v/cm-1 3455 (N-H), 1692 (C=O), 1566 (C=N). 1H-NMR (400 MHz, DMSO-d6) δ 12.11 (s, 1 H), 8.79 (d, J 4.10 Hz, 2 H), 8.46 (s, 1 H), 7.83 (d, J 3.90 Hz, 2 H), 7.78-7.74 (m, 2 H), 7.47 (br. s., 3 H). 13C-NMR (100.60 MHz, DMSO-d6) 5 161.65, 150.33, 149.07, 140.47, 134.01, 130.41, 128.90, 127.27, 121.53.
Benzaldehyde p-nitrobenzoyl hydrazone (B-2): Using the method described above, the compound B-2 was obtained with a 79% yield.
M.p.: 260-262°C. Elemental analysis calcd. (%) for C14H11N3O3: C, 62.45; H, 4.12; N, 15.61; found: C, 61.65; H, 4.15; N, 15.35. FT-IR (KBr) v/cm-1 3450 (N-H), 1656 (C=O), 1554 (C=N). 1H-NMR (400 MHz, DMSO-d6) δ 12.17 (s, 1 H), 8.47 (s, 1 H), 8.37 (d, J 8.59 Hz, 2 H), 8.15 (d, J 8.78 Hz, 2 H), 7.78-7.73 (m, 2 H), 7.49-7.45 (m, 3 H). 13C-NMR (100.60 MHz, DMSO-d6) δ 161.61, 149.31, 149.03, 139.11, 134.06, 130.44, 129.21, 128.93, 127.31, 123.69.
Benzaldehyde p-dimethylamino-benzoyl hydrazone (B-3): Using the method described above, the compound was synthesized and obtained in a 82% yield. M.p.: 283-285°C. Elemental analysis calcd. (%) for C16H17N3O: C, 71.89; H, 6.41; N, 15.72; found: C,71.48; H, 6.50; N, 15.82. FT-IR (KBr) v/cm-1 3223 (N-H), 1614 (C=O), 1524 (C=N). 1H-NMR (400 MHz, DMSO-d6) δ 11.57 (br. s., 1 H), 8.42 (br. s., 1 H), 7.82 (d, J 8.78 Hz, 2 H) 7.70 (d, J 6.63 Hz, 2 H), 7.487.40 (m, 3 H), 6.76 (d, J 8.98 Hz, 2 H), 3.00 (s, 6 H). 13C-NMR (100.60 MHz, DMSO-d6) δ 163.07, 152.48, 145.96, 134.68, 129.69, 128.79, 126.84, 119.44, 110.81.
Benzaldehyde nicotinoyl hydrazone (B-4): Using the method described above, the compound was synthesized and obtained in a 70% yield. M.p.: 129-130°C. Elemental analysis calcd. (%) for C13H11N3O: C, 69.32; H, 4.92; N, 18.66; found: C, 67.47; H, 4.94; N, 17.68. FT-IR (KBr) v/cm-1 3270 (N-H), 1653 (C=O), 1550 (C=N).
1H-NMR(400 MHz,DMSO-d6) δ 12.01 (s, 1 H), 9.07 (d, J 1.17 Hz, 1 H), 8.77 (d, J 3.90 Hz, 1 H), 8.46 (s, 1 H), 8.26 (d, J 7.80 Hz, 1 H), 7.79-7.71 (m, 2 H), 7.57 (dd, J 7.80, 4.88 Hz, 1 H), 7.50-7.45 (m, 3 H). 13C-NMR (100.60 MHz, DMSO-d6) δ 161.67, 152.26, 148.54, 135.41, 134.10, 130.24, 129.22, 128.84, 128.52, 127.17, 123.58.
Competitive reactions of acylhydrazines 1-4 with aldehydes A and B
Two competitive reactions, named DCL-1 and DCL-2, were carried out from the hydrazides 1-4 and aldehyde A (DCL-1) or B (DCL-2) by mixing equimolar amounts of the respective building blocks in a NMR tube and using a deuterated solvent (Figure 2). The libraries with A and B were monitored for 320 min by 1H-NMR spectroscopy, further time did not showed any changes in the relative concentration of the DCL. The relative amount of acylhydrazone formed was calculated from the relative intensities of the corresponding signals and compared to an internal standart (1,4-dioxane). As shown in Figure 4 for DCL-1 and Figure 5 for DCL-2, the appearance of four new signals in the aforementioned region shows the formation of the four corresponding acylhydrazones (A-1, A-2, A-3 and A-4).
From the NMR data, kinetic traces for acylhydrazones formation were ploted. Aditionally, equillibrium distributions of the different acylhydrazones are shown in Figure 6. From these results, it is observed that A-3 and B-3 are the acylhydrazones kinetically and thermodynamically favored in their respective DCLs. The latter is understood based on the greater nucleophilicity of acylhydrazine 3.
Likewise, the highest rate of formation and stability of A-3 and B-3 may also be explained if we consider that the precursors of the other acylhydrazones have in their structure either one electron withdrawing group or an electronegative nitrogen in the aromatic ring, which by both inductive and resonance effects generate an electronic deficiency in the molecule, making it less reactive towards the nucleophilic attack of the nitrogen to the carbonyl group of the aldehyde (2).
While in A-3 and B-3, the dimethylamine group makes the molecule more electron-rich, thus confering a higher reactivity for the nucleophilic attach which is reflected in the greater proportion and the greater stability of this acylhydrazones. Although equillibrium was confirmed by a control experiment using different starting concentrations of previously prepared acylhydrazones reaching the same final distributions, it is important to remark that those distributions are reached in longer times which implies a slow amine interchange in the DCL. Despite that amine interchange can be increased by changing the nature of the solvent, this was not considered in this study to avoid issues with the solubility of the reagents.
DCL-3: hydrazides 1-4 plus aldehydes A and B
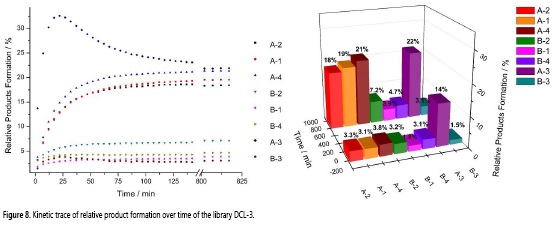
Aldehydes A-B were added to an equimolar mixture of hydrazides 1-4. The reaction was monitored for 817 min, resulting in 168 FH-NMR spectra in total (some of them are shown in Figure 7).
Figure 8 shows the kinetic trace of the competition DCL reaction. According to the results, A-3 is the kinetic product, not only for the larger nucleophilicity of acylhydrazine 3 but also for the larger electrophilicity of aldehyde A, which plays an important role in the reaction kinetics. Likewise, acylhydrazones formed from A were found in larger amounts than the ones formed from B.
This can be attributed to the electronegative nitrogen present in A, which by inductive effect causes the carbonyl group to be more electron deficient, making it more susceptible to nucleophilic attack. Noteworthy, data fit to a kinetic model is quite difficult for this system, however, within the first 8% of the reaction, the DCL follows a second order reaction with a 4-5% error, this allows to estimate that acylhydrazone A-3 is generated in around 12-fold faster than its B-3 counterpart. Interestingly, electrophilicity of the aldehyde is more important than acylhydrazine nucleophilicity in both, kinetic and thermodynamic control of the DCL. Upon equilibrium, A-containing acylhydrazones exhibit similar concentrations, which supports the dynamic character of the DCL.
The evident acylhydrazine interchange is probably due to the conjugation of the hydrazonic nitrogen (-NH-) with the carbonyl group which reduces the conjugation of this one with the imino group (C=N), making the latter a more reactive bond towards nucleophiles such as hydrazides or water (27). Therefore, the exchange reaction promotes another product to be formed at the expense of A-3, but still, at the end of the experiment (t = 817 min), this acylhydrazone continues to be the one with the highest percentage yield, therefore A-3 is the thermodynamic product in the library. The difference between the greater proportion of acylhydrazones formed from A, as compared with the generated from B, confirms the higher reactivity of 2-pyridinecarboxaldehyde over benzaldehyde, due to the presence of an electronegative nitrogen atom in the ring.
Effect of UV light irradiation
Acylhydrazones formed from the aldehyde A exhibit, in the Z configuration, a thermodynamic stabilization by the formation of an intramolecular hydrogen bond between the amine hydrogen and the pyridine nitrogen upon photochemical isomerization. Meanwhile, the Z configuration of acylhydrazones from B do not exhibit this thermodynamic stabilization (25, 26). With this in mind, it was interesting to observe the effect of UV light irradiation on the acylhydrazone distribution of the DCLs. For this purpose, the library was formed only with hydrazides 1 and 4 as well as the aldehydes A and B, due to their solubility in MeOH-d4.
The latter was used instead of DMSO-d6 because photoisomerization experiments in DMSO-d6 did not exhibit any appreciable changes, even after 150 min of UV irradiation, contrary to MeOH-d4 (Figure 9). This contrasting result is due to the viscosity of DMSO which slows down the photoisomerization of hydrazone-based compounds (25-28).
In a typical procedure, a competition reaction was carried out until equillibrium was reached. Afterwards, the mixture was irradiated with UV light using a mercury lamp of 250 W. The competition reaction was monitored for 228 min. The relative concentrations of each acylhydrazone were calculated only at the end of the experiment and the results were 39/15/29/17% of A-1/A-4/ B-1/B-4, respectively. The fact that those products containing the hydrazide 1 are in greater proportion, suggests that hydrazide 4 is less nucleophilic by the overall inductive effect that the N of the pyridine ring in position 3 has on the R group. Once the equilibrium was reached, the library was subjected to UV irradiation for 1 h and then was monitored by 1H-NÍMR. It is remarkable the appearance of new signals in the spectra shown in Figures 10-12 which correspond to the Z isomers of compounds A-1 and A-4.
The relative percentages shown in Table 1 were calculated by integrating those signals obtained in Figure 11 that are not overlapped and then by the substraction between these and the overlapped ones, the integrals and therefore the percentages of the latters were obtained. From the distribution of acylhydrazones it can be observed that the product which is amplified after 60 min of UV light irradiation is the Z isomer of A-1, suggesting the adaptation of the library when a stimulus is applied. Vantomme et al. (27) also observed the same photoselection in a different DCL with similar yields of photoisomerization.
A second DCL was generated from the same building blocks (acylhydrazines 1 and 2 and aldehydes A and B) in the presence of UV light irradiation. For this purpose, the NMR tubes were irradiated with a mercury vapor lamp during 1 h before the reaction started. Afterwards, the 1H-NMR spectra were taken to observe the distribution of the library (Figure 13). The amplified product for this DCL was the acylhydrazone B-1 (Table 1). The presence of A-1 and A-4 Z isomers on the library proved that UV light is part of the system, however, this also indicates that whether UV light is added at the beginning or at the end of the reaction, the amplified product will be a different one.
Noteworthy, in both cases the resulting product distribution is quite difficult to analyze even by the use of 2D NMR experiments, besides, once the UV light is removed the products concentrations do not go back to the previous distributions. These results imply that the system is in a metastable thermodynamic point due to the hydrogen bond formation (22, 25). In any case, these experiments proved the use of UV light as an irreversible template in DCL amplification and deserves further exploration.
Effect of the introduction of metal cations on DCL distributions
Finally, we wanted to explore the role of metal ions on DCL distributions, since it is well known that hydrazones and acylhydrazones derived from 2-pyridinecarboxaldehydes and 2-pyridinehydrazines or acylhydrazines are able to coordinate metal cations in a terpyrdine-like fashion (22, 25, 26). In this regard it can be thought that the introduction of metal ions can be used as a template to amplify acylhydrazones derived from aldehyde A. Accordingly, hydrazines 1 and 4 and aldehydes A y B were used to form the DCL; in addition, 0.5 eq of Zn(OTf)2 were added to the mixture, the reaction was monitored for 820 min. Comparing this library with other DCL in the present work (Figure 14) it was observed from the beginning of the reaction the apearence of only four N-H signals instead of eight corresponding to the formation of every possible acylhydrazone.
The ones that disappear, correspond to those acylhydrazones derived from aldehyde A, those which have a propitious structure to form a complex with Zn2+ by their tridentate NNO coordination site (28-32).
These signals disappearance are proof of the formation of ML2 type complexes usually formed with this kind of ligands (22, 25-27). When they form a complex, these ligands are deprotonated, either because there is a relatively basic environment or because the enol form of the ligand predominates (32) Although it is known that the addition of this template (M2+ ions) results in the formation of such complexes, it is not possible to know which component is amplified, because the signals from each product are highly overlapped Therefore, it is necessary to determine the binding and stability constants to have a clearer idea of what it is inside the solution
Although DCLs have been studied with some detail over the last years, it is difficult to compare our results with the literature Since, on one hand, most reports deal with the use of biological chemical templates and only one article introduces UV light to a DCL (based on aldehydes and hydrazines) obtaining similar results (27) On the other hand, metal ion selection has been studied for a more simple system (23) and similar to the present work a metalloselection was observed.
Conclusions
Generation of dynamic combinatorial libraries derived from acylhydrazines 1-4 and aldehydes A and B were monitored by 1H-NMR spectroscopy, achieving the calculation of the products distribution in time in most cases. The acylhydrazone A-3 was both the kinetic and thermodynamic product of two of the formed libraries, confirming the greater nucleophilicity of the corresponding hydrazide due to the electron releasing character of the dimethylamino group and the higher electrophilicity of 2-pyridin-carboxaldehyde as compared with benzaldehyde, because of the presence of an electronegative nitrogen atom in its structure.
In competitive DCL reactions acylhydrazone products derived from hydrazide 1 (versus hydrazide 4) were found in higher yields, suggesting that the reduced nucleophilicity of hydrazide 4 is due to the overall inductive effect that the pyridine ring N in position 3 has on the R group (R=-(CO)-NH-NH2). Exposure to a physical stimulus such as irradiation with UV light, demostrated that DCLs respond or adapt themselves to that stimulus, reorganizing and leading to the formation of a new library. In this particular case, it was also observed that depending on the time when the stimulus is added, the amplified product change, because the formation of the E isomer occurs first that the Z one, still in the presence of UV light.
Finally, the disappearance of the signals corresponding to the acylhydrazones NHv fragment derived from 2-pyridinecarboxaldehyde, by adding Zn2+, demostrates the formation of ML2 type complexes and its amplification.
Acknowledgements
Authors are grateful to Vicerrectoría de Investigaciones and Centro de Excelencia en Nuevos Materiales (CENM) from Universidad del Valle (Colombia) for the financial support of this project. We also thank professor Julien Wist for his collaboration regarding NMR spectroscopy experiments.
References
1. Corbett, P. T.p Leclaire, J.; Vial, L. West, K. R.; Wietor, J. L. Sanders, J. K. M. et al. Dynamic Combinatorial Chemistry. Chem. Rev. (Washington, DC, U. S.) 2006, 706, 36-52. DOI: https://doi.org/10.1002/chin.200648268.
2. Cougnon, F. B. L.; Sanders, J. K. M. Evolution of Dynamic Combiantioral Chemistry. Acc. Chem. Res. 2012, 45, 2211. DOI: https://doi.org/10.1021/ar200240m.
3. Custelcean, R. Dynamic chemistry of anion recognition. Top. Curr. Chem. 2012, 322, 193. DOI: https://doi.org/10.1007/128_2011_197.
4. Bru, M.; Alfonso, I.; Burguete, M. I.; Luis, S. V. Anion-Templated Syntheses of Pseudopeptidic Macrocycles Angew. Chemie Int. Ed. 2006, 45, 6155. DOI: https://dx.doi.org/10.1002/anie.200602206.
5. Bru, M.; Alfonso, I.; Bolte, M.; Burguete, M. I.; Luis, S. V. Structurally disfavoured pseudopeptidic macrocycles through anion templation Chem. Commun. 2011, 47, 283. DOI: https://doi.org/10.1039/c0cc01784a.
6. Besenius, P.; Cormack, P. A. G.; Ludlow, R. F; Otto, S.; Sherrington, D. C. Polymersupported cationic templates for molecular recognition of anionic hosts in water. Chem. Commun. 2008, 2809. DOI: https://doi.org/10.1039/b802982b.
7. Saggiomo, V.; Lüning, U. Transport of calcium ions through a bulk membrane by use of a dynamic combinatorial library. Chem. Commun. 2009, 3771. DOI: https://doi.org/10.1039/b902847a.
8. Verma, A.; Rotello, V. M. Surface recognition of biomacromolecules using nanoparticle receptors. Chem. Commun. 2005, 303. DOI: https://doi.org/10.1039/b410889b.
9. Ingerman, L . A.; Cuellar, M . E.; Waters, M . L . A small molecule receptor that selectively recognizes trimethyl lysine in a histone peptide with native protein-like affinity. Chem. Commun. 2010, 46, 1839. DOI: https://doi.org/10.1039/c000255k.
10. Shi, B.; Stevenson, R. ; Campopiano, D . J. ; Greaney, M . F. Discovery of Glutathione S-Transferase Inhibitors Using Dynamic Combinatorial Chemistry. J. Am. Chem. Soc. 2006, 728, 8459. DOI: https://doi.org/10.1021/ja058049y.
11. Vial, L.; Sanders, J . K . M.; Otto, S . A catalyst for an acetal hydrolysis reaction from a dynamic combinatorial library. New J. Chem. 2005, 29, 1001. DOI: https://doi.org/10.1039/b505316a.
12. Gasparini, G.; Prins, L . J.; Scrimin, P. Exploiting Neighboring-Group Interactions for the Self-Selection of a Catalytic Unit. Angew. Chemie Int. Ed. 2008, 47, 2475 . DOI: https://doi.org/10.1002/anie.200703857.
13. Prins, L . J. ; Scrimin, P. Covalent Capture: Merging Covalent and Noncovalent Synthesis Angew. Chemie Int. Ed. 2009, 48, 2288 DOI: https://doi.org/10.1002/anie.200803583.
14. Belowich, M . E.; Valente, C.; Stoddart, J. F. Template-Directed Syntheses of Rigid Oligorotaxanes under Thermodynamic Control Angew. Chemie Int. Ed 2010, 49, 7208 DOI: https://doi.org/10.1002/anie.201004304.
15. Belowich, M . E.; Valente, C.; Smaldone, R. A.; Friedman, D . C.; Thiel, J ; Cronin, L ; Stoddart, J F Positive Cooperativity in the Template-Directed Synthesis of Monodisperse Macromolecules J. Am. Chem. Soc. 2012, 734, 5243. DOI: https://doi.org/10.1021/ja2107564.
16. Chung, M . -K.; White, P. S.; Lee, S . J. ; Gagné, M . R. Synthesis of Interlocked 56-Membered Rings by Dynamic Self-Templating Angew. Chemie Int. Ed 2009, 48, 8683 DOI: https://doi.org/10.1002/anie.200903478.
17. Mal, P. ; Breiner, B.; Rissanen, K.; Nitschke, J. R. White Phosphorus Is Air-Stable Within a Self-Assembled Tetrahedral Capsule Science 2009, 324, 1697. DOI: https://doi.org/10.1126/science.1175313.
18. Horiuchi, S.; Murase, T. ; Fujita, M . Noncovalent Trapping and Stabilization of Dinuclear Ruthenium Complexes within a Coordination Cage J. Am. Chem. Soc. 2011, 733, 12445 DOI: https://doi.org/10.1021/ja205450a.
19. Pluth, M . D.; Bergman, R . G.; Raymond, K . N. Acid Catalysis in Basic Solution: A Supramolecular Host Promotes Orthoformate Hydrolysis . Science 2007, 376, 85. DOI: https://doi.org/10.1126/science1138748.
20. Sadownik, J . W. ; Philp, D . A Simple Synthetic Replicator Amplifies Itself from a Dynamic Reagent Pool Angew. Chemie Int. Ed 2008, 47, 9965 . DOI: https://doi.org/10.1002/anie.200804223.
21. Von Delius, M.; Geertsema, E . M.; Leigh, D . A . A synthetic small molecule that can walk down a track. Nat. Chem. 2010, 2, 96 . DOI: https://doi.org/10.1038/nchem.481.
22. Chaur, M N ; Collado, D ; Lehn, J -M Configurational and Constitutional Information Storage: Multiple Dynamics in Systems Based on Pyridyl and Acyl Hydrazones Chem. A Eur. J 2011, 77, 248. DOI: https://doi.org/10.1002/chem.201002308.
23. Vantomme, G.; Lehn, J.-M. Photo- and Thermoresponsive Supramolecular Assemblies: Reversible Photorelease of K+ Ions and Constitutional Dynamics. Angew. Chem. Int. Ed. 2013, 52, 3940. DOI: https://doi.org/10.1002/anie.201210334.
24. Vantomme, G.; Hafezi, N.; Lehn, J.-M. A light-induced reversible phase separation and its coupling to a dynamic library of imines. Chem. Sci. 2014, 5, 1475. DOI: https://doi.org/10.1039/c3sc53130a.
25. Romero, E.; D'Vries, R.; Zuluaga, F.; Chaur, M. Multiple Dynamics of Hydrazone Based Compounds. J. Braz. Chem. Soc. 2015, 26, 1265. DOI: https://doi.org/10.5935/0103-5053.20150092.
26. Chaur, M.N. Aroylhydrazones as potential systems for information storage: photoisomerization and metal complexation. Rev. Colomb. Quim. 2012, 41, 349-358. DOI: https://doi.org/10.15446/rev.colomb.quim.v43n1.50540.
27. Vantomme, G.; Jiang, S.; Lehn, J.-M. Adaptation in Constitutional Dynamic Libraries and Networks, Switching between Orthogonal Metalloselection and Photoselection Processes. J. Am. Chem. Soc. 2014,136, 9509. DOI: https://doi.org/10.1021/ja504813r.
28. Lehn, J.-M. Conjecture: Imines as Unidirectional Photodriven Molecular Motors-Motional and Constitutional Dynamic Devices. Chemistry 2006, 12, 5910. DOI: https://doi.org/10.1002/chem.200600489.
29. Stadler, A.-M.; Harrowfield, J. Bis-acyl-/aroyl-hydrazones as multidentate ligands. Inorganica Chim. Acta 2009, 362, 4298. DOI: https://doi.org/10.1016/j.ica.2009.05.062.
30. Pouralimardan, O.; Chamayou, A.-C.; Janiak, C.; Hosseini-Monfared, H. Hydrazone Schiff base-manganese(II) complexes: Synthesis, crystal structure and catalytic reactivity. Inorganica Chim. Acta 2007, 360, 1599. DOI: https://doi.org/10.1016/j.ica.2006.08.056.
31. Mangalam, N. A.; Sivakumar, S.; Sheeja, S. R.; Prathapachandra Kurup, M. R.; Tiekink, E. R. T. Chemistry of molecular and supramolecular structures of vanadium(IV) and dioxygen bridged V (V) complexes incorporating tridentate hydrazone ligands. Inorganica Chim. Acta 2009, 362, 4191. DOI: https://doi.org/10.1016/j.ica.2009.06.029.
32. Bernhardt, P. V; Chin, P.; Sharpe, P. C.; Richardson, D. R. Hydrazone chelators for the treatment of iron overload disorders: iron coordination chemistry and biological activity. Dalton Trans. 2007, 9226, 3232. DOI: https://doi.org/10.1039/b704102k.
Referencias
Corbett, P. T.; Leclaire, J.; Vial, L.; West, K. R.; Wietor, J. L.; Sanders, J. K. M. et al. Dynamic Combinatorial Chemistry. Chem. Rev. (Washington, DC, U. S.) 2006, 106, 3652. DOI: http://dx.doi.org/10.1002/chin.200648268.
Cougnon, F. B. L.; Sanders, J. K. M. Evolution of Dynamic Combiantioral Chemistry. Acc. Chem. Res. 2012, 45, 2211. DOI: https://doi.org/10.1021/ar200240m.
Custelcean, R. Dynamic chemistry of anion recognition. Top. Curr. Chem. 2012, 322, 193. DOI: 10.1007/128_2011_197.
Bru, M.; Alfonso, I.; Burguete, M. I.; Luis, S. V. Anion-Templated Syntheses of Pseudopeptidic Macrocycles. Angew. Chemie Int. Ed. 2006, 45, 6155. DOI: https://doi.org/10.1002/anie.200602206.
Bru, M.; Alfonso, I.; Bolte, M.; Burguete, M. I.; Luis, S. V. Structurally disfavoured pseudopeptidic macrocycles through anion templation. Chem. Commun. 2011, 47, 283. DOI: https://doi.org/10.1039/c0cc01784a.
Besenius, P.; Cormack, P. A. G.; Ludlow, R. F.; Otto, S.; Sherrington, D. C. Polymer-supported cationic templates for molecular recognition of anionic hosts in water. Chem. Commun. 2008, 2809. DOI: https://doi.org/10.1039/b802982b.
Saggiomo, V.; Lüning, U. Transport of calcium ions through a bulk membrane by use of a dynamic combinatorial library. Chem. Commun. 2009, 3711. DOI: https://doi.org/10.1039/b902847a.
Verma, A.; Rotello, V. M. Surface recognition of biomacromolecules using nanoparticle receptors. Chem. Commun. 2005, 303. DOI: https://doi.org/10.1039/b410889b.
Ingerman, L. A.; Cuellar, M. E.; Waters, M. L. A small molecule receptor that selectively recognizes trimethyl lysine in a histone peptide with native protein-like affinity. Chem. Commun. 2010, 46, 1839. DOI: https://doi.org/10.1039/c000255k.
Shi, B.; Stevenson, R.; Campopiano, D. J.; Greaney, M. F. Discovery of Glutathione S-Transferase Inhibitors Using Dynamic Combinatorial Chemistry. J. Am. Chem. Soc. 2006, 128, 8459. DOI: https://doi.org/10.1021/ja058049y.
Vial, L.; Sanders, J. K. M.; Otto, S. A catalyst for an acetal hydrolysis reaction from a dynamic combinatorial library. New J. Chem. 2005, 29, 1001. DOI: https://doi.org/10.1039/b505316a.
Gasparini, G.; Prins, L. J.; Scrimin, P. Exploiting Neighboring-Group Interactions for the Self-Selection of a Catalytic Unit. Angew. Chemie Int. Ed. 2008, 47, 2475. DOI: https://doi.org/10.1002/anie.200703857.
Prins, L. J.; Scrimin, P. Covalent Capture: Merging Covalent and Noncovalent Synthesis. Angew. Chemie Int. Ed. 2009, 48, 2288. DOI: https://doi.org/10.1002/anie.200803583.
Belowich, M. E.; Valente, C.; Stoddart, J. F. Template-Directed Syntheses of Rigid Oligorotaxanes under Thermodynamic Control. Angew. Chemie Int. Ed. 2010, 49, 7208. DOI: https://doi.org/10.1002/anie.201004304.
Belowich, M. E.; Valente, C.; Smaldone, R. A.; Friedman, D. C.; Thiel, J.; Cronin, L.; Stoddart, J. F. Positive Cooperativity in the Template-Directed Synthesis of Monodisperse Macromolecules. J. Am. Chem. Soc. 2012, 134, 5243. DOI: https://doi.org/10.1021/ja2107564.
Chung, M.-K.; White, P. S.; Lee, S. J.; Gagné, M. R. Synthesis of Interlocked 56-Membered Rings by Dynamic Self-Templating. Angew. Chemie Int. Ed. 2009, 48, 8683. DOI: https://doi.org/10.1002/anie.200903478.
Mal, P.; Breiner, B.; Rissanen, K.; Nitschke, J. R. White Phosphorus Is Air-Stable Within a Self-Assembled Tetrahedral Capsule. Science 2009, 324, 1697. DOI: https://doi.org/10.1126/science.1175313.
Horiuchi, S.; Murase, T.; Fujita, M. Noncovalent Trapping and Stabilization of Dinuclear Ruthenium Complexes within a Coordination Cage. J. Am. Chem. Soc. 2011, 133, 12445. DOI: https://doi.org/10.1021/ja205450a.
Pluth, M. D.; Bergman, R. G.; Raymond, K. N. Acid Catalysis in Basic Solution: A Supramolecular Host Promotes Orthoformate Hydrolysis. Science 2007, 316, 85. DOI: https://doi.org/10.1126/science.1138748.
Sadownik, J. W.; Philp, D. A Simple Synthetic Replicator Amplifies Itself from a Dynamic Reagent Pool. Angew. Chemie Int. Ed. 2008, 47, 9965. DOI: https://doi.org/10.1002/anie.200804223.
Von Delius, M.; Geertsema, E. M.; Leigh, D. A. A synthetic small molecule that can walk down a track. Nat. Chem. 2010, 2, 96. DOI: https://doi.org/10.1038/nchem.481.
Chaur, M. N.; Collado, D.; Lehn, J.-M. Configurational and Constitutional Information Storage: Multiple Dynamics in Systems Based on Pyridyl and Acyl Hydrazones. Chem. A Eur. J. 2011, 17, 248. DOI: https://doi.org/10.1002/chem.201002308.
Vantomme, G.; Lehn, J.-M. Photo- and Thermoresponsive Supramolecular Assemblies: Reversible Photorelease of K+ Ions and Constitutional Dynamics. Angew. Chem. Int. Ed. 2013, 52, 3940. DOI: https://doi.org/10.1002/anie.201210334.
Vantomme, G.; Hafezi, N.; Lehn, J.-M. A light-induced reversible phase separation and its coupling to a dynamic library of imines. Chem. Sci. 2014, 5, 1475. DOI: https://doi.org/10.1039/c3sc53130a.
Romero, E.; D’Vries, R.; Zuluaga, F.; Chaur, M. Multiple Dynamics of Hydrazone Based Compounds. J. Braz. Chem. Soc. 2015, 26, 1265. DOI: https://doi.org/10.5935/0103-5053.20150092.
Chaur, M.N. Aroylhydrazones as potential systems for information storage: photoisomerization and metal complexation. Rev. Colomb. Quim. 2012, 41, 349-358. DOI: https://doi.org/10.15446/rev.colomb.quim.v43n1.50540.
Vantomme, G.; Jiang, S.; Lehn, J.-M. Adaptation in Constitutional Dynamic Libraries and Networks, Switching between Orthogonal Metalloselection and Photoselection Processes. J. Am. Chem. Soc. 2014, 136, 9509. DOI: https://doi.org/10.1021/ja504813r.
Lehn, J.-M. Conjecture: Imines as Unidirectional Photodriven Molecular Motors—Motional and Constitutional Dynamic Devices. Chemistry 2006, 12, 5910. DOI: https://doi.org/10.1002/chem.200600489.
Stadler, A.-M.; Harrowfield, J. Bis-acyl-/aroyl-hydrazones as multidentate ligands. Inorganica Chim. Acta 2009, 362, 4298. DOI: https://doi.org/10.1016/j.ica.2009.05.062.
Pouralimardan, O.; Chamayou, A.-C.; Janiak, C.; Hosseini-Monfared, H. Hydrazone Schiff base-manganese(II) complexes: Synthesis, crystal structure and catalytic reactivity. Inorganica Chim. Acta 2007, 360, 1599. DOI: https://doi.org/10.1016/j.ica.2006.08.056.
Mangalam, N. A.; Sivakumar, S.; Sheeja, S. R.; Prathapachandra Kurup, M. R.; Tiekink, E. R. T. Chemistry of molecular and supramolecular structures of vanadium(IV) and dioxygen-bridged V(V) complexes incorporating tridentate hydrazone ligands. Inorganica Chim. Acta 2009, 362, 4191. DOI: https://doi.org/10.1016/j.ica.2009.06.029.
Bernhardt, P. V; Chin, P.; Sharpe, P. C.; Richardson, D. R. Hydrazone chelators for the treatment of iron overload disorders: iron coordination chemistry and biological activity. Dalton Trans. 2007, 9226, 3232. DOI: https://doi.org/10.1039/b704102k.
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Kilian Caillaud, Catherine Ladavière. (2022). Water‐Soluble (Poly)acylhydrazones: Syntheses and Applications. Macromolecular Chemistry and Physics, 223(15) https://doi.org/10.1002/macp.202200064.
2. Mónica A. Gordillo, Mónica Soto‐Monsalve, Christian C. Carmona‐Vargas, Gustavo Gutiérrez, Richard F. D'vries, Jean‐Marie Lehn, Manuel N. Chaur. (2017). Photochemical and Electrochemical Triggered Bis(hydrazone) Switch. Chemistry – A European Journal, 23(59), p.14872. https://doi.org/10.1002/chem.201703065.
3. Meixia He, Jean-Marie Lehn. (2019). Time-Dependent Switching of Constitutional Dynamic Libraries and Networks from Kinetic to Thermodynamic Distributions. Journal of the American Chemical Society, 141(46), p.18560. https://doi.org/10.1021/jacs.9b09395.
4. David Peña, Antonio López-Piñeiro, Damian Fernández, Mark E. Light, Juan Manuel Prieto, Lucía Santisteban, Richardo Xhavier Valladares, Pedro Cintas, Reyes Babiano. (2023). A new series of acylhydrazones derived from metribuzin with modulated herbicidal activity. Heliyon, 9(11), p.e21313. https://doi.org/10.1016/j.heliyon.2023.e21313.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons (CC. Atribución 4.0) que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).